Abstract
Objective: To examine the neuropsychological profile of dementia patients from a community-based autopsy sample of dementia, comparing Alzheimer disease (AD), Lewy body pathology (LBP) alone, and LBP with coexistent AD (AD/LBP). Methods: The authors reviewed 135 subjects from a community-based study of dementia for whom autopsy and brain tissue was available. Diagnostic groups were determined according to standard neuropathologic methods and criteria, and the presence of LBs was determined using α-synuclein immunostaining. Neuropathologically defined diagnostic groups of AD, AD/LBP, and LBP were examined for differences on neuropsychological test performance at the time of initial study enrollment. Results: There were 48 patients with AD alone, 65 with LB and AD pathology (AD/LBP), and 22 with LBP alone (LBP alone). There were no significant differences between groups demographically or on performance of enrollment Mini-Mental State Examination (MMSE) or Dementia Rating Scale (DRS). AD patients performed worse than the LBP patients on memory measures (Fuld Object Memory Evaluation Delayed Recall, Wechsler Memory Scale Logical Memory Immediate and Delayed Recall; p < 0.05) and a naming task (Consortium to Establish a Registry for Alzheimer's Disease Naming; p < 0.05). LBP patients were more impaired than AD patients on executive function (Trail Making Test Part B; p < 0.05) and attention tasks (Wechsler Adult Intelligence Scale–Revised Digit Span; p < 0.05). Decline in MMSE and DRS scores over time were greatest in the patients with AD/LBP. Conclusions: In a community-based sample of older, medically complicated patients with dementia, there are neuropsychological differences between dementia subtypes at the time of diagnosis. In particular, patients with Alzheimer disease (AD) alone and AD/Lewy body pathology (LBP) had more severe memory impairment than patients with LBP. LBP alone was associated with more severe executive dysfunction. Patients with AD/LBP had the most rapid rate of cognitive decline.
It has been suggested that dementia with Lewy bodies (LBs) is the second most common form of dementia in old age.1 The high frequency and extent of LB-associated pathology in dementia patients has recently been confirmed and extended by the use of α-synuclein (ASN) immunohistochemistry and sampling of regions such as the amygdala.2-4 However, despite improved techniques in neuropathologically confirming the presence of LB and associated ASN pathology, clinically differentiating between patients with only LB pathology (LBP alone), Alzheimer disease (AD) alone, or AD with LB (AD/LBP) remains challenging. This is clinically relevant, as there is evidence that these patients may differ in terms of response to medications and prognosis.5,6 In addition, it is important to improve the diagnostic accuracy for clinical studies of the LB-associated dementias.
Previous studies examining the neuropsychological characteristics of the LB-associated dementias have noted that these patients generally demonstrate more severe impairments in visuoconstructive and visuospatial abilities, working memory, attention, language, initiation, and “executive” functions with some evidence of relatively preserved episodic memory when compared with AD.1,7-15 However, there have been major methodologic problems with these studies, such as using cases without autopsy confirmation or without the use of ASN and extranigral staining. Also, most studies have not differentiated LBP alone from AD/LBP. In general, these studies have used patients from highly selected research samples, which may significantly bias results and may not be generalizable to the usually older and more medically complicated dementia patient in the general medical community.
To address some of these issues, we examined the neuropsychological characteristics of patients from a large community-based sample of older adults with cognitive complaints.16 This study examined only autopsy-confirmed cases from this sample and utilized ASN immunostaining to systematically identify LBP change. We divided cases into three diagnostic groups (AD, LBP alone, AD/LBP).
Methods
Subjects were part of the University of Washington/Group Health Cooperative Alzheimer's Disease Patient Registry, which is a population-based registry of incident dementia cases.16 In brief, 1,028 patients with clinical history of memory and cognitive complaints or documented impairments were enrolled from 1987 to 1996. Patients underwent a complete standardized diagnostic workup and neuropsychological evaluation followed by a consensus diagnosis using National Institute of Neurological and Communication Disorders and Stroke/Alzheimer's Disease and Related Disorders Association17 and Diagnostic and Statistical Manual for Mental Disorders (3rd rev. ed.)18 criteria for AD and dementia. Patients received annual follow-up consisting of a physical examination and abbreviated neuropsychological evaluation, namely, Mini-Mental State Examination (MMSE) and Dementia Rating Scale (DRS). To date, 291 subjects from this sample have come to autopsy. Of these autopsied cases, 135 cases met criteria for inclusion in the current study. One hundred thirty-two cases were excluded based on insufficient neuropsychological data, inadequate tissue, limited AD pathology, or advanced dementia at intake.
Tests administered at the time of entry included the following: DRS,19 Wechsler Memory Scale–Revised (WMS-R) Logical Memory and Visual Reproduction Subtests,20 Fuld Object Memory Evaluation,21 Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Span,20 WAIS-R Comprehension Subtest,20 WAIS-R Proverb items from Comprehension Subtest,20 WAIS-R Similarities Subtest,20 WAIS-R Block Design Subtest,20 Trail Making Test,22 Consortium to Establish a Registry of Alzheimer's Disease (CERAD) Test Battery Naming Subtest,23 and MMSE.24
Neuropathologic evaluation was performed on specific brain regions including frontal, temporal, parietal, and calcarine cortex, cingulate gyrus, substantia innominata, basal ganglia, claustrum, insula, amygdaloid nucleus, hypothalamus, hippocampus and parahippocampus, midbrain, pons, medulla, and cerebellum. Braak staging25 was based on a modified Bielshowsky stain. Evaluation of LBP included hematoxylin and eosin staining in the substantia nigra and immunohistochemical staining for ASN (LB 509; Zymed, San Francisco, CA) in the amygdala and substantia nigra. A blinded assessment (blind to diagnosis, Braak staging, and ASN immunostaining in other regions) of ASN-immunostained sections was performed for the presence or absence of intraneuronal ASN-immunopositive inclusions and neurites.
Participants were categorized into the following diagnostic groups: AD (Braak stage IV or higher and plaque scores consistent with CERAD definite AD); AD/LBP (AD as above with ASN-positive inclusions in either the amygdala or the substantia nigra); LBP alone (Braak stage III or less and ASN-positive inclusions in either the amygdala or the substantia nigra). This criterion for setting a cut-off score of Braak stage IV for clinically relevant AD is based on a previous description of neuropathology in cognitively normal elderly subjects.26 Others have suggested that the amygdala is the most sensitive region for identifying ASN LB pathology,2,3,27 and in this study, some subjects in the AD/LBP group were found to have LB inclusions only in the amygdala and not in the brainstem (amygdala-only AD/LBP). Currently, the clinical and pathophysiologic importance of AD with LBP restricted to the amygdala alone is unclear (without other brainstem, limbic, or neocortical LBP). Therefore, in addition to the group membership as described above, an additional analysis was performed with the amygdala-only AD/LBP patients excluded from the AD/LBP group. Following neuropathologic diagnosis, subsequent analyses were performed on neuropsychological data obtained at intake. Because these diagnostic groups are defined neuropathologically and because this study was started before the consensus criteria for dementia with LBs, clinical data about fluctuation, parkinsonian features, and visual hallucinations were not adequately generated and were not available to analyze. Patients without AD or LBP were not included in this study, and patients with an MMSE score of <14 at their initial evaluation were excluded to select a population of subjects not already in the late stages of a dementing illness. To examine the change in patients' cognitive function over time, final MMSE and DRS scores were subtracted from their initial (entry) scores and divided by years of observation. Yearly follow-up data from other neuropsychological tests were not available for analysis.
Results
Between-group comparisons for education, age at onset, and MMSE and DRS scores at entry to the study (average of 1 year after onset of memory problems) revealed no significant differences between groups (table 1). A multivariate analysis of variance (ANOVA) with groups (AD, AD/LBP, and LBP) as the between factor and tests (DRS, WMS Logical Memory and Visual Reproduction, Fuld Object Memory Evaluation, WAIS-R Digit Span, WAIR-R Comprehension, WAIS-R Similarities, WAIS-R Block Design, WAIS-R Proverbs, Trail Making Test, CERAD Naming, and MMSE) as dependent factors was significant (F[5, 22] = 4.65, p < 0.05). To ensure a larger sample size, subsequent ANOVAS were performed for each individual test.
Table 1.
Demographics
| AD, n = 48 | AD/LBP, n = 65 | LBP, n = 22 | Total, n = 135 | |
|---|---|---|---|---|
| Age at onset, mean | 77.47 ± 7.34 | 74.77 ± 6.59 | 76.45 ± 5.34 | 75.99 ± 6.75 |
| Sex, M/F | 18/30 | 24/41 | 16/6 | 58/77 |
| Intake MMSE | 20.60 ± 3.87 | 20.68 ± 3.72 | 20.73 ± 3.81 | 20.66 ± 3.76 |
| Education | ||||
| <HS | 13 (0.27) | 13 (0.20) | 4 (0.18) | 30 (0.22) |
| HS | 7 (0.15) | 26 (0.40) | 5 (0.23) | 38 (0.28) |
| >HS | 27 (0.56) | 26 (0.40) | 13 (0.59) | 66 (0.49) |
| Duration of disease | 6.03 ± 2.62 | 6.81 ± 2.52 | 5.65 ± 2.23 | 6.16 ± 2.50 |
| Braak stage | 4.69 ± 0.59 | 4.88 ± 0.57 | 2.14 ± 0.77 | 4.36 ± 1.16 |
Sex = frequency of male and female patients; intake MMSE = initial Mini-Mental State Examination score out of 30; <HS = less than high school; HS = high school or equivalent; >HS = greater than high school; duration of disease = age at death minus age of onset; Braak stage = mean stage of neurofibrillary tangles.
Table 2 displays the means and SE of intake neuropsychological test results and change in DRS and MMSE by diagnostic group. AD patients performed worse than LBP patients on memory measures (Fuld Object Memory Delayed Recall: F[1,90] = 4.04, p < 0.05; WMS Logical Memory Delayed Recall: F[2,12] = 12.24, p < 0.05) and a verbal task (CERAD Naming: F[2,126] = 5.45, p < 0.05). LBP patients were more impaired than AD patients on an executive function task (Trails B Time: F[2,31] = 6.03, p < 0.05) and an attention task (WAIS-R Digit Span: F[1,95] = 8.26, p < 0.05) (see table 2).
Table 2.
Means and standard errors of intake neuropsychological test results by diagnostic group
| AD (Mean ± SE) | AD/LBP (Mean ± SE) | LBP (Mean ± SE) | p Value | |
|---|---|---|---|---|
| Attention | ||||
| DRS – Attention | 34.4 ± 0.3 | 34.6 ± 0.3 | 34.3 ± 0.4 | 0.89 |
| Trail Making Test – Part A | 98.7 ± 11.2 | 100.3 ± 8.9 | 114.0 ± 13.9 | 0.67 |
| WAIS-R Digit Span | 9.1 ± 0.4 | 8.2 ± 0.4 | 7.2 ± 0.5 | 0.01** |
| Language | ||||
| CERAD Naming | 6.9 ± 0.4 | 6.1 ± 0.3 | 7.9 ± 0.4 | 0.01** |
| Memory | ||||
| DRS – Memory | 15.1 ± 0.6 | 15.1 ± 0.6 | 17.5 ± 1.1 | 0.08 |
| Fuld Object Memory – Retrieval | 17.6 ± 1.4 | 19.1 ± 1.4 | 20.2 ± 2.0 | 0.56 |
| Fuld Object Memory – Del. Recall | 3.6 ± 0.3 | 4.3 ± 0.4 | 4.9 ± 0.5 | 0.05* |
| WMS – Logical Memory Immediate | 3.1 ± 0.3 | 3.2 ± 0.3 | 5.3 ± 0.7 | 0.00** |
| WMS – Logical Memory Delayed | 0.5 ± 0.1 | 0.8 ± 0.2 | 2.4 ± 0.5 | 0.00** |
| WMS – Visual Reproduction. Immediate | 2.0 ± 0.3 | 1.3 ± 0.2 | 1.6 ± 0.4 | 0.12 |
| WMS – Visual Reproduction. Delayed | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.48 |
| Visuo-constructional | ||||
| DRS – Construction | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.7 ± 0.3 | 0.89 |
| WAIS-R – Block Design | 7.4 ± 0.5 | 7.2 ± 0.5 | 6.5 ± 0.5 | 0.59 |
| Executive Function | ||||
| DRS – Initiation/Perseveration | 26.1 ± 0.9 | 26.7 ± 0.8 | 24.4 ± 1.3 | 0.35 |
| DRS – Conceptualization | 33.8 ± 0.9 | 33.1 ± 0.6 | 33.3 ± 1.1 | 0.79 |
| Trail Making Test – Part B | 168.7 ± 18.1 | 183.8 ± 14.8 | 297.5 ± 45.1 | 0.01** |
| WAIS-R Comprehension | 8.6 ± 0.5 | 8.6 ± 0.4 | 8.2 ± 0.6 | 0.87 |
| WAIS-R – Proverbs | 1.9 ± 0.3 | 2.1 ± 0.3 | 1.2 ± 0.3 | 0.17 |
| WAIS-R – Similarities | 9.9 ± 0.4 | 8.8 ± 0.4 | 8.9 ± 0.6 | 0.11 |
| Other | ||||
| DRS – Total | 114.7 ± 2.1 | 114.2 ± 1.8 | 114.2 ± 2.7 | 0.99 |
| MMSE – Total | 20.6 ± 0.6 | 20.7 ± 0.5 | 20.7 ± 0.8 | 0.99 |
| Rate of Decline (DRS) – points per year | 9.6 ± 1.5 | 15.3 ± 1.9 | 8.8 ± 1.7 | 0.03* |
| Rate of Decline (MMSE) – points per year | 3.5 ± 0.4 | 5.0 ± 0.5 | 3.4 ± 0.7 | 0.04*† |
p Values: A one-way ANOVA for group difference.
This finding is reduced to a statistical trend (p < 0.07) with removal of patients in the AD/LBP group who have Lewy body pathology restricted to the Amygdala (N = 17).
DRS – attention subscale = 37 points possible; Trail Making Test – Part A = total time in seconds to complete trails test A; WAIS-R Digit Span subscale = age corrected scaled score; CERAD Naming = 10 item naming task; DRS-Memory subscale = 25 points possible; Fuld Object Memory – Retrieval = 50 points possible; Fuld Object Memory – Delayed Recall = 10 points possible; WMS-Logical Memory Immediate = 50 points possible; WMS-Logical Memory Delayed = 50 points possible; WMS-Visual Reproduction – Immediate = 41 points possible; WMS-Visual Reproduction – Delayed = 41 points possible; DRS – Construction subscale = 6 points possible; WAIS-R Block Design subscale = age corrected scaled score; DRS-Initiation/Perseveration subscale = age corrected scaled score; DRS-Conceptualization subscale = age corrected scaled score; Trail Making Test – Part B = total time in seconds to complete trails B; WAIS-R Comprehension subscale = age corrected scaled score; WAIS-R Comprehension subscale = age corrected scaled score; WAIS-R Similarities subscale = age corrected scaled score; WAIS-R Proverb items taken from comprehension subscale = age corrected scaled score; DRS-Total = total score of the DRS out of 144; Rate of Decline (DRS) = average points per year of decline; Rate of Decline (MMSE) = average points per year of decline.
AD = Alzheimer disease; LBP = Lewy body pathology; DRS = Dementia Rating Scale; WAIS-R = Wechsler Adult Intelligence-Scale Revised; CERAD = Consortium to Establish a Registry of AD; WMS = Wechsler Memory Scale; MMSE = Mini-Mental State Examination.
A comparison of MMSE and DRS scores over time revealed a difference between groups (DRS: F[2,97] = 3.69, p < 0.05; MMSE: F[2,116] = 3.44, p < 0.05), with AD/LBP patients demonstrating the greatest degree of change, followed by AD patients, and then LBP (figure). The groups did not differ in terms of their duration of disease, defined as the amount of time that elapsed between age at onset and age at death, so it is unlikely that cognitive change over time was affected by survival rates (p = 0.315). Although we recognize that rate of decline may not have a truly linear progression and may be influenced by factors such as education, age at diagnosis, duration of disease at first testing, etc., this simple calculation of change per year seems to reflect a more rapid change in general cognitive function over time.
Figure.
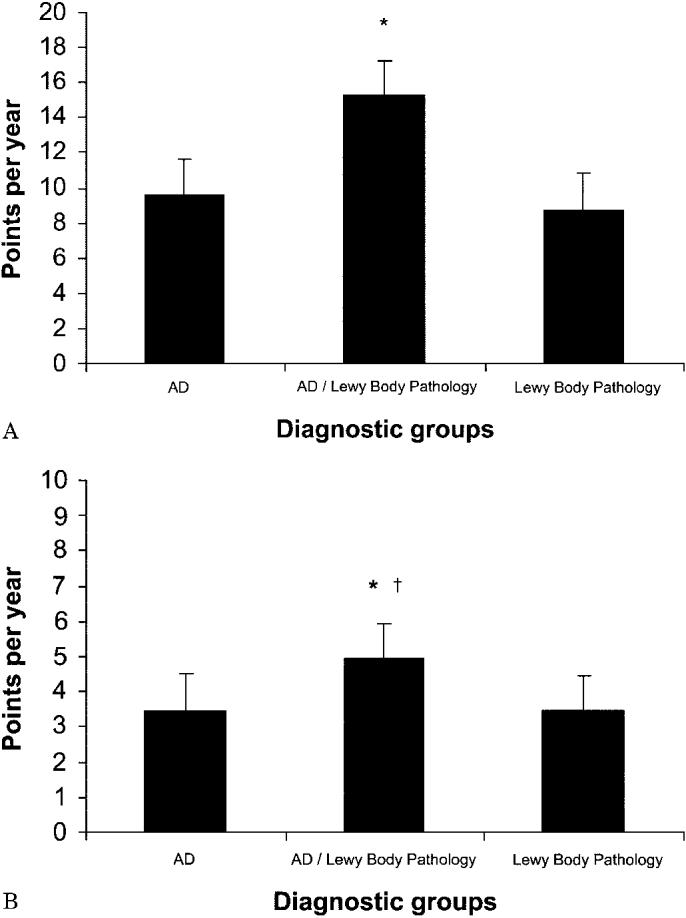
(A) Repeated follow-up change in cognitive testing scores (Dementia Rating Scale [DRS]). Change in DRS scores over time. Decline (points/year) was calculated by subtracting final scores from initial scores and dividing by the number of years the patient was followed. Patients with Alzheimer disease (AD)/Lewy body pathology (LBP) had a significantly greater rate of decline (p < 0.05) than both AD and LBP patients. *p < 0.05. (B) Repeated follow-up change in cognitive testing scores (Mini-Mental State Examination [MMSE]). Change in MMSE scores over time. Decline (points/year) was calculated by subtracting final scores from initial scores and dividing by the number of years the patient was followed. AD/LBP patients had a significantly greater rate of decline (p < 0.05) than both AD and LBP patients. *p < 0.05. †This finding is reduced to p < 0.07 with the removal of patients in the AD/LBP group who have LBP restricted to the amygdala.
Because the clinical and pathophysiologic importance of amygdala-only LB pathology is currently unclear, we excluded these cases from our analysis. AD/LBP patients with LB inclusions restricted to the amygdala (n = 17) were removed from the AD/LBP group, and ANOVAS as described above were repeated. These results were essentially unchanged from those described above (and as noted in figure and table 2), with the exception of MMSE change over time, which was only approaching significance (p < 0.07). To examine whether concomitant diseases or vascular dementia contributed to the findings of unique cognitive deficits between groups, we reviewed neuropathology reports and counted the total number of additional findings for each subject (e.g., infarcts, lacunes, hemorrhage, etc.). A comparison of this count (a score of 1 for each additional event) between groups was not significant.
Discussion
We used only autopsy-confirmed cases from a community-based sample of dementia and used ASN immunohistochemistry and extranigral sampling. The number of cases in each group was large, and the LB-associated dementias were divided by the presence or absence of significant coexistent AD pathologic change. Our results indicate that patients with LBP alone evidence relatively less severe impairments than patients with AD and AD/LBP on measures of verbal memory and confrontation naming when mild to moderately demented. These findings were not due to differences of demographics such as age at onset and education or dementia severity, as the initial MMSE and DRS scores were not significantly different between dementia subgroups. Consistent with previous findings, patients with LBP alone performed worse than those with AD and AD/LBP on measures of executive function (Trails B) and attention (WAIS-R Digit Span). Part A of the Trail Making Test did not differ among groups, which suggests that LBP patients were more impaired in terms of divided attention and not simply performing the task more slowly owing to motor impairments.
Other studies have failed to find cognitive differences between LBP and AD patients at an early disease stage.28 LBP patients with high Braak stage ratings have been shown to be less likely to express the clinical features of LBP.29 Thus, clinical recognition and differentiation of LBP vs AD can be difficult. Consistent with these findings, our study also found that patients with AD/LBP did not differ significantly from AD patients on any of the neuropsychological measures used. It is possible that because both groups share a high degree of overlap with regard to AD pathology, clinical symptoms and cognitive differences, should they exist, may be too subtle to detect with current methods.
In terms of cognitive decline, patients with AD/LBP clearly stood out as having the greatest degree of change in both the DRS and the MMSE per year. Even when the analysis excluded the patients in the AD/LBP group where LB inclusions were found only in the amygdala, there was a significant change in the DRS per year. A greater degree of change in cognitive function over time in the AD/LBP group could be explained as the result of two concomitant disease processes or that ASN pathology is a marker for a more aggressive form of AD. Other groups have described a rapidly progressive course for patients with AD/LBP.8,30 Our study confirms that finding but emphasizes that it is only the AD cases with LB that have this more rapid loss of cognitive function regardless of the localization of LBP. In fact, those cases with LBP alone had the least amount of change over time on the MMSE and DRS.
It has been suggested that early diagnosis of LBD and differentiation from AD may have therapeutic implications in terms of avoiding neuroleptic medications,5 and the use of cholinesterase inhibitors has been recommended.6 Our study provides additional support for the previously described symptoms, cognitive impairments, and functional disabilities that specifically characterize LBP and provides further evidence for some initial cognitive differences among the groups. Our study sample is community based, and patients were not excluded based on other disease processes. Thus, this sample may better represent dementia in the general medical community.
Limitations of this study may include selection bias between patients who elected to participate in the initial evaluation as well as for those who consented for autopsy. It is unknown whether these differences are systematic.
Footnotes
The Alzheimer's Disease Patient Registry was supported in part by a grant from the National Institute on Aging (UO1-AG06781).
References
- 1.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down's syndrome brains with Alzheimer's disease. Ann Neurol. 1999;45:353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.McKeith I, Fairbairn A, Perry R, Thompson P, Perry E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. Br Med J. 1992;305:673–678. doi: 10.1136/bmj.305.6855.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62:181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 8.Heyman A, Fillenbaum GG, Gearing M, et al. Comparison of Lewy body variant of Alzheimer's disease with pure Alzheimer's disease: Consortium to Establish a Registry for Alzheimer's Disease, part XIX. Neurology. 1999;52:1839–1844. doi: 10.1212/wnl.52.9.1839. [DOI] [PubMed] [Google Scholar]
- 9.Ballard C, O'Brien J, Gray A, et al. Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2001;58:977–982. doi: 10.1001/archneur.58.6.977. [DOI] [PubMed] [Google Scholar]
- 10.Larner AJ. MMSE subscores and the diagnosis of dementia with Lewy bodies. Int J Geriatr Psychiatry. 2003;18:855–856. doi: 10.1002/gps.990. [DOI] [PubMed] [Google Scholar]
- 11.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. 2004;19:60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 12.Pasquier F. Early diagnosis of dementia: neuropsychology. J Neurol. 1999;246:6–15. doi: 10.1007/s004150050299. [DOI] [PubMed] [Google Scholar]
- 13.Salmon DP, Galasko D, Hansen LA, et al. Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn. 1996;31:148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- 14.Collerton D, Burn D, McKeith I, O'Brien J. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual–perceptual and attentional–executive dementia. Dement Geriatr Cogn Disord. 2003;16:229–237. doi: 10.1159/000072807. [DOI] [PubMed] [Google Scholar]
- 15.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Larson E, Kukull W, Teri L, et al. The University of Washington Alzheimer's Disease Patient Registry (ADPR): 1987–1988. Aging. 1990;2:404–408. [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDSADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . 3rd ed. American Psychiatric Press; Washington, DC: 1987. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 19.Mattis S. Psychological Assessment Resources; Odessa, FL: 1988. Dementia Rating Scale. [Google Scholar]
- 20.Wechsler D. Psychological Corp.; New York: 1981. Wechsler Adult Intelligence Scale-rRevised manual. [Google Scholar]
- 21.Fuld PA. Stoetling; Chicago, IL: 1977. Fuld Object–Memory Evaluation. [Google Scholar]
- 22.Reitan R. Validity of the Trail-Making Test as an indicator of organic brain damage. Percept Mot Skills. 1985;19:199–206. [Google Scholar]
- 23.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry in Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 27.Marui W, Iseki E, Nakai T, et al. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195:153–159. doi: 10.1016/s0022-510x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 28.Mormont E, Laurier-Grymonprez L, Baisset-Mouly C, Pasquier F. The profile of memory disturbance in early Lewy body dementia differs from that in Alzheimer's disease. Rev Neurol (Paris) 2003;159:762–766. [PubMed] [Google Scholar]
- 29.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 30.Olichney JM, Galasko D, Salmon DP, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]


