Abstract
Ranolazine is a novel anti-anginal agent capable of producing anti-ischemic effects at plasma concentrations of 2–6 μM without reducing heart rate or blood pressure. The present study examines its electrophysiologic effects in isolated canine ventricular myocytes, tissues and arterially-perfused left ventricular wedge preparations.
Methods
Transmembrane action potentials (AP) from epicardial and midmyocardial (M) regions and a pseudo-electrocardiogram were simultaneously recorded from wedge preparations. APs were also recorded from epicardial and M tissues. Whole cell currents were recorded from epicardial and M myocytes.
Results
Ranolazine inhibited IKr (IC50=11.5μM), late INa, late ICa, peak ICa, and INa-Ca (IC50=5.9, 50, 296 and 91 μ M, respectively) and IKs (17% at 30 μM), but caused little or no inhibition of Ito or IK1. In tissues and wedge preparations, ranolazine produced a concentration-dependent prolongation of AP duration of epicardial, but abbreviation of that of M cells, leading to reduction or no change in transmural dispersion of repolarization (TDR). At [K+]o=4 mM, 10μ M ranolazine prolonged QT interval by 20 ms but did not increase TDR. Extrasystolic activity or spontaneous Torsade de Pointes (TdP) were never observed and stimulation-induced TdP could not be induced at any concentration of ranolazine, either in normal or low [K+]o. Ranolazine (5–20 μ M) suppressed early afterdepolarizations (EADs) and reduced the increase in TDR induced by selective IKr blocker, d-sotalol.
Conclusion
Ranolazine produces ion channel effects similar to those observed following chronic amiodarone (reduced IKr, IKs, late INa and ICa). Ranolazine’s actions to suppress EADs and reduce TDR suggest that, in addition to its anti-anginal actions, the drug may possess antiarrhythmic activity.
Abridged Abstract
Ranolazine is a novel anti-anginal agent capable of producing anti-ischemic effects without reducing heart rate or blood pressure. The present study examines its electrophysiologic effects in isolated canine ventricular myocytes, tissues and arterially-perfused left ventricular wedge preparations. Ranolazine produces ion channel effects similar to those observed following chronic amiodarone (reduced IKr, IKs, late INa and ICa). Preferential prolongation of epicardial vs M cell action potentials results in modest QT interval prolongation but reduction in transmural dispersion of repolarization (TDR). Ranolazine’s effects to reduce TDR and suppress EADs suggest that, in addition to its anti-anginal actions, the drug may possess antiarrhythmic activity.
Keywords: Anti-ischemic, IKr blocker, Transmural dispersion of repolarization, QT prolongation, Early afterdepolarizations
Ranolazine is the first of a new class of compounds shown to exert anti-anginal effects without causing significant bradycardia and/or lowering systemic blood pressure.1–3 The drug has been shown to increase left ventricular function in animals with chronic heart failure4 and two clinical trials (MARISA and CARISA) have established its effectiveness as an anti-anginal agent. 1–3 Ranolazine is known to produce a modest prolongation of the QT interval, yet little is known about the electrophysiologic actions of the drug.1 The present study was designed to assess the electrophysiological effects of ranolazine in myocytes, tissues and arterially-perfused wedge preparations isolated from the canine left ventricle.
Methods
Dogs weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with pentobarbital (35 mg/kg, i.v.). The chest was opened via a left thoracotomy, the heart excised and placed in a cold cardioplegic solution ([K+]0 = 8 mmol/L, 4°C). All protocols were in conformance with guidelines established by the Institutional Animal Care and Use Committee.
Voltage Clamp Studies in Isolated Canine Ventricular Myocytes
Myocytes were isolated by enzymatic dissociation from a wedge-shaped section of the left ventricular free wall supplied by the left circumflex coronary artery5. We used epicardial and M cells for most ion channel studies, the exception being measurement of IKs, which was done exclusively in epicardial cells, because of the low density of this current in M cells. We did not note any differences between the two cell types in response to ranolazine. Tyrode’s solution used in the dissociation contained (in mM): 135 NaCl, 5.4 KCl, 1 MgCl2, 0 or 0.5 CaCl2, 10 glucose, 0.33 NaH2PO4, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and the pH was adjusted to 7.4 with NaOH.
External solutions
| IKrand IK1(mM) | IKs(mM) | INa-Ca, Late INaand ICaWhole cell/Perf-patch (mM) | ItoWhole cell (mM) |
|---|---|---|---|
| 11 glucose | 11 glucose | 10 glucose | 10 glucose |
| 4 KCl | 4 KCl | - | 4 KCl |
| 1.2 MgSO4 | 1.8 MgCl2 | 1 MgCl2 | 1 MgCl2 |
| 2 CaCl2 | 1.8 CaCl2 | 2 CaCl2 | 2 CaCl2 |
| 132 NaCl | 145 NaCl | 140 Na-methanesulfonate | 140 N-methyl-D-glucamine-Cl |
| 1 NaH2PO4 | |||
| 20 HEPES | 10 HEPES | 10 HEPES | 10 HEPES |
| pH 7.4 with NaOH | pH 7.4 with NaOH | pH 7.4 with methane sulfonic acid | pH 7.4 with HCl |
Internal solutions
| ICa, Late INa, (mM) | INa-Ca (mM) | Ito (mM) | IKs, IKrand IK1 (mM) |
|---|---|---|---|
| 140 Cs-aspartate | 140 Cs-aspartate | 130 K-aspartate | 20 KCl |
| - | - | 20 KCl | 125 K-Aspartate |
| 10 NaOH | 10 NaOH | - | 1 MgCl2 |
| 1 MgCl2 | 1 MgCl2 | 1 MgCl2 | 10 EGTA |
| 5 MgATP | 5 MgATP | 5 MgATP | 5 MgATP |
| 10 HEPES | 10 HEPES | 10 HEPES | 5 HEPES |
| 10 EGTA | 0.1 EGTA | 5 EGTA | pH 7.1 with KOH |
| pH 7.1 with CsOH | pH 7.1 with CsOH | pH 7.1 with KOH |
L-type calcium current (ICa), late sodium current (Late INa), transient outward current (Ito), inward rectifier potassium current (IK1), slowly (IKs) and rapidly (IKr) activating delayed rectifier potassium current, and sodium-calcium exchange current (INa-Ca) were recorded at 37°C using standard patch electrodes. The composition of the external and pipette solutions is shown in the Tables above. IK1 was measured in presence of 3 μM ouabain and 5 μM nifedipine to block the sodium-potassium pump and L-type calcium current (ICa,L), respectively. IKs was measured in the presence of 5 μM E-4031 and 300 μM cadmium to block IKr and ICa. IKr was measured in the presence of 5 μM nifedipine.
Isolated myocytes were placed in a temperature controlled 0.5 ml chamber (Medical Systems, Greenvale, NY) on the stage of an inverted microscope and superfused at a rate of 2 ml/min. An eight-barrel quartz micromanifold (ALA Scientific Instruments Inc., Westbury, NY) placed 100 μm from the cell was used to apply ranolazine at concentrations of (in μM): 0.1, 0.5, 1.0, 5.0, 10 and 100 and in some cases 300 and 900. An Axopatch 1D amplifier (Axon Instruments, Foster City, CA) was operated in voltage clamp mode to record currents. Whole cell currents were filtered with a 3-pole low-pass Bessel filter at 5 kHz, digitized between 2 – 5 kHz (Digidata 1200A, Axon Instruments) and stored on a computer. Clampex 7 acquisition and analysis software (Axon Instruments) was used to record and analyze ionic currents. Pipette tip resistance was 1.0– 2.0 MΩ and seal resistance was greater than 5 GΩ. Electronic compensation of series resistance averaged 76 %. Voltages reported in the text were corrected for patch electrode tip potentials6. A 3 M KCl-agar bridge was used between the Ag/AgCl ground electrode and external solution to avoid development of a ground potential when switching to experimental solution.
IKs was elicited by depolarization to +40 mV for 2 sec from a holding potential of −50 mV followed by a repolarization step to −30 mV. The time-dependent tail current elicited by the repolarization was termed IKs. Ito was not blocked, but it had little influence on our measurement of IKs because of its fast and complete inactivation. All measurements were obtained 5–12 min after patch rupture because no significant run-down of IKs is generally observed during this interval. IKr was measured as the time-dependent tail current elicited at a potential of − 40 mV following a short 250 ms depolarizing pulse to 30 mV. IK1 was recorded during 900 ms of 10 mV voltage steps applied from a holding potential of − 40 mV to test potentials ranging from −100 mV to 0 mV, and was characterized as the 5 ms average of the steady state current at the end of the test pulse.
Peak ICa was defined as maximum inward current minus the current at the end of the test pulse. External solution contained 10 μM TTX to block the steady state component of late INa. Cells were rested for 20 seconds at −90 mV before evoking an 800 ms ramp to −60 mV and a 15 ms step to −50 mV to inactivate sodium channels and maintain voltage control immediately followed by a 500 ms step to 0 mV to record ICa in control solutions. This protocol was repeated 5 times at a rate of 0.5 Hz for each of the drug concentrations. Late ICa was defined as the sum of the slow and fast components calculated 300 ms after the start of the test pulse.
To trigger INa-Ca by means of the normal calcium transient, a 5 ms step to 0 mV to activate ICa and a calcium transient was immediately followed by a pulse to −80 mV to record INa-Ca. INa-Ca was quantified as total charge transported (pA × ms). Voltage clamp protocols were preceded by a train of ten pulses to 20 mV delivered at a rate of 0.5 Hz followed by a rest of 6 sec to maintain calcium loading of the SR.
Late INa was measured during a train of 30 pulses at repetition rates of 300 and 2000 ms. Currents during the last 5 pulses of the trains were averaged to reduce noise, and late INa was defined as the TTX-sensitive current. Protocols were repeated in drug-free solution, 2–4 min after adding ranolazine, and immediately after 10 μM TTX was added to completely block late INa. Action potentials, rather than square pulses were used to voltage clamp late INa. At a basic cycle length (BCL) of 300 ms, measurements were made midway through the plateau at a voltage of 13 mV and during phase 3 repolarization at a voltage of −28 mV. At a BCL of 2000 ms, measurements were made at voltages of 20 mV and −28 mV.
Action Potential Studies in Isolated Canine Ventricular Epicardial and M region Tissues
Epicardial and midmyocardial (M) cell preparations (tissue slices approximately 1 × 0.5 × 0.15 cm) were isolated from the left ventricle. The tissue slices were placed in a tissue bath (5 ml volume with flow rate of 12 ml/min) and allowed to equilibrate for at least 4 hours while superfused with an oxygenated Tyrode’s solution (pH=7.35, t0 =37±0.5°C) and paced at a basic cycle length (BCL) of 500 ms using field stimulation. The composition of the Tyrode’s solution was (in mM): NaCl 129, KCl 2 or 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5. Transmembrane potentials were recorded using standard glass microelectrodes filled with 2.7 M KCl (10–20 MΩDC resistance). Action potentials were recorded from isolate free-running Purkinje fibers as well as epicardial and M cell preparations. In the case of ventricular myocardial slices, control recordings were obtained after a 4–6 hour equilibrium period. The effects of ranolazine were determined at concentrations of 1, 5, 10, 50, and 100 μM, with recordings started 30 minutes after the addition of each concentration of the drug. Rate-dependence of ranolazine’s actions were determined by recording transmembrane action potentials at basic pacing cycle lengths (ec) of 300, 500, 800, 1000, 2000, 5000 ms. Time controls demonstrated the stability of the preparations over a period of 6 hours. The rate of rise of the action potential (Vmax) was recorded as a differentiated signal or measured from the digitally recorded signal (at 50 KHz).
Action Potential Studies in Arterially-Perfused Canine Left Ventricular Wedge Preparations
Transmural left ventricular wedges with dimensions of approximately 12 mm × 35 mm × 12 mm were dissected from the mid-to-basal anterior region of the left ventricular wall and a diagonal branch of the left anterior descending coronary artery was cannulated to deliver the perfusate (Tyrode’s solution as defined above). Transmembrane action potentials were recorded from epicardial and subendocardial M cell regions using floating microelectrodes. A transmural pseudo-electrocardiogram (ECG) was recorded using two Ag/AgCl half cells (2 mm diam. × 4 mm) placed approx. 1 cm. from the epicardial(+) and endocardial(−) surfaces of the preparation and along the same axis as the transmembrane recordings. Ventricular wedges were allowed to equilibrate in the chamber for 2 hrs while paced at BCL of 2000 ms using silver bipolar electrodes contacting the endocardial surface. Perfusion pressure was maintained at 40–50 mmHg and temperature at 35 or 37.5 °C. The preparations were fully immersed in the extracellular solution throughout the course of the experiment.
Programmed electrical stimulation (PES)
Premature stimulation was applied to the epicardial surface before and after each concentration of drug in an attempt to induce arrhythmias. Single pulses (S2) were delivered once after every tenth basic beat (S1) at cycle lengths of 2000 ms. The S1–S2 coupling interval was progressively reduced until refractoriness was encountered (S2 stimuli were of 2–3 ms duration with an intensity equal to 3–5 times the diastolic threshold).
DRUGS
Ranolazine, E-4031 and d-sotalol were all dissolved in distilled water.
STATISTICS
Statistical analysis was performed using one way repeated measures analysis of variance (ANOVA) followed by Bonferroni’s test, as appropriate. Mean values were considered to be different when p < 0.05.
RESULTS
Effect of Ranolazine on IKr, IKs, IK1, Ito, late INa, ICa, late ICa and INa-Ca
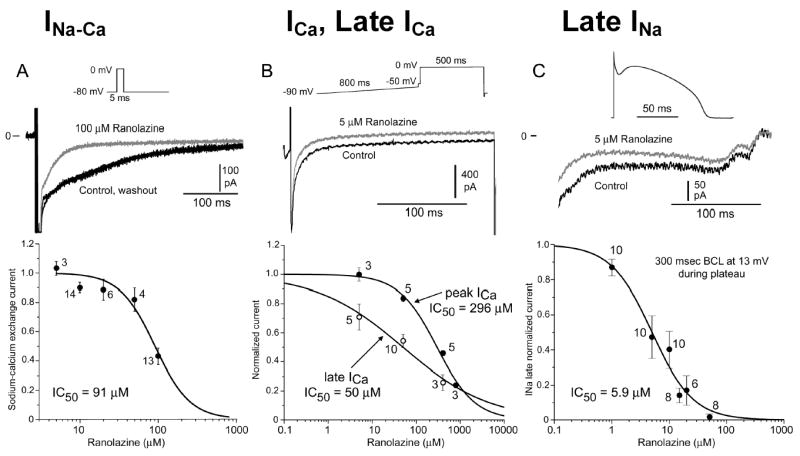
Figures 1–3 show the effect of ranolazine to inhibit IKr, late INa, ICa, late ICa and INa-Ca in canine LV myocytes (midmyocardial and epicardial cells). Ranolazine inhibited both inward depolarizing and outward repolarizing currents. The potency (IC50) of drug inhibition of late INa ranged between 5 and 21 μM, with a greater potency at more positive voltage-clamp test potentials and higher frequency of depolarization. Although ranolazine inhibited late ICa,L with an IC50 of 50 μM, significant inhibition (25–30 %) occurred within the therapeutic range (2–6 μM). The drug weakly inhibited INa-Ca (inward sodium-calcium exchange current), peak ICa,L, and the slow component of the delayed rectifier potassium current (IKs-17% inhibition at 30 μM and 20% at 900 μM), but produced no effect on the outward rectifier potassium current (IK1), or the transient outward potassium current (Ito).
Figure 1.
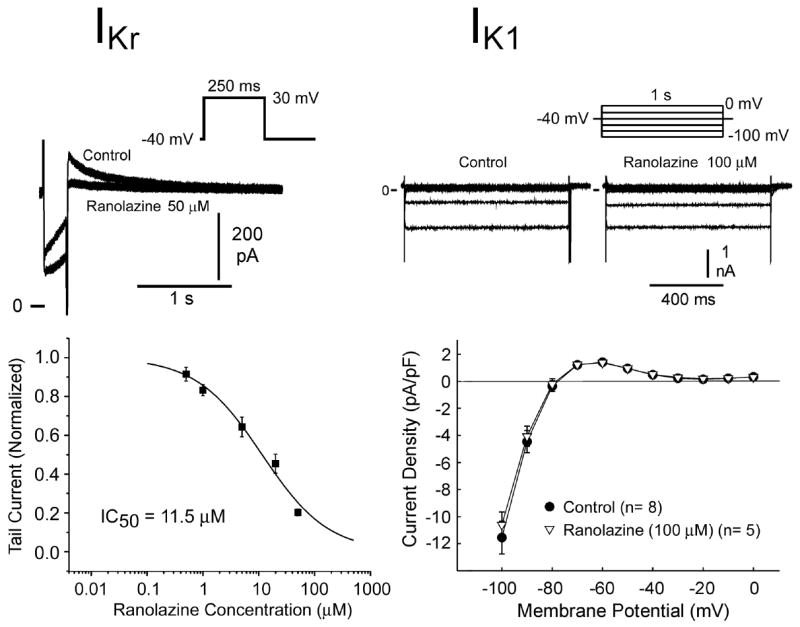
Effect of ranolazine on the rapidly activating component of the delayed rectifier current (IKr, left) and the inward rectifier current (IK1, right) in canine left ventricular myocytes. Top panels illustrate the voltage protocol and representative current traces. Bottom panels are the concentration-response relationships. Data are presented as mean ± S.E.M. (n = 5–8).
Figure 3.
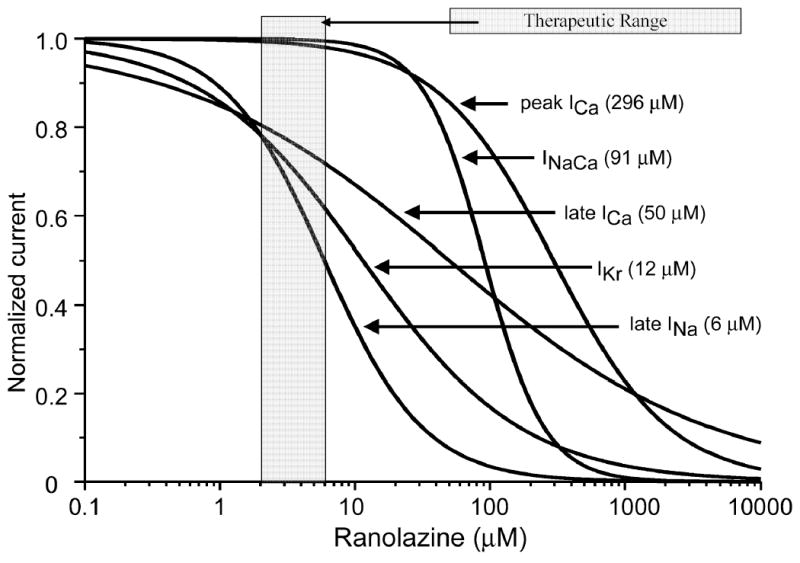
Summary of the concentration–response relationships for the effect of ranolazine to inhibit inward and outward ion channel currents in canine ventricular myocytes. Numbers inside the parentheses are IC50 values for the effect of ranolazine to inhibit the rapidly activating delayed rectifier potassium current (IKr), late sodium current (late INa), peak calcium current (ICa), late ICa, and the sodium-calcium exchange current (INa-Ca).
Figure 3 shows the superimposed concentration-response curves for all of the currents for which such a relationship could be obtained. The plot highlights the overlap between the inhibition by ranolazine of IKr, late INa and late ICa, at clinically relevant therapeutic concentrations (2–6 μM), suggesting that the effect of the drug to block outward repolarizing current, which prolongs APD, is substantially counterbalanced by its effect to inhibit the inward current, which is expected to abbreviate the action potential.
Effect of Ranolazine in Isolated Canine Ventricular Tissues
Ranolazine caused no change in resting membrane potential, action potential amplitude or overshoot in either M or epicardial cells, except at very high concentrations (100 μM) considerably above the plasma concentrations encountered in clinical use (Table 1).
TABLE 1.
Effects of ranolazine on phase 0 amplitude, resting membrane potential (RMP), and overshoot of action potential in M cell and epicardial preparations at a BCL of 500 ms.
| Ranolazine Concentration (μM)
|
||||||
|---|---|---|---|---|---|---|
| M Cell | ||||||
| Control | 1 | 5 | 10 | 50 | 100 | |
| Amplitude | 107±6 | 109±4 | 114±4 | 113±4 | 104±3 | 91±8* |
| RMP | −86±2 | −86±1 | −86±1 | −88±1 | −84±2 | −82±3 |
| Overshoot | 21±6 | 23±5 | 27±3 | 25±3 | 19±1 | 9±6 |
| Epicardial Cell | ||||||
|---|---|---|---|---|---|---|
| Control | 1 | 5 | 10 | 50 | 100 | |
| Amplitude | 95±2 | 93±2 | 101±1 | 94±2 | 86±6 | 93±2 |
| RMP | −84±2 | −84±2 | −89±1 | −88±1 | −86±1 | −85±2 |
| Overshoot | 11±1 | 10±2 | 12±1 | 8±2 | 0±6 | 8±4 |
Data are all in mV and expressed as mean±SEM, n=5 for all,
- p<0.05 vs. control
Figure 4 illustrates the effect of ranolazine on the maximal rate of rise of the upstroke (Vmax) of the action potential recorded from free-running canine Purkinje fibers. Significant inhibition, presumably due to the effect of ranolazine to block peak INa, was observed only at concentrations ≥50 μM. Similar inhibition was observed in canine ventricular M cells (not shown). Shown in the bottom panel of Figure 4 is the effect of ranolazine on the APD of canine Purkinje fibers. At a BCL of 2000 ms, ranolazine produced a concentration dependent abbreviation of APD which was greater at 50% than at 90% repolarization. At a BCL of 500 ms, the drug produced little change in APD90, but a concentration-dependent depression of the action potential plateau.
Figure 4.
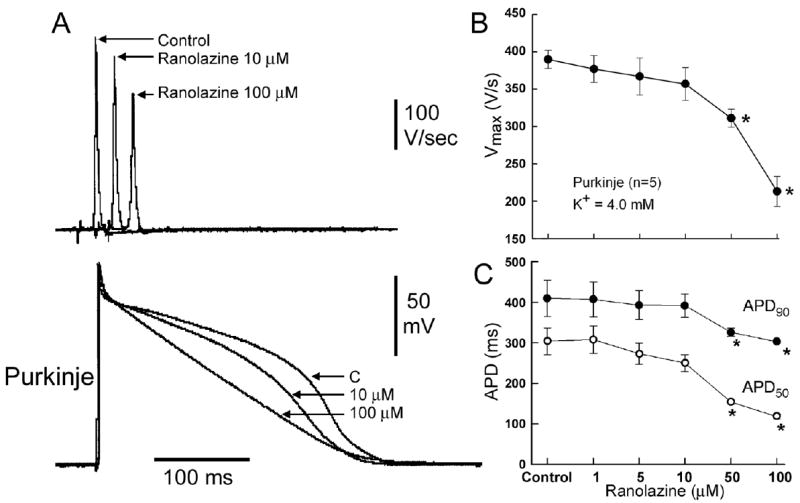
Concentration -dependent effects of ranolazine on the rate of rise of the upstroke of a Purkinje fiber action potential (Vmax). A: Shown are superimposed action potentials and corresponding differentiated upstrokes (dV/dt) recorded in the absence and presence of ranolazine (1–100 μM) (BCL=500 ms). B: Concentration-response relationship of ranolazine’s effect to reduce Vmax. Data are presented as mean ± S.E.M. (n=5).
C: Concentration-dependent effect of ranolazine on action potential duration at 50 and 90% repolarization (APD50and APD90). BCL=2000 ms. Values are mean ± SEM. * − 0.05 vs. control
The effects of ranolazine on the action potential of canine ventricular M cell and epicardial tissues are illustrated in Figure 5. The drug caused a small concentration-dependent prolongation of APD in epicardium, but an abbreviation or biphasic effect in M cell preparations. Thus, ranolazine caused a concentration-dependent reduction of transmural dispersion of action potential duration. Higher concentrations of the drug produced a depression of the plateau of the action potential, likely due to the effect of ranolazine to inhibit ICa and late ICa. The preferential prolongation of epicardial APD was more apparent at a [K+]o of 2 mM than at 4 mM (Figure 5, right panels). Early afterdepolarizations (EADs) were not observed at any concentration of ranolazine, not even under bradycardic and hypokalemic conditions known to potentiate the effect of IKr blocker to induce EADs.
Figure 5.
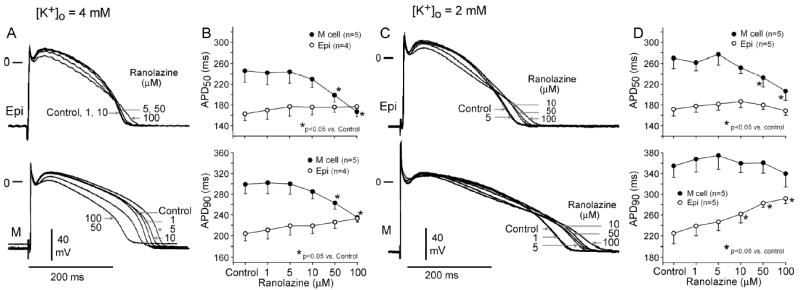
Left Panel: 4 mM [K+]o. Effects of ranolazine on epicardial and M cell action potentials. A: Superimposed transmembrane action potentials recorded under control conditions and following the addition of progressively higher concentrations of ranolazine (1–100 μM). B: Concentration-response curves for the effect of ranolazine on action potential duration (APD50 and APD90). Right Panels: 2 mM [K+]o. Effects of ranolazine on epicardial and M cell action potentials recorded at a pacing cycle length of 2000 ms and [K+]0 = 2 mM. C: Shown are superimposed transmembrane action potentials recorded in the absence and presence of ranolazine (1–100 μM). D: Concentration-dependent effect of ranolazine on action potential duration (APD50 and APD90). BCL=2000 ms. Data presented as mean ± SEM.
These concentration and rate-dependent characteristics of ranolazine distinguish it from other agents that block IKr. Whereas selective IKr blockers invariably produce a concentration - dependent prolongation of APD that is greater in Purkinje fibers and M cells than in epicardial cells, the multi-ion channel block produced by ranolazine causes a concentration dependent abbreviation of Purkinje and M cell APD, but a slight prolongation of APD of epicardial cells.
Figure 6 illustrates another important distinction between typical IKr blockers and ranolazine. E-4031, a highly selective IKr blocker, caused a reverse rate-dependent prolongation of APD that is much greater in M cells than in epicardial cells, leading to a bradycardia-dependent accentuation of transmural dispersion of APD (TD-APD), typical of the actions of IKr blockers. In contrast, ranolazine produced a largely rate-independent prolongation of APD in epicardium and abbreviation in M cells, leading to a rate-independent reduction of transmural dispersion of APD. The reverse rate-dependent prolongation of APD and accentuation of TD-APD by E-4031 were still more dramatic at a [K+]o of 2 mM (Fig 6, right panel). In contrast, ranolazine persisted in causing a rate-independent reduction of TD-APD at the lower [K+]o. These results are consistent with the clinical finding that prolongation of QTc by ranolazine, unlike that of pure IKr blockers, is not reverse-rate-dependent.7 Thus, although ranolazine modestly prolongs APD and QTc, it is fundamentally different from other IKr blockers, which are typically associated with proarrhythmic actions.
Figure 6.
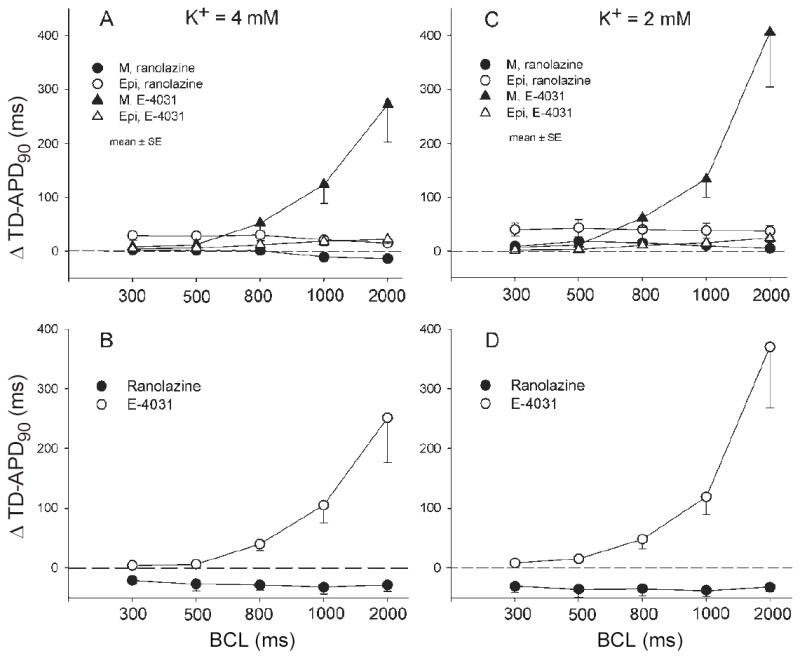
Rate-dependent effect of ranolazine (10 μM) and E-4031 (1 μM) on action potential duration (APD) in canine ventricular M cell and Epicardial tissue slices in the presence of 4 mM (A and B) and 2 mM (C and D) [K+]o. A and C: Rate-dependence of E-4031 and ranolazine-induced change in APD90 of M and epicardial cells, respectively. B and D: Rate-dependence of drug-induced change in transmural dispersion of action potential duration (TD-APD90). Values are mean ± SEM.
Effect of Ranolazine in Canine Left Ventricular Wedge Preparation
Table 2 shows the effect of ranolazine in isolated canine left ventricular wedge preparations. In the presence of 4 mM [K+]o and at a BCL of 2000 ms, ranolazine caused a small concentration dependent prolongation of epicardial APD and a small biphasic effect in the M region, resulting in a 30 ms QT prolongation at the highest concentration tested (100 μM). It is noteworthy that much of the QT interval prolongation observed at the higher concentrations of ranolazine is due a slowing of conduction secondary to sodium channel inhibition. Transmural dispersion of repolarization (TDR) showed a tendency to diminish, although the decrease was not statistically significant. Under hypokalemic conditions ([K+]o=3 mM), ranolazine produced a much greater prolongation of the QT interval, but TDR showed a similar tendency to decrease.
Table 2.
Summary of the effects of ranolazine on the action potential duration (APD), QT interval and transmural dispersion of repolarization (TDR) in the canine arterially-perfused left ventricular wedge preparation.
| 4 mM [K+]o | ||||||
|---|---|---|---|---|---|---|
| Epicardium | M region | |||||
| Concentration | APD50 | APD90 | APD50 | APD90 | QT | TDR |
| control | 180.4 ± 8.0 | 219.7 ± 9.6 | 218.0 ± 7.5 | 270.3 ± 9.6 | 276.7 ± 8.7 | 33.4 ± 3.2 |
| 1 μM | 185.6 ± 7.5 | 228.0 ± 9.5 | 216.6 ± 5.0 | 271.4 ± 8.6 | 283.8 ± 8.6* | 28.7 ± 2.5 |
| 5 μM | 189.5 ± 9.4 | 233.0 ± 9.5 | 220.6 ± 6.1 | 281.1 ± 9.1 | 295.1 ± 9.5* | 31.0 ± 3.8 |
| 10 μM | 188.9 ± 10.1 | 238.3 ± 10.2 | 219.6 ± 5.8 | 284.9 ± 9.8 | 301.3 ± 9.0* | 30.7 ± 4.5 |
| 100 μM | 164.3 ± 7.6 | 233.0 ± 7.5 | 188.6 ± 6.0* | 278.0 ± 8.1 | 306.9±11.7‡* | 28.2 ± 5.3 |
| 3 mM [K+]o | ||||||
|---|---|---|---|---|---|---|
| Epicardium | M region | |||||
| Concentration | APD50 | APD90 | APD50 | APD90 | QT | TDR |
| Control | 186.4 ± 8.6 | 234.9 ± 10.3 | 228.6 ± 12.9 | 285.9 ± 14.3 | 293.0 ± 12.6 | 29.5 ± 7.7 |
| 1 μM | 192.2 ± 9.2 | 240.0 ± 11.0 | 229.8 ± 13.9 | 289.7 ± 14.6 | 300.3 ± 12.8 | 28.7 ± 7.2 |
| 5 μM | 193.6 ± 9.1 | 250.6 ± 10.8 | 226.5 ± 11.7 | 298.4 ± 13.7 | 314.4 ± 11.5* | 26.0 ± 5.4 |
| 10 μM | 198.9 ± 7.7 | 262.1 ± 8.2 | 236.4 ± 8.9 | 307.9 ± 7.0 | 318.8 ± 8.8 | 25.0 ± 5.6 |
| 100 μM | 181.6 ± 14.7 | 285.3 ± 10.1* | 198.4 ± 12.0 | 312.2 ± 9.6 | 347.7 ± 16.2‡* | 12.9 ± 3.7 |
p<0.05 vs. control. n=7 (unless otherwise noted);
n=5 All values are mean ± SEM in ms; BCL=2000 ms
p<0.05 vs. control. n=5 (unless otherwise noted);
n=3 All values are mean ± SEM in ms; BCL=2000 ms
Ranolazine did not induce either spontaneous or programmed electrical stimulation-mediated TdP during endocardial or epicardial pacing of the canine left ventricular wedge preparation at any concentration up to 100 μM. TdP did not develop nor could it be induced, even in the presence extremely low [K+]o (2 or 3 mM) and high concentrations of ranolazine. In contrast, both spontaneous and stimulation induced TdP have been shown to develop in the perfused wedge preparation in response to a wide variety of IKr blockers.8
Neither early or delayed afterdepolarizations were observed in either tissue or wedge preparations pretreated with any concentration of ranolazine. On the contrary, as illustrated in Figure 7, ranolazine proved to be effective in suppressing EADs in M cell and Purkinje fiber preparations pretreated with other IKr blockers such as d-sotalol. D-Sotalol (100 μM) produced a remarkable prolongation of repolarization and induced EADs in both M cell and Purkinje fiber preparations. Ranolazine caused a concentration-dependent abbreviation of the action potential and abolished the EADs. A similar effect of ranolazine (5–20 μM) to suppress EAD activity and abbreviate APD was observed in 4/4 M cell and 3/3 Purkinje fiber preparations. Moreover, ranolazine was found to be effective in reducing TD-APD, the interval between the peak and end of the T wave (Tpeak-Tend) and TDR under long QT conditions (D-sotalol, moxifloxacin and ATX-II)(data not shown).
Figure 7.
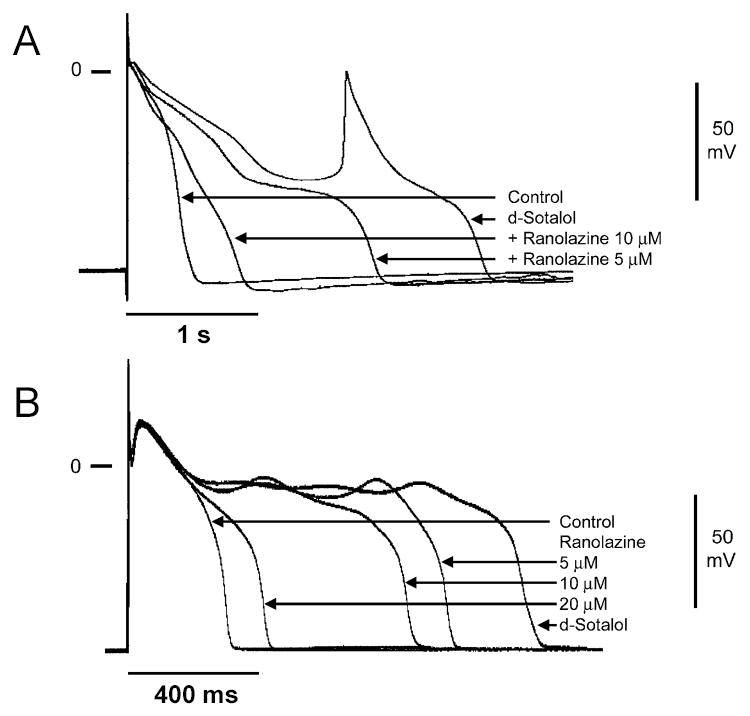
Effect of ranolazine to suppress d-sotalol-induced early afterdepolarizations (EAD) in M cell and Purkinje fiber preparations. A: Shown are superimposed transmembrane action potentials recorded from a Purkinje fiber preparation in the presence of IKr block (100 μM d-sotalol), and following addition of increasing concentrations of ranolazine (5 and 10 μM) in the continued presence of d-sotalol. [K+]o=3 mM. BCL=8000 ms and B: Superimposed transmembrane action potentials recorded from an M cell preparation under control conditions, in the presence of IKr block (100 μM d-sotalol), and following increasing concentrations of ranolazine (5, 10, and 20 μM) in the continued presence of d-sotalol. BCL=2000 ms.
Discussion
The effects of ranolazine on cardiac ion currents at concentrations within the therapeutic range (i.e., 2–6 μM) include inhibition of IKr, late INa and late ICa,L. Ranolazine inhibition of IKr prolongs APD and its effect to inhibit late INa and late ICa,L, abbreviates APD. The net effect and clinical consequence of inhibition of these ion channel currents is a modest increase in the mean QTc interval over the therapeutic range. The drug differs significantly from other agents that block IKr and induce TdP. Ranolazine-induced prolongation of the APD is rate independent (i.e., does not display reverse rate dependent prolongation of APD), and is not associated with EADs, triggered activity, an increase in spatial dispersion of repolarization or polymorphic VT. Indeed, rather than displaying arrhythmogenic activity, ranolazine, via its actions to suppress EADs and reduce TDR, possesses significant antiarrhythmic activity, acting to suppress the arrhythmogenic effects induced by a variety of other QT-prolonging drugs.
Drugs with QT prolonging properties have attracted considerable attention in recent years due to their proclivity to induce life-threatening cardiac arrhythmias, such as Torsade de Pointes9–11. More than 50 commercially available or investigational non-cardiovascular and 20 cardiovascular non-antiarrhythmic drugs have been shown or are suspected to have proarrhythmic effects.
The mechanisms underlying TdP have long been a matter of debate. Recent studies have identified dispersion of repolarization secondary to accentuation of electrical heterogeneities intrinsic to ventricular myocardium as the substrate and EADs as the trigger for the development of TdP. 8, 10, 12–15
Ventricular myocardium is comprised of at least three electrophysiologically distinct cell types: epicardial, M and endocardial. M cells are distinguished by having action potentials that prolong disproportionately relative to the action potentials of other ventricular myocardial cell types in response to a slowing of rate and/or in response to many QT-prolonging drugs.16–18 The ionic basis for these features include the presence of a smaller slowly activating delayed rectifier current (IKs) 19, a larger late INa 20, and INa-Ca 21. IKr and IK1 are similar in the three transmural cell types. M cells, like Purkinje fibers, develop EADs in response to agents and pathophysiologic conditions that reduce the repolarization reserve of the ventricular myocardium. Epicardial and endocardial cells generally do not.
Most drugs that prolong the QT interval accentuate the normal transmural heterogeneity of final ventricular repolarization by causing a preferential prolongation of the action potential of M cells. IKr blockers, including d-sotalol, almokalant, E-4031, moxifloxacin and erythromycin augment transmural dispersion of repolarization as a consequence. These agents cause relatively little prolongation of the APD of epicardial and endocardial cells, because these cell types possess a much more prominent IKs as compared to the M cell. A similar preferential prolongation of the M cell APD is seen with agents that increase calcium current ( ICa) such as Bay K 8644 as well as with agents that increase late INa such as ATX-II, anthopleurin-A and DPI 201-106. An exception to this rule applies to agents that block IKs, which cause a similar percentage of APD prolongation in the three transmural cell types.
A more complex electrophysiological effect is observed with drugs affecting two or more ion channels, such as amiodarone, sodium pentobarbital, quinidine, cisapride and azimilide. Amiodarone is a potent antiarrhythmic agent used in the management of both atrial and ventricular arrhythmias. In addition to its β-blocking properties, amiodarone is known to block late INa, ICa, IKr and IKs. The efficacy of the amiodarone and its low incidence of proarrhythmia relative to other agents with Class III actions are attributable to this complex multi-channel inhibition.22 When administered chronically, amiodarone increases QT without augmenting spatial dispersion of repolarization, unlike other IKr blockers. 23–25 In some cases transmural dispersion of repolarization is reduced.23 Chronic amiodarone therapy can also suppress the effect of other IKr blocker, like d-sotalol, to increase TDR or induce EADs. 23 Thus, chronic amiodarone alters cellular electrophysiology of ventricular myocardium so as to reduce TDR and suppress EADs, especially under conditions in which they are accentuated. The drugs potent inhibition of late INa is thought to play a key role.
The multi-channel inhibition, particularly the ability to potently block late INa, has been suggested to underlie the effect of IKr blockers to prolong QT without creating the substrate or trigger for the development of TdP. Indeed, this feature could contribute to the suppression of EADs and reduction of spatial dispersion of repolarization, the substrate and trigger for TdP. This is the case for amiodarone and sodium pentobarbital, as well as for high concentrations of quinidine and cisapride. 24, 26–28 Our data suggest that ranolazine fits this pharmacologic profile as well. Like amiodarone and sodium pentobarbital, ranolazine produces a preferential prolongation of epicardial APD90, leading to a reduction in transmural dispersion of repolarization. The opposite effects of ranolazine on M and Purkinje fiber to that of epicardial APD is likely due to the more prominent late INa in the M cell and Purkinje fiber than in epicardial cells 20. Ranolazine is among the most potent late INa blockers reported. It causes a decrease in net inward current in the M cells and Purkinje fiber, but a decrease in net outward current in epicardium. The effect of ranolazine to block late INa and late ICa likely underlie its effect to suppress EAD activity. Thus, unlike other IKr blockers, ranolazine does not lead to the development of TdP, either spontaneous or stimulation-induced. Of note, ranolazine has recently been evaluated in an anesthetized dog model with acute complete atrioventricular block, a model susceptible to drug-induced polymorphic VT. At doses that prolonged the QT interval by approximately 5% to 11% above control, ranolazine did not cause spontaneous TdP or TdP facilitated by iv bolus of phenylephrine (which increases susceptibility to TdP) in five dogs, whereas sotalol induced TdP in all five dogs under these conditions.29 Recent studies involving isolated guinea pig and rabbit hearts have also reported failure of ranolazine to induce TdP, but it effectiveness to suppress TdP induced by selective IKr blockers (E-4031) and agents that augment late INa (ATX-II). 29 In summary, the available data suggest that ranolazine, in addition to its anti-anginal actions, may possess important antiarrhythmic activity.
Figure 2.
Effect of ranolazine on the sodium calcium exchange current (INa-Ca, left), peak and late calcium channel current (ICa and late ICa, middle) and late sodium current (late INa, right). Top panels show the voltage protocols used and representative current traces. Bottom panels are the concentration-response relationships. Data are presented as mean ± S.E.M. (n = 3 – 14 as indicated over data points)
Footnotes
Supported by grant HL47678 from NHLBI (CA) and grants from the American Heart Association (AB, JF and CA), CV Therapeutics (CA) and NYS and Florida Grand Lodges F.& A.M.
References
- 1.Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- 2.Louis AA, Manousos IR, Coletta AP, et al. Clinical trials update: The Heart Protection Study, IONA, CARISA, ENRICHD, ACUTE, ALIVE, MADIT II and REMATCH. Impact Of Nicorandil on Angina Combination Assessment of Ranolazine In Stable Angina ENhancing Recovery In Coronary Heart Disease patients Assessment of Cardioversion Using Transoesophageal Echocardiography AzimiLide post-Infarct surVival Evaluation Randomised Evaluation of Mechanical Assistance for Treatment of Chronic Heart failure . EurJHeart Fail. 2002;4:111–116. doi: 10.1016/s1388-9842(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 3.Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Ranolazine Study Group. AmJCardiol. 1999;84:46–50. doi: 10.1016/s0002-9149(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 4.Chandler MP, Stanley WC, Morita H, et al. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. CircRes. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 5.Liu DW, Gintant GA, Antzelevitch C. Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. CircRes. 1993;72:671–687. doi: 10.1161/01.res.72.3.671. [DOI] [PubMed] [Google Scholar]
- 6.Zygmunt AC, Goodrow RJ, Antzelevitch C. Sodium effects on 4-aminopyridine-sensitive transient outward current in canine ventricular cells. AmJPhysiol. 1997;272:H1–H11. doi: 10.1152/ajpheart.1997.272.1.H1. [DOI] [PubMed] [Google Scholar]
- 7.Okada Y, Ogawa S, Sadanaga T, et al. Assessment of reverse use-dependent blocking actions of class III antiarrhythmic drugs by 24-hour Holter electrocardiography. J Am Coll Cardiol. 1996;27:84–89. doi: 10.1016/0735-1097(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 8.Belardinelli L, Antzelevitch C, Vos M. Potential Predictors of drug-induced Torsade de Pointes: early afterdepolarizations, ectopic beats and increased dispersion of ventricular repolarization. TIPS. 2003;24:619–625. doi: 10.1016/j.tips.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Haverkamp W, Breithardt G, Camm AJ, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: Clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res. 2000;47:219–233. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 10.Antzelevitch C, Shimizu W. Cellular mechanisms underlying the Long QT syndrome. CurrOpinCardiol. 2002;17:43–51. doi: 10.1097/00001573-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Bednar MM, Harrigan EP, Anziano RJ, et al. The QT interval. ProgCardiovascDis. 2001;43:1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 12.El-Sherif N. Polymorphic ventricular tachycardia and torsades de pointes: beyond etymology. JCardiovascElectrophysiol. 2001;12:695–696. doi: 10.1046/j.1540-8167.2001.00695.x. [DOI] [PubMed] [Google Scholar]
- 13.Kozhevnikov DO, Yamamoto K, Robotis D, et al. Electrophysiological mechanism of enhanced susceptibility of hypertrophied heart to acquired torsade de pointes arrhythmias: tridimensional mapping of activation and recovery patterns. Circulation. 2002;105:1128–1134. doi: 10.1161/hc0902.104711. [DOI] [PubMed] [Google Scholar]
- 14.Akar FG, Rosenbaum DS. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. CircRes. 2003;93:638–645. doi: 10.1161/01.RES.0000092248.59479.AE. [DOI] [PubMed] [Google Scholar]
- 15.Vos MA, van Opstal JM, Leunissen JD, et al. Electrophysiologic parameters and predisposing factors in the generation of drug-induced Torsade de Pointes arrhythmias. PharmacolTher. 2001;92:109–122. doi: 10.1016/s0163-7258(01)00162-0. [DOI] [PubMed] [Google Scholar]
- 16.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle: The M cell. CircRes. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 17.Antzelevitch C, Sicouri S, Litovsky SH, et al. Heterogeneity within the ventricular wall: Electrophysiology and pharmacology of epicardial, endocardial and M cells. CircRes. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 18.Anyukhovsky EP, Sosunov EA, Rosen MR. Regional differences in electrophysiologic properties of epicardium, midmyocardium and endocardium: In vitro and in vivo correlations. Circulation. 1996;94:1981–1988. doi: 10.1161/01.cir.94.8.1981. [DOI] [PubMed] [Google Scholar]
- 19.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial and endocardial myocytes: A weaker IKs contributes to the longer action potential of the M cell. CircRes. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 20.Zygmunt AC, Eddlestone GT, Thomas GP, et al. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. AmJPhysiol. 2001;281:H689–H697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 21.Zygmunt AC, Goodrow RJ, Antzelevitch C. INa-Ca contributes to electrical heterogeneity within the canine ventricle. AmJPhysiol. 2000;278:H1671–H1678. doi: 10.1152/ajpheart.2000.278.5.H1671. [DOI] [PubMed] [Google Scholar]
- 22.Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated proarrhythmic effects. A review with special reference to torsade de pointes tachycardia. AnnInternMed. 1994;121:529–535. doi: 10.7326/0003-4819-121-7-199410010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sicouri S, Moro S, Litovsky SH, et al. Chronic amiodarone reduces transmural dispersion of repolarization in the canine heart. JCardiovascElectrophysiol. 1997;8:1269–1279. doi: 10.1111/j.1540-8167.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 24.van Opstal JM, Schoenmakers M, Verduyn SC, et al. Chronic Amiodarone evokes no Torsade de Pointes arrhythmias despite QT lengthening in an animal model of acquired Long-QT Syndrome. Circulation. 2001;104:2722–2727. doi: 10.1161/hc4701.099579. [DOI] [PubMed] [Google Scholar]
- 25.Cui G, Sen L, Sager PT, et al. Effects of amiodarone, sematilide, and sotalol on QT dispersion. AmJCardiol. 1994;74:896–900. doi: 10.1016/0002-9149(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 26.Antzelevitch C, Shimizu W, Yan GX, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. JCardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 27.Di Diego JM, Belardinelli L, Antzelevitch C. Cisapride-induced Transmural Dispersion of Repolarization and Torsade de Pointes in the Canine Left Ventricular Wedge Preparation During Epicardial Stimulation. Circulation. 2003;108:1027–1033. doi: 10.1161/01.CIR.0000085066.05180.40. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu W, McMahon B, Antzelevitch C. Sodium pentobarbital reduces transmural dispersion of repolarization and prevents torsade de pointes in models of acquired and congenital long QT syndrome. JCardiovascElectrophysiol. 1999;10:156–164. doi: 10.1111/j.1540-8167.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 29.CV Therapeutics Ranexa (Ranolazine) FDA Review Documents. NDA 21–256 December 09 2003,


