Abstract
The E2F and pocket protein families are known to play an important role in the regulation of both cellular proliferation and terminal differentiation. In this study, we have used compound E2F and pocket protein mutant mouse embryonic fibroblasts to dissect the role of these proteins in adipogenesis. This analysis shows that loss of E2F4 allows cells to undergo spontaneous differentiation. The ability of E2F4 to prevent adipogenesis seems to be quite distinct from the known properties of E2F. First, it can be separated from any change in either E2F-responsive gene expression or cell cycle regulation. Second, it is a specific property of E2F4, and not other E2Fs, and it occurs independently of E2F4's ability to interact with pocket proteins. In addition, E2F4 loss does not override the differentiation defect resulting from pRB loss even though it completely suppresses the proliferation defect of Rb−/− mouse embryonic fibroblasts. This finding definitively separates the known, positive role of pRB in adipogenesis from its cell cycle function and shows that this pocket protein is required to act downstream of E2F4 in the differentiation process.
The E2F transcription factors play a key role in regulating the cell cycle by controlling the transcription of genes encoding key components of the cell cycle and DNA replication machinery (reviewed in refs. 1 and 2). The activity of E2F is controlled by the pocket protein family that includes the retinoblastoma protein (pRB), a known tumor suppressor, and its relatives p107 and p130. The pocket proteins bind to E2F and inhibit the activation of E2F-responsive genes through two mechanisms (reviewed in refs. 1 and 2). First, their binding interferes with the function of the E2F transactivation domain. Second, the resultant E2F⋅pocket protein complexes can actively repress transcription through recruitment of histone deacetylase and methylase activities. Consequently, the pocket proteins are key regulators of the G1 to S transition.
The E2F proteins can be divided into three subgroups based on structural and functional homology (reviewed in refs. 1 and 2). E2F6 is the sole member of one subgroup. It does not interact with the pocket proteins and has been shown to play a role in vertebrate patterning through interaction with the mammalian polycomb complex (3, 4). The second E2F subgroup includes E2F1, E2F2, and E2F3a. These E2Fs are specifically regulated by pRB, and not p107 and p130, in vivo. Combined analysis of both overexpressing and deficient cells indicates that they play a key role in activating E2F-responsive genes and thereby promoting S-phase progression (5–7). E2F3b, a recently identified second product of the E2F3 gene, is most closely related to E2F1, -2, and -3a, but its biological properties remain to be established (8–10).
The final E2F subgroup, comprising E2F4 and E2F5, is the focus of this study. E2F5 binds specifically to p130 in vivo (reviewed in refs. 1 and 2). In contrast, E2F4 associates with pRB, p107, and p130 and comprises the majority of the E2F⋅pocket protein complexes in vivo (11). These E2Fs are thought to play a key role in the repression of responsive genes through the recruitment of pocket proteins and their associated histone deacetylases. Chromatin immunoprecipitation assays show that the E2F4/5⋅p107/p130 complexes specifically associate with E2F-responsive promoters during G0/G1 when these genes are repressed and are then replaced by the activating E2Fs E2F1, -2, and -3, as cells reenter the cell cycle (12). Importantly, cells that lack either E2F4 and E2F5 or p107 and p130 have a defect in their ability to exit the cell cycle in response to a variety of growth arrest signals (13–15). In the case of p107−/−;p130−/− cells, this defect correlates with a failure to mediate the normal repression of certain E2F-responsive genes (15, 16). The repressive E2F complexes are also required for normal development. Mice lacking E2F4 or E2F5 display distinct developmental defects in one or a few tissues that in each case result in neonatal lethality (17–19). It is currently unclear whether the developmental defects reflect a requirement for E2F4 and/or E2F5 in cell cycle control or whether these proteins play a more direct role in the differentiation process. In contrast, there is considerable evidence that the essential requirement for p107 and p130 in long bone development is due to their role in mediating cell cycle exit (20, 21).
pRB is also an important developmental regulator (as reviewed in refs. 2 and 22). pRB-deficient embryos die in mid-gestation because of a combination of inappropriate proliferation and apoptosis in a wide variety of tissues. These defects are suppressed by the loss of either E2F1 or E2F3, indicating that they result from the inappropriate release of the activating E2Fs. However, the analysis of Rb;E2f1 and Rb;E2f3 double mutant embryos reveals a key role for pRB in development that is unrelated to the regulation of E2F. In vitro differentiation assays support a role for pRB in myogenesis, osteogenesis, and adipogenesis, and pRB can function as a coactivator for transcription factors that promote these differentiation processes (as reviewed in ref. 22).
In this study, we investigated the role of the repressive E2F complexes in terminal differentiation. We have focused on adipogenesis because this pathway is well defined and it involves both a proliferation and a differentiation phase. Specifically, confluence-arrested mouse embryonic fibroblasts (MEFs) can be triggered to undergo differentiation by treatment with the hormone insulin, the glucocorticoid receptor agonist dexamethasone, and the cAMP phosphodiesterase inhibitor methyl-isobutylxanthine (23). After treatment, the cells express CCAAT/enhancer-binding protein β (C/EBPβ) and C/EBPδ and then undergo one to two rounds of mitotic clonal expansion before arresting again and inducing expression of the C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ) transcription factors that are required for lipid accumulation and adipocyte induction. The E2F and pocket protein complexes have already been linked to adipogenesis at several different levels. First, hormonal induction correlates with the dissociation of the repressive E2F4⋅p130 complex and the expression of the activating E2Fs (24, 25). E2F activity induces clonal expansion and the simultaneous transcriptional activation of PPARγ, an E2F-responsive gene (25). Consistent with this scheme, p107−/−;p130−/− MEFs have an increased propensity to undergo hormone-induction adipogenesis (26). Second, pRB functions as a coactivator for C/EBPβ and thereby plays a positive role in adipogenesis (24, 27, 28). Finally, C/EBPα and PPARγ can repress E2F activity and may thereby contribute to the switch from the clonal expansion to the differentiation phase (29–31).
Materials and Methods
Cell Culture and Analysis.
MEFs were generated as described (6, 32). For each genotype, two to four independently derived, passage 4 MEF lines were analyzed. Confluent monolayers were fed every other day with MEF media (DMEM containing 10% FCS, 1% penicillin/streptomycin, 1% glutamine) in either the absence (cell cycle and spontaneous differentiation assays) or presence (hormone-induced adipogenesis) of 1 μM dexamethasone (Sigma), 0.5 mM 3-isobutyl-1-methylxanthine (Sigma), and 5 μg/ml insulin (Sigma). Lipid accumulation was assessed by quantification of Oil Red O staining (33). Cell cycle progression was determined by Northern blotting (6) or 24-h incubation in media containing 3 mg/ml BrdUrd and 0.3 mg/ml 5-fluorodeoxyuridine (FdUrd) to assess DNA synthesis.
E2F4 Expression and Immunoblotting.
pBabe-E2F4ΔC was generated by digesting pCMV-E2F444 (34) with BamHI and HindIII and subcloning into the pBABE vector. 293 T cells were infected at 60% confluence with 1 μg pBabe vector and 1 μg of pCL-Eco packaging construct (35) by using FuGENE 6 (Roche Diagnostics). The media was replaced 24 h later, and supernatants were harvested at 48 and 72 h, filtered, and used to infect MEFs with 8 μg polybrene. Infected cells were selected by culturing in 2 μg/ml puromycin for 48 h. Western blotting assays were performed by using 50 μg whole cell lysates and the LLF4-1 monoclonal antibody (11).
Results
It has previously been shown that the loss of E2F4 or p107/p130 promotes the ability of primary fibroblasts to undergo adipogenesis in response to hormone induction (25, 26). We wished to determine whether this finding reflects the shared role of these proteins in the formation of repressive E2F complexes and whether it correlates with, or can be separated from, regulation of cell cycle exit. To address these questions, we intercrossed the E2f4 (19) and p107;p130 (20) mutant mouse strains to allow the generation of compound E2f4−/−;p107−/−;p130−/− (TKO) mutant MEFs. Fig. 1A depicts how these mutations would affect the various E2F and pocket protein complexes that exist in normal cells. The properties of the TKO cells were directly compared with those of the parental p107−/−;p130−/− (DKO) and E2f4−/− mutant MEFs.
Figure 1.
E2F4 pocket protein repression is required for repression of adipogenesis. (A) Cartoon indicating the spectrum of E2F complexes that exist in normal cells and the components (denoted by the Xs) that are affected by the combined mutation of E2f4, p107, and p130. (B) WT (+/+), E2f4−/−, DKO, and TKO MEFs were induced to differentiate for 2 wk, stained with Oil Red O, and quantitated. (C) MEFs of all four genotypes maintained at confluence for 3 wk, stained with Oil Red O, and quantitated. (D) WT (+/+), E2f4−/−, and TKO MEFs stained with Oil Red O after 6 wk at confluence.
E2F4-Deficent MEFs Undergo Spontaneous Adipogenesis.
We first examined the ability of the TKO, DKO, and E2f4−/− MEFs to differentiate into adipocytes by using the standard hormone induction conditions. After 2 wk of induction, the degree of differentiation was determined by staining with Oil Red O, a lipophillic dye that can be extracted and quantified to assess for induction efficiency. As reported (25, 26), the absence of either E2F4 or p107 and p130 led to a 2- to 5-fold increase in adipogenesis (Fig. 1B). Strikingly, the combined loss of all three proteins yielded a significant increase in lipid accumulation (10-fold). The relative effects of the E2f4−/−, DKO, and TKO genotypes were observed with numerous preparations of MEFs, indicating that it is highly reproducible. Thus, E2F4 and the pocket proteins seem to have additive, negative effects on the differentiation process. Clearly, this effect could result from their contribution to either the same or to distinct regulatory processes.
During these studies we discovered that E2f4−/− MEFs were predisposed to undergoing adipogenesis when they were maintained at confluence without hormone induction (Fig. 1 C and D). Importantly, this spontaneous differentiation was observed with every preparation of E2f4−/− MEFs but never with the WT control MEFs (Fig. 1D and data not shown). Given this finding, we also tested the ability of DKO and TKO MEFs to accumulate lipids without hormone induction. In this assay, DKO cells behaved differently from E2f4−/− MEFs in that they consistently failed to undergo spontaneous adipogenesis. This result suggests that E2F4 and p107/p130 repress adipocyte induction through distinct mechanisms. This hypothesis is supported by the phenotype of the TKO MEFs. These cells were highly predisposed to undergo spontaneous differentiation, consistently yielding many more Oil Red O-positive cells than the E2f4−/− MEFs (Fig. 1 C and D). Taken together, these data indicate that E2F4 plays a gate-keeping role to block spontaneous adipocyte differentiation whereas p107/p130 inhibit induced differentiation in a distinct manner.
E2F4's Role in Adipogenesis Can Be Separated from Its Role in Cell Cycle Regulation.
The induction of adipogenesis in vitro involves transitions in cell cycle regulation from confluence arrest, to mitotic clonal expansion, and then terminal cell cycle exit. The p107−/−;p130−/− MEFs have a well documented defect in their ability to arrest in response to certain growth conditions (14, 15). It has previously been suggested that this finding could account for, or at least contribute to, the altered differentiation properties of these cells (26). Given this finding, we wished to establish whether there is any correlation between the differentiation and cell cycle phenotypes of the various mutant genotypes.
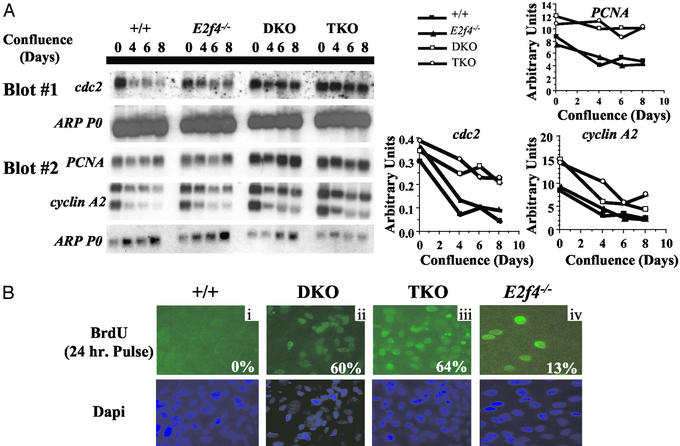
Because the in vitro adipogenesis is thought to require confluence arrest (reviewed in ref. 23), we wished to establish the cell cycle properties of the E2f4−/−, DKO, and TKO MEFs under these conditions (Fig. 2). First, we determined how the mutation of the E2F and pocket proteins affected the regulation of E2F-responsive genes (Fig. 2A). As confluence arrest proceeded, the levels of known E2F-responsive mRNAs declined with identical kinetics in the WT and E2f4−/− MEFs (Fig. 2A). This result is entirely consistent with previous conclusions that there are no defects in the regulation of either E2F target genes or cell cycle exit and reentry in E2f4−/− MEFs in serum-starvation/restimulation assays (17, 19). In contrast, we found that specific E2F-responsive mRNAs were maintained at high levels in the DKO MEFs even 8 days after confluence was established (Fig. 2A). This finding correlates with the known defect in the down-regulation of a subset of E2F-responsive genes in serum-starved DKO MEFs (15). Significantly, the pattern of target gene expression in the TKO MEFs was identical to that observed in the DKO MEFs (Fig. 2A). Thus, mutation of E2f4 does not alter the regulation of E2F-responsive genes in WT cells or modulate (either qualitatively or quantitatively) the gene expression defect that results from the loss of p107/p130.
Figure 2.
Loss of repression leads to defects in cell cycle control and deregulation of E2F target gene expression. (A) WT (+/+), E2f4−/−, DKO, and TKO MEFs were grown and analyzed at 0, 4, 6, and 8 days of confluence for expression of E2F responsive genes. Gene expression was quantitated by using the loading control (ARP PO). (Bi) WT (+/+), (Bii) DKO, (Biii) TKO, and (Biv) E2f4−/− MEFs were grown and maintained at confluence for 8 days and then pulsed with BrdUrd for 24 h. The percentage of cycling cells was based on counting 300 DAPI (4′,6-diamidino-2-phenylindole)-positive nuclei for each genotype.
In parallel with the gene expression studies, we also monitored the confluent monolayers for evidence of inappropriate cell cycle entry by culturing them in the presence of BrdUrd for 24 h (Fig. 2B). As expected, there was no evidence of proliferating cells in the WT controls. In contrast, we observed sporadic cells, or pairs of cells, within the E2f4−/− monolayer that were BrdUrd-positive. Importantly, this cell division specifically occurred in E2f4−/− cells that were subjected to confluence and not serum starvation arrest (Fig. 2B; refs. 17 and 19). Given this specificity, and the lack of any detectable gene-expression defect, we believe that the presence of proliferating cells within the confluent E2f4−/− monolayer is indicative of cells that are undergoing the clonal expansion phase of the adipocyte differentiation process as opposed to evidence of an intrinsic cell cycle defect. This hypothesis is supported by our finding that E2f4 mutation had no detectable effect on the magnitude of the cell cycle defect in the DKO MEFs. Even after 8 days of confluence arrest, a similar high proportion of the DKO (60%) and TKO (64%) monolayers underwent inappropriate DNA synthesis during the 24 h labeling window (Fig. 2B). The arrest defects of the DKO and TKO MEFs were also similar to one another at shorter BrdUrd labeling times (data not shown). We therefore conclude that the DKO and TKO MEFs display indistinguishable defects in their ability to down-regulate E2F-responsive genes and arrest in G0/G1 in response to confluence arrest.
Importantly, these data strongly suggest the role of E2F4 in differentiation can be separated from its role in cell cycle regulation. E2f4−/− cells have an increased propensity to undergo spontaneous adipogenesis without intrinsic defects in the regulation of E2F-responsive genes and therefore cell cycle entry/exit. Moreover, E2F4 loss greatly increases the differentiation of DKO MEFs without modulating their cell cycle phenotype.
p130 Is the Predominant Mediator of the p107/p130 Differentiation Effect.
We wished to establish whether the enhanced differentiation of TKO versus E2f4−/− MEFs depends on the combined mutation of p107 and p130 or whether it can be attributed to the mutation of one of these genes. To address this question, we compared the differentiation phenotypes of E2f4−/−;p107−/− and E2f4−/−;p130−/− MEFs with those of E2f4−/− and TKO controls (Fig. 3A and data not shown). p107 loss had no effect on the differentiation of E2f4−/− MEFs. In contrast, the E2f4−/−;p130−/− MEFs displayed a wide range of phenotypes in this assay: some were equivalent to E2f4−/− MEFs whereas others phenocopied the TKO MEFs (Fig. 3A and data not shown). This degree of variation was specifically observed with E2f4−/−;p130−/− MEFs and not those derived from any other mutant genotype. Moreover, it only arose between E2f4−/−;p130−/− MEF lines isolated from different embryos and never the same embryo (data not shown). It has recently been reported that the p130 mutant allele used in this study has some residual activity (21) and strain-specific modifiers of p130 have been described (36). It therefore seems likely that this phenotypic range arises because the mixed (C57BL/6 × 129S/v) genetic background of the MEFs modulates the residual p130 activity such that it is either above or below a critical threshold level.
Figure 3.
Analysis of E2F4-mediated adipocyte repression. (A) E2f4−/−;p107−/− and E2f4−/−;p130−/− MEFs were induced to differentiate in response to hormones for 2 wk, stained with Oil Red O, quantitated, and compared with WT (+/+), E2f4−/−, and TKO MEFs. (B) WT, E2f5−/−, E2f4−/−, and E2f4−/−;E2f5−/− MEFs were induced to differentiate in response to hormones for 2 wk and analyzed as in A. (C) Full-length or a C-terminal truncated (E2F4ΔC) E2F4 were expressed in control and E2f4−/− MEFs by retroviral-mediated infection. (D) Infected cells were induced by hormones to differentiate for 2 wk and analyzed as above. (E) WT (+/+), E2f4−/−, Rb−/−, and E2f4−/−; Rb−/− MEFs were differentiated in response to hormones for 2 wk and analyzed as above.
Importantly, at least in certain settings, our data show that the p130 mutation was sufficient to enhance the E2f4−/− differentiation phenotype as well as the combined mutation of p107 and p130. Because, p107 loss has no detectable effect in these assays, this strongly suggests that p130 must be the predominant mediator of the p107/p130 differentiation role. We also examined the cell cycle properties of E2f4−/−;p107−/− and E2f4−/−;p130−/− confluent monolayers, as judged by both BrdUrd incorporation and E2F-responsive gene expression (data not shown). Regardless of their differentiation phenotype, all preparations of E2f4−/−;p107−/− and E2f4−/−;p130−/− MEFs had cell cycle phenotypes that were indistinguishable from that of the E2f4−/− MEFs. This suggests that the ability of p130 mutation to enhance the differentiation phenotype of the E2F4-deficient cells occurs independently of p130's role in cell cycle control.
Pocket Protein Binding Is Not Required for the Repression of Adipogenesis by E2F4.
E2F4 and -5 have many overlapping properties in vivo including regulation by p130 (reviewed in ref. 2). We therefore tested whether E2F5 inhibits adipogenesis in a manner analogous to E2F4 (Fig. 3B). Significantly, E2F5 loss had little or no effect on the differentiation phenotype of either wild-type or E2f4−/− MEFs. Thus, the inhibition of adipogenesis seems to be a unique property of E2F4 and not E2F5.
It was important to establish whether the increased differentiation of E2f4−/− MEFs results for the loss of E2F4 and not a subsequent change in the resulting cell lines. To address this issue, we introduced full length E2F4 into wild type and E2f4−/− MEFs by using retroviral infection (Fig. 3C). We then compared the ability of control (pBABE) and E2F4-expressing cells to undergo adipogenesis (Fig. 3D). Significantly, wild-type E2F4 reduced the differentiation potential of E2f4−/− MEFs to wild type levels. Thus, the differentiation defect of the E2f4−/− MEFs is due to the loss of E2F4.
Our data indicates that E2F4 and p130 act synergistically to inhibit adipocyte differentiation. There is also extensive evidence to indicate that pRB is essential for adipogenesis (27, 28). Given these findings, we wanted to determine whether E2F4's ability to repress differentiation depends on its ability to bind to pocket proteins. To address this question, we generated a mutant form of E2F4 that lacks the C-terminal pocket protein binding domain (E2F4ΔC) and introduced this into wild type or E2f4−/− MEFs by retroviral infection (Fig. 3C). Although this truncated protein is unable to bind pRB-family members (data not shown), it suppressed the differentiation defect of the E2f4−/− MEFs almost as efficiently as the wild-type E2F4 protein. This suggests that E2F4 inhibits differentiation independently of pocket protein binding.
pRB Is Required for Adipogenesis in the Absence of E2F4.
Previous studies have shown that Rb−/− cells fail to significantly undergo adipogenesis (27, 28). pRB has been shown to serve as a coactivator for C/EBPβ, suggesting that pRB loss impairs differentiation by reducing the activity of C/EBPβ. However, it is also possible that the differentiation defect is a consequence of the requirement for pRB in mediating cell cycle arrest. We have recently shown that E2F4 loss completely suppresses the inappropriate proliferation of pRB-deficient cells under growth arrest conditions (32). Given this finding, we wished to establish whether E2F4 loss would modulate the differentiation phenotype of Rb−/− MEFs. Consistent with our previous studies, the E2f4−/− MEFs differentiated much better that the wild-type controls in response to hormone induction (Fig. 3E). In contrast, the E2f4−/−;Rb−/− MEFs failed to undergo hormone-induced differentiation (Fig. 3E). Moreover, confluence arrested E2f4−/−;Rb−/− MEFs never spontaneously differentiated (data not shown). We therefore conclude that E2F loss is unable to override the differentiation defect of the Rb−/− MEFs even though it suppresses the cell cycle defect. This finding supports the prevailing view that pRB promotes differentiation independently of its role in cell cycle regulation. Furthermore, our data indicate that, in adipogenesis, pRB is required to act downstream of E2F4.
Discussion
In this study, we have continued to investigate the roles of the E2F and pocket proteins in the regulation of adipocyte differentiation. It was previously shown that hormone-induced adipogenesis is promoted by the loss of either E2F4 or p107 and p130 (25, 26). It seemed highly likely that the shared activity of E2F4 and p107/p130 simply reflects their participation in transcriptionally repressive complexes. However, our current analyses of compound mutant MEFs do not support this hypothesis. Instead, they suggest that the E2F and pocket proteins contribute to the regulation of adipocyte differentiation through three distinct mechanisms. Moreover, each one of these can be separated from effects on cell cycle control.
The first mechanism involves the E2F4 transcription factor. We have found that E2F4 loss predisposes MEFs to undergo adipogenesis. This phenotype includes increasing the proportion of cells that differentiate in response to the standard hormone treatment as well as enabling confluent monolayers to undergo spontaneous differentiation. We do not understand the precise mechanism by which E2F4 mediates this apparent gatekeeper function, but our analysis suggests that it is quite distinct from the known properties of E2F. First, the increased differentiation of the E2F4-deficient cells can be separated from any gross changes in either E2F-responsive gene expression or cell cycle regulation. Second, this differentiation phenotype is neither shared nor augmented by the loss of E2F5, with which it is known to cooperate in the control of cell cycle exit, or any other E2F family member (ref. 25; Fig. 3; unpublished findings), indicating that it is a specific activity of E2F4. Finally, E2F4's ability to suppress differentiation can occur in the absence of its C terminus sequences. This deleted region includes the region of the protein that mediates both transcriptional activation and pocket protein binding functions. Thus, E2F4's ability to inhibit differentiation must occur independently of its ability to either directly activate target genes, or to mediate their repression through recruitment of the pocket proteins and their associated histone deactylates and/or methylases.
Our observations do not rule out the possibility that E2F4 contributes to the regulation of adipogenesis by influencing, either positively or negatively, the activity of other transcription factors. Indeed, there is some precedence for this mode of action. Chen et al. (37) recently showed that the E2F4/p107 complex binds to the Smad proteins in a transforming growth factor β (TGFβ)-responsive manner and that the resulting complex represses the c-myc gene. Because E2F4's role in adipogenesis does not require pocket protein binding, it cannot function through this precise mechanism, but one can envisage variations on this theme. Importantly, the finding that E2F4 participates in both TGFβ signaling and adipogenesis indicates that its role extends well beyond the regulation of classical E2F-responsive genes. It also raises the possibility that E2F4 might play a direct role in the differentiation of other cell lineages. In this regard, it is interesting to note that the erythroid defect in the E2F4-deficient mice seems to result from a failure in terminal differentiation without any obvious affect on cell cycle regulation (17, 19).
The second mechanism by which the E2F and pocket proteins contribute to the regulation of adipocyte differentiation involves p107 and p130. Consistent with previous reports (26), we find that DKO MEFs undergo hormone-induced differentiation with increased efficiency. Our analysis of compound mutant MEFs provides additional insight into their action. First, the loss of these proteins cannot trigger the differentiation process in an analogous manner to the absence of E2F4. However, their loss significantly enhances adipogenesis that has been induced by either classic hormone induction or E2F4 loss. Although our data show that E2F4 can function in a pocket protein-independent manner, it is entirely possible that p107 and p130 repress adipocyte induction in association with one or more E2F proteins, including E2F4. Because E2F4 and p107/p130 both regulate adipogenesis negatively, we cannot order the action of these proteins. However, we were able exploit the cooperative effects of E2F4 and p107/p130 deficiency to determine the relative contribution of p107 and p130. Whereas p107 loss had no detectable effect on the differentiation phenotype of the E2f4−/− MEFs, p130 mutation could increase it, as well as the combined mutation of p107 and p130. This finding strongly suggests that p130 is the predominant mediator of the p107/p130 differentiation function.
These observations provide important insight into the relative roles of p107 and p130. To date, the analysis of both development and cell cycle defects in the p107;p130 compound mutant mice have largely highlighted the overlapping properties of these proteins (14–16, 20, 21). For example, the p107−/− and p130−/− mice have no obvious developmental defects, but the p107−/−;p130−/− compound mutants are neonatal lethal (20, 38). In contrast, our differentiation studies provide clear evidence for differential properties of p130 and p107 that is commensurate with the specificity of their expression in arrested (p130) vs. cycling (p107) cells. Given these findings, it will be interesting to compare the phenotypes of the E2f4−/−;p130−/− and E2f4−/−;p107−/− mutant mice to determine whether the differential roles of p130 and p107 can be observed in vivo and whether they are specific for adipocyte differentiation or extend to other tissues.
The analysis of the E2f4−/−;p130−/− MEFs also raises some question of the role of cell cycle effects. The DKO MEFs have a major defect in their ability to exit the cell cycle, and this was thought to account for, or at least contribute to, the propensity of these cells to undergo adipogenesis (14, 15, 26). However, the E2f4−/−;p130−/− MEFs do not have an obvious cell cycle defect even though they display a differentiation defect as profound as TKO MEFs. Thus, at least in the absence of E2F4, the effect of p130 mutation seems largely cell cycle independent.
The final mechanism by which the E2F and pocket proteins contribute to the regulation of adipocyte differentiation involves pRB. Numerous studies have shown that pRB is required for the differentiation of MEFS in vitro. This finding is believed to be due to pRB's role as a coactivator for C/EBPβ, but it could also result from the profound cell cycle defect of the Rb−/− cells (24, 27, 28). Indeed, Fajas and coworkers (39) recently reported that pRB also negatively regulates adipogenesis via inhibition of PPARγ and suggested that pRB's dominant positive role might primarily depend on its key role in cell cycle exit. Because E2F4 loss suppresses the inappropriate proliferation of pRB-deficient cells (32), we have also examined the differentiation phenotype of the E2f4−/−;Rb−/− MEFs. Significantly, these cells are completely impaired in their ability to undergo either spontaneous or hormone-induced differentiation. This finding represents evidence that the requirement for pRB in adipogenesis is independent of any cell cycle effect. Moroever, it shows that pRB acts downstream of E2F4 in the regulation of adipogenesis. Because C/EBP and E2F bind to the same region of pRB, we initially suspected that E2F4 would inhibit adipogenesis by preventing the pRB-C/EBPβ interaction. However, our data show that E2F4's inhibitory activity does not require the C-terminal, pocket protein-binding domain. Thus, whereas this competitive mechanism may operate in some situations, it cannot fully account for the adipogenic properties of the E2F4-deficient cells.
Our data clearly show that the three distinct mechanisms by which the E2F and pocket proteins regulate adipogenesis can be separated from one another and, in certain situations, from cell cycle control. However, in vivo, it seems highly likely that there will be extensive cross-talk between these pathways to ensure ordered progression through sequential stages of the differentiation process and link each one to the appropriate cell cycle condition. Thus, the future challenge will be to establish the underlying basis of each mechanism and determine how they are coordinated in vivo.
Acknowledgments
We thank Tyler Jacks for kindly providing the p107 and p130 mutant mouse strains, and members of the Lees laboratory for helpful discussions during this study and the preparation of this manuscript. R.L.L. was supported by a fellowship from the Anna Fuller Fund. This work was supported by National Institutes of Health Grant RO1-GM53204 (to J.A.L.).
Abbreviations
- pRB
retinoblastoma protein
- MEF
mouse embryonic fibroblast
- C/EBPβ
CCAAT/enhancer-binding protein β
- PPARγ
peroxisome proliferator-activated receptor γ
- TKO
E2f4−/−;p107−/−;p130−/−
- DKO
p107−/−;p130−/−.
References
- 1.Dyson N. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 2.Trimarchi J M, Lees J A. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi J M, Fairchild B, Wen J, Lees J A. Proc Natl Acad Sci USA. 2001;98:1519–1524. doi: 10.1073/pnas.041597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storre J, Elsasser H P, Fuchs M, Ullmann D, Livingston D M, Gaubatz S. EMBO Rep. 2002;3:695–700. doi: 10.1093/embo-reports/kvf141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helin K. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 6.Humbert P O, Verona R, Trimarchi J M, Rogers C, Dandapani S, Lees J A. Genes Dev. 2000;14:690–703. [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L, Timmers C, Maiti B, Saavedra H I, Sang L, Chong G T, Nuckolls F, Giangrande P, Wright F A, Field S J, et al. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Armanious M K, Thomas M J, Cress W D. Oncogene. 2000;19:3422–3433. doi: 10.1038/sj.onc.1203682. [DOI] [PubMed] [Google Scholar]
- 9.Adams M R, Sears R, Nuckolls F, Leone G, Nevins J R. Mol Cell Biol. 2000;20:3633–3639. doi: 10.1128/mcb.20.10.3633-3639.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leone G, Nuckolls F, Ishida S, Adams M, Sears R, Jakoi L, Miron A, Nevins J R. Mol Cell Biol. 2000;20:3626–3632. doi: 10.1128/mcb.20.10.3626-3632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moberg K, Starz M A, Lees J A. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi Y, Rayman J B, Dynlacht B D. Genes Dev. 2000;14:804–816. [PMC free article] [PubMed] [Google Scholar]
- 13.Gaubatz S, Lindeman G J, Ishida S, Jakoi L, Nevins J R, Livingston D M, Rempel R E. Mol Cell. 2000;6:729–735. doi: 10.1016/s1097-2765(00)00071-x. [DOI] [PubMed] [Google Scholar]
- 14.Bruce J L, Hurford R K, Jr, Classon M, Koh J, Dyson N. Mol Cell. 2000;6:737–742. doi: 10.1016/s1097-2765(00)00072-1. [DOI] [PubMed] [Google Scholar]
- 15.Hurford R K, Jr, Cobrinik D, Lee M H, Dyson N. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan G J, Wong J, Jacks T. Mol Cell Biol. 1998;18:206–220. doi: 10.1128/mcb.18.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rempel R E, Saenz-Robles M T, Storms R, Morham S, Ishida S, Engel A, Jakoi L, Melhem M F, Pipas J M, Smith C, Nevins J R. Mol Cell. 2000;6:293–306. doi: 10.1016/s1097-2765(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 18.Lindeman G J, Dagnino L, Gaubatz S, Xu Y, Bronson R T, Warren H B, Livingston D M. Genes Dev. 1998;12:1092–1098. doi: 10.1101/gad.12.8.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humbert P O, Rogers C, Ganiatsas S, Landsberg R L, Trimarchi J M, Dandapani S, Brugnara C, Erdman S, Schrenzel M, Bronson R T, Lees J A. Mol Cell. 2000;6:281–291. doi: 10.1016/s1097-2765(00)00029-0. [DOI] [PubMed] [Google Scholar]
- 20.Cobrinik D, Lee M H, Hannon G, Mulligan G, Bronson R T, Dyson N, Harlow E, Beach D, Weinberg R A, Jacks T. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- 21.Rossi F, MacLean H E, Yuan W, Francis R O, Semenova E, Lin C S, Kronenberg H M, Cobrinik D. Dev Biol. 2002;247:271–285. doi: 10.1006/dbio.2002.0691. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski M M, Jacks T. Oncogene. 1999;18:7873–7882. doi: 10.1038/sj.onc.1203244. [DOI] [PubMed] [Google Scholar]
- 23.Rosen E D, Spiegelman B M. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Richon V M, Lyle R E, McGehee R E., Jr J Biol Chem. 1997;272:10117–10124. doi: 10.1074/jbc.272.15.10117. [DOI] [PubMed] [Google Scholar]
- 25.Fajas L, Landsberg R L, Huss-Garcia Y, Sardet C, Lees J A, Auwerx J. Dev Cell. 2002;3:39–49. doi: 10.1016/s1534-5807(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 26.Classon M, Kennedy B K, Mulloy R, Harlow E. Proc Natl Acad Sci USA. 2000;97:10826–10831. doi: 10.1073/pnas.190343597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen P L, Riley D J, Chen Y, Lee W H. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 28.Charles A, Tang X, Crouch E, Brody J S, Xiao Z X. J Cell Biochem. 2001;83:414–425. doi: 10.1002/jcb.1239. [DOI] [PubMed] [Google Scholar]
- 29.Slomiany B A, D'Arigo K L, Kelly M M, Kurtz D T. Mol Cell Biol. 2000;20:5986–5997. doi: 10.1128/mcb.20.16.5986-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porse B T, Pedersen T A, Xu X, Lindberg B, Wewer U M, Friis-Hansen L, Nerlov C. Cell. 2001;107:247–258. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- 31.Altiok S, Xu M, Spiegelman B M. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee E Y, Cam H, Ziebold U, Rayman J B, Lees J A, Dynlacht B. Cancer Cell. 2002;2:463–472. doi: 10.1016/s1535-6108(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez-Zacarias J L, Castro-Munozledo F, Kuri-Harcuch W. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 34.Verona R, Moberg K, Estes S, Starz M, Vernon J P, Lees J A. Mol Cell Biol. 1997;17:7268–7282. doi: 10.1128/mcb.17.12.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LeCouter J E, Kablar B, Whyte P F, Ying C, Rudnicki M A. Development (Cambridge, UK) 1998;125:4669–4679. doi: 10.1242/dev.125.23.4669. [DOI] [PubMed] [Google Scholar]
- 37.Chen C R, Kang Y, Siegel P M, Massague J. Cell. 2002;110:19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- 38.Lee M H, Williams B O, Mulligan G, Mukai S, Bronson R T, Dyson N, Harlow E, Jacks T. Genes Dev. 1996;10:1621–1632. doi: 10.1101/gad.10.13.1621. [DOI] [PubMed] [Google Scholar]
- 39.Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril M-B, Mirad S, Auwerx J. Dev Cell. 2002;3:903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]