Abstract
The enzyme CYP17 primarily regulates androgen production by mediating four reactions: conversion of pregnenolone and progesterone to 17-hydroxypregnenolone and 17-hydroxyprogesterone, respectively (17α-hydroxylase activity), followed by conversion of the 17-hydroxylated steroids to dehydroepiandrosterone and androstenedione, respectively (17,20-lyase activity). Most mammalian CYP17 isoforms have high 17α-hydroxylase relative to 17,20-lyase activities and preferentially mediate one of the two 17,20-lyase reactions. In contrast, Xenopus laevis CYP17 potently regulates all four reactions in the frog ovary. CYP17 isoforms generally rely on the cofactor cytochrome b5 for the 17,20-lyase reaction, suggesting that the high lyase activity of Xenopus CYP17 might be due to a lesser dependence on b5. The kinetics of Xenopus CYP17 expressed in yeast microsomes were therefore examined in the absence and presence of Xenopus on human b5. Xenopus CYP17 mediated both 17,20-lyase reactions in the absence of b5, confirming that the activity did not require b5. However, both Xenopus and human b5 slightly enhanced Xenopus CYP17-mediated lyase activity, indicating that the enzyme was still at least partially responsive to b5. Surprisingly, only the human b5 cofactor enhanced human CYP17-mediated lyase activity, implying that the human enzyme had more specific cofactor requirements than Xenopus CYP17. Studies using human/Xenopus chimeric b5 proteins revealed that human b5 residues 16–41 were important for the specific regulation of the lyase activity of HuCYP17, possibly serving as an interacting domain with the enzyme. CYP17 may therefore have evolved from a general producer of sex steroids in lower vertebrates to a more tightly regulated producer of both sex steroids and glucocorticoids in mammals.
The development and maintenance of male and female fertility depends on the normal function of androgens and their cognate androgen receptor. Androgens induce male sexual differentiation before birth, promote sexual maturation during puberty, and maintain male reproductive function in adults (1). In women, androgens serve as precursors for estrogen biosynthesis (2) while also playing significant roles in normal as well as pathologic ovarian development (3–8).
Androgen production depends on the enzyme CYP17 (9). CYP17 mediates two enzymatic reactions. Its 17-hydroxylase activity primarily converts pregnenolone (Δ5 pathway) and progesterone (Δ4 pathway) to 17-hydroxypregnenolone and 17- hydroxyprogesterone, respectively. The 17,20-lyase activity of CYP17 then converts the 17-hydroxylated pregnenolone and progesterone to dehydroepiandrosterone (DHEA)1 and androstenedione, respectively. Notably, the cofactor cytochrome b5 has been implicated as a critical component of the 17,20-lyase reaction. Nearly all mammalian CYP17 isoforms that have been examined have little 17,20-lyase activity in the absence of b5. The addition of b5 markedly enhances lyase activity, although it usually remains lower than the hydroxylase activity under similar conditions (10). Furthermore, most mammalian isoforms of CYP17 respond to b5 by preferentially promoting lyase activity in only one of the two pathways (Δ4 versus Δ5) (11). Together, these b5-dependent responses ensure that mammalian CYP17s will regulate the production of a variety of steroids, including both androgen precursors such as DHEA and androstenedione, as well as 17-hydroxylated progestins such as 17-hydroxyprogesterone and 17-hydroxypregnenolone.
We have previously shown that ovarian androgen production is extremely important in the female frog Xenopus laevis, as testosterone and androstenedione appear to be the physiologic regulators of oocyte maturation, or re-entry into meiosis. These androgens trigger maturation by signaling in an unusual non-genomic (transcription-independent) fashion that involves at least in part classical androgen receptors (12, 13). Interestingly, Xenopus ovarian androgen production is dependent upon both ovarian germ and somatic cells, as CYP17 activity is found almost exclusively in oocytes, whereas all other steroidogenic enzymes are expressed in the surrounding follicle cells. Initial characterization of CYP17 activity in frog oocytes revealed remarkably high 17,20-lyase activity in both the Δ4 and the Δ5 pathways, with lyase rates at least equaling those of the 17α- hydroxylase reactions (14). Thus, Xenopus ovaries produced virtually no detectable progesterone, 17-hydroxyprogesterone, or 17-hydroxypregnenolone upon stimulation with gonadotropins, whereas ovarian androgens such as androstenedione and testosterone levels were very high.
The unusually high lyase activity of Xenopus CYP17 (Xe-CYP17) suggested that the enzyme was either more sensitive to or less dependent on cytochrome b5. To address this issue, we designed a series of experiments that would examine the b5 dependence of XeCYP17. As a comparison, similar studies were performed in parallel using the well characterized human isoform of CYP17 (HuCYP17). Finally, the effects of both Xenopus and human b5 (Xb5 and Hb5, respectively) on each isoform of CYP17 were examined to study the specificity of the CYP17/b5 interactions.
We found that XeCYP17 contained significant 17,20-lyase activity in both the Δ4 and the Δ5 pathways in the absence of b5, although these activities were slightly enhanced upon the addition of either the Xenopus or the human b5. In contrast, HuCYP17-mediated 17,20-lyase activity was extremely low in both pathways in the absence of b5 and was markedly enhanced by the human, but not the Xenopus b5, cofactor. Finally, studies with Xenopus/human b5 chimeras revealed residues within Hb5 that were important for its specific interactions with HuCYP17. In addition to uncovering a potential CYP17-binding domain in b5, these studies suggest that CYP17 from lower vertebrates such as frogs may be more potent regulators of androgen production in part due to their lesser dependence on cytochrome b5 as a regulator of the 17,20-lyase reaction.
MATERIALS AND METHODS
Synthesis of Recombinant Wild-type Xb5 and Human/Xenopus b5 Chimeras
The expression vector pT7-7 and cDNA of Hb5 were generous gifts from Drs. Ron Estabrook and Richard Auchus (University of Texas Southwestern Medical Center, Dallas, TX). The cDNA encoding Xb5 was obtained by PCR amplification from a Xenopus oocyte cDNA library (GenBankTM accession number AY775052). The 5′ primer introduced a NdeI site and four histidine codons prior to the start codon (Supplementary Table). The Xb5 cDNA was cloned into pT7-7, and the DNA was transformed into the Rosetta bacterial strain (Dr. R. Auchus, University of Texas Southwestern Medical Center). The recombinant human/Xenopus b5 chimeras (see Fig. 1C) were obtained by PCR using primers described in the Supplementary Table.
Fig. 1. Cytochrome b5 wild-type and chimeric proteins.
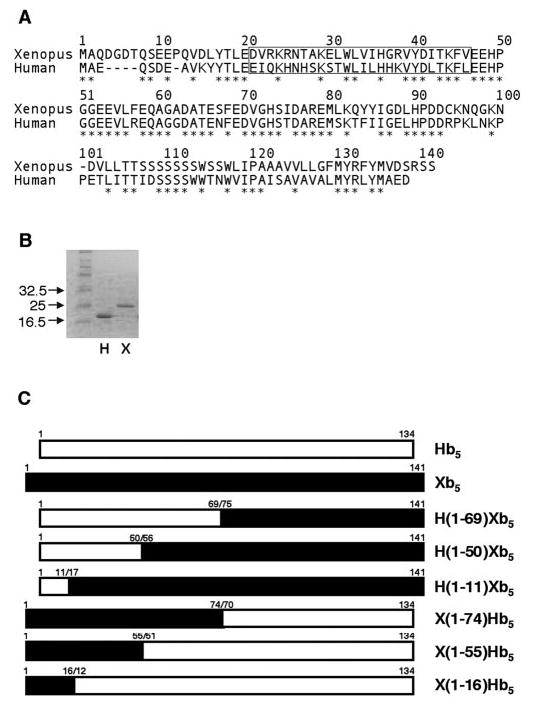
A, alignments of the Xenopus and human cytochrome b5 protein sequences. Xenopus b5 is a 141-amino acid protein that shares ~55% identity with human b5, a 134-amino-acid protein. The * indicates an identical match of amino acids. The boxed area represents Hb5 residues 16–41. B, SDS-polyacrylamide gel electrophoresis analysis of Hb5 and Xb5. Equal volumes of bacterially purified Hb5 (H) and Xb5 (X) were examined by SDS-polyacrylamide gel electrophoresis under reducing conditions using a 14% gel that was stained with Coomassie Blue. C, design of the chimeric b5 proteins. Closed and open rectangles represent Xb5 and Hb5, respectively.
Expression of the Wild-type Xb5 and Recombinant Human/Xenopus b5 Chimeras
The cytochrome b5 proteins were expressed as described (15). Briefly, 10-ml cultures of Rosetta bacterial cells transformed with plasmid pT7-7 containing cDNAs encoding the wild-type or chimeric b5 proteins were grown at 30 °C in medium (2 g/liter peptone, 12 g/liter tryptone, 24 g/liter yeast extract, 4 ml/liter glycerol, 17.5 mm KH2PO4, and 72.5 mm K2HPO4) containing ampicillin (0.2 mg/ml), chloramphenicol (34 μg/ml), and glucose (0.2%). The overnight cultures were diluted to an A600 = 0.03 in 1 liter of the same medium except without glucose. The cells were incubated at 30 °C with shaking until an A600 = 0.8–1.5 (7–10 h) was reached, at which time 0.1 mm isopropyl-1-thio-β-d-galactopyranoside was added. Cells were then incubated at 24 °C overnight (about 15 h).
Isolation and Purification of Wild-type Xb5 and Recombinant Human/Xenopus b5 Chimeras
Cells from cultures expressing b5 were harvested by centrifugation at 5000 rpm for 10 min. Pelleted cells were suspended in 2 volumes (w/v) of buffer A (75 mm Tris-HCl, pH 8.0, 10 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride) and broken by emulsification (Department of Biochemistry, University of Texas Southwestern Medical Center). Membranes were harvested by centrifugation at 12,000 × g for 10 min and resuspended in 10 ml of buffer B (20 mm Tris-HCl, pH 8.0, containing 2 mm β-mercaptoethanol and 20% glycerol) with a Dounce homogenizer. The membrane fraction was harvested by centrifugation (32,000 rpm for 50 min) and stored frozen at −80 °C. The frozen membrane fraction was then thawed, and CHAPS (Pierce) was slowly added to the cold suspension of stirred membranes until a concentration of 1% (w/v) was attained. The suspension was stirred for an additional hour, and the solubilized membranes were centrifuged for 50 min at 32,000 rpm. The supernatant was carefully decanted and diluted with an equal amount of buffer B supplemented with 0.1% CHAPS (B/CHAPS). The sample was loaded onto a Ni2+-nitrilotriacetic acid-agarose column followed by 10 column volumes of buffer B/CHAPS. The b5 was eluted with buffer B/CHAPS containing 100 mm imidazole (Sigma). The fractions containing b5 were combined and dialyzed overnight against a buffer containing 10 mm potassium phosphate, pH 7.5, 20% glycerol, 0.5 mm EDTA, 0.1 mm dithiothreitol, and 0.05% CHAPS. SDS-PAGE, and mass spectrometry confirmed the identity of b5.
Yeast Transformation and Microsome Preparation
Saccharomyces cerevisiae strains YiV(B) and plasmid V60, which contains the cDNA encoding oxidoreductase, were gifts of Dr. R. Auchus (University of Texas Southwestern Medical Center). YiV(B) yeast were transformed with 1 μg of plasmid V60 containing the cDNA encoding either wild-type HuCYP17 or wild-type XeCYP17. Microsomes were prepared as described (16, 17), and microsomal proteins were quantified colorimetrically. CYP17 concentrations were determined using difference spectroscopy (Department of Biochemistry, University of Texas Southwestern Medical Center). For the studies here, the concentration of HuCYP17 was 348.88 pmol/mg of total microsomal protein, whereas the concentration of XeCYP17 was 40.71 pmol/mg of total microsomal protein.
CYP17 Enzyme Assays
17α-hydroxylase and 17,20-lyase activities in yeast microsomes were measured as described previously (10). Microsomes were assayed under initial rate kinetics by incubation in 50 mm potassium phosphate buffer (pH 7.4) with 0.03–1 μm steroid (Sigma and Steraloids, Newport, RI) and 1 mm cofactor NADPH (Sigma) in 500 μl of total volume at room temperature for 30 min. Each reaction contained 25 μg of microsomes (final concentrations of 17.4 pmol/ml HuCYP17 and 2.0 pmol/ml XeCYP17) and 50,000 cpm of [7-3H(N)]pregnenolone, [1,2,6,7-3H]17α-hydroxypregnenolone, [1,2,6,7-3H(N)]progesterone, or [1,2,6,7-3H(N)]17α-hydroxyprogesterone (PerkinElmer Life Sciences). Unless otherwise indicated, reactions contained 2.5 μg/ml (~150 pmol/ml) final concentration of cytochrome b5. Recombinant Hb5 was purchased from Pan Vera (Madison, WI). Steroids were extracted with 3 ml of 3:2 ethyl acetate:hexane, concentrated under nitrogen, and separated by TLC using 3:1 chloroform:ethyl acetate. Relative steroid amounts were determined by cutting out the steroid spots on the TLC plate and measuring radioactivity using liquid scintillation. Kinetic behavior was approximated using Michaelis-Menten analysis.
Computer Modeling
Graphics for the cytochrome b5 modeling were created on a Silicon Graphics Octane/S.E. R12000 work station (Mountain View, CA) using the ribbonjr program of MidasPlus software version 2.1. The coordinates of bovine cytochrome b5 (Protein Data Bank ID1CYO) were used as a template, and residue side chains were replaced with the swapaa command of MidasPlus without additional model refinement.
RESULTS
XeCYP17 Catalyzed the 17,20-Lyase Reaction in Both a b5- independent and b5-dependent Manner
Previous work in our laboratory demonstrated that Xenopus oocyte membranes contained high 17,20-lyase activity in both the Δ4 and the Δ5 pathways (14). However, these experiments using crude membrane preparations did not prove that XeCYP17 was the primary mediator of this activity, nor could they be used for detailed quantitative analyses of XeCYP17-mediated catalysis. Additionally, oocyte membranes likely contained cytochrome b5; thus, they could not be used to quantify the b5 dependence of the XeCYP17-mediated lyase reaction. To address these issues, XeCYP17 was expressed in yeast microsomes, and its enzymatic activities were examined both in the absence and in the presence of cytochrome b5. Xb5 protein containing 4 amino-terminal histidines was expressed in bacteria and purified using Ni2+-nitrilotriacetic acid-agarose. The purity of the final Xb5 preparation was assessed by SDS-PAGE under reducing conditions followed by staining with Coomassie Blue (Fig. 1B). Unexpectedly, although the predicted mass of Xb5 is ~17 kDa, the predominant band in the gel migrated at a slightly higher molecular mass of ~25 kDa. This differed from the Hb5, which migrated at the predicted mass of 17 kDa (Fig. 1B). Mass spectroscopy and proteolysis studies confirmed that the dominant band from the Xenopus preparation was Xb5 and that its true molecular mass was indeed 17 kDa (data not shown). Thus, the slower than predicted electrophoretic mobility of Xb5 appeared to be due to SDS- and reduction-resistant structural differences.
Interestingly, XeCYP17 contained significant 17,20-lyase activities in both the Δ5 and the Δ4 pathways independent of Xb5 as radiolabeled 17-hydroxypregnenolone and 17-hydroxyprogesterone were converted to DHEA and androstenedione, respectively, in a time-dependent fashion (Fig. 2, A and B). Xe-CYP17 contained b5-dependent lyase activity as well since the addition of Xb5 enhanced both reactions (Fig. 2, A and B). To further quantify the enzymatic activities of XeCYP17 and to precisely determine the contribution of b5 to the lyase reaction, detailed kinetic analyses of XeCYP17 were performed (Table I). The Vmax values for XeCYP17-mediated lyase activities in the absence of b5 were substantial and relatively similar in both the Δ4 and the Δ5 pathways (0.51 + 0.11 and 0.41 + 0.12 min−1, respectively), confirming that XeCYP17 could mediate the 17,20-lyase reaction in the absence of b5. Xb5 at the predetermined concentration yielding maximum activity (2.5 μg/ml, data not shown) only slightly enhanced 17,20-lyase activity, increasing the rate of the Δ4 lyase reaction (17-hydroxyprogesterone to androstenedione) by ~2-fold and the rate of the Δ5 lyase reaction (17-hydroxypregnenolone to DHEA) by ~4-fold (Table I). As expected, Xb5 did not significantly alter XeCYP17- mediated hydroxylase activities in either pathway, nor did it markedly affect the calculated Km values of any of the reactions examined. Of note, the Vmax and Km values for XeCYP17- mediated 17α-hydroxylase activity in the Δ4 pathway were higher than in the Δ5 pathway, suggesting that progesterone might be the preferred substrate for XeCYP17 in vitro when substrate concentrations are high. In contrast, recent studies have demonstrated that virtually no progesterone is produced by the Xenopus ovary in vivo, most likely due to the extremely slow conversion of pregnenolone to progesterone, indicating that pregnenolone is in fact the dominant physiologic substrate of XeCYP17 (14).
Fig. 2. XeCYP17 has b5-independent and b5-dependent 17,20- lyase activity.
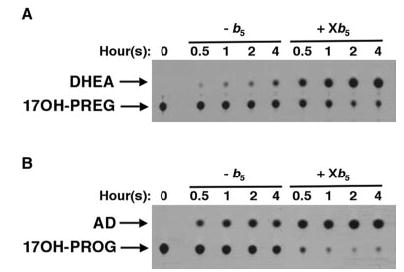
Radiolabeled 17-hydroxypregnenolone (A, 17OH-PREG) and 17-hydroxyprogesterone (B, 17OH-PROG) were added at concentrations of 4 nm to yeast microsomes containing XeCYP17 with or without Xb5 (2.6 μg/ml). Steroids were extracted at the indicated time points and resolved by TLC as described under “Materials and Methods.” Experiments were performed at least three times with nearly identical results.
Table I. Kinetic parameters of 17α-hydroxylase (H) and 17, 20-lyase (L) activity of XeCYP17 in yeast microsomes.
The kinetic parameters of XeCYP17 expressed in yeast microsomes were measured as described in the Experimental Procedures section. The Km and Vmax values for 17α-hydroxylase (H) and 17, 20-lyase (L) activities in both the Δ 4 and Δ 5 pathways are indicated. All experiments were performed at least three times. Values are expressed as mean ± S.D. (n = 3).
| XeCYP17 | Km | Vmax |
|---|---|---|
| nM | min−1 | |
| Δ4 | ||
| −b5 | ||
| H | 856 ± 250 | 3.14 ± 0.97 |
| L | 183 ± 48 | 0.51 ± 0.11 |
| +Xb5 | ||
| H | 697 ± 129 | 3.07 ± 0.52 |
| L | 313 ± 119 | 1.33 ± 0.41 |
| +Hb5 | ||
| H | 674 ± 156 | 3.85 ± 0.34 |
| L | 151 ± 9 | 2.13 ± 0.07 |
| Δ5 | ||
| −b5 | ||
| H | 23 ± 8 | 0.20 ± 0.03 |
| L | 634 ± 283 | 0.41 ± 0.12 |
| +Xb5 | ||
| H | 22 ± 2 | 0.27 ± 0.03 |
| L | 240 ± 22 | 1.96 ± 0.02 |
| +Hb5 | ||
| H | 44 ± 5 | 0.40 ± 0.04 |
| L | 332 ± 94 | 1.71 ± 0.32 |
Hb5 Enhanced XeCYP17 Lyase Activity but Xb5 Did Not Affect HuCYP17
Since Xb5 enhanced the 17,20-lyase activity of XeCYP17, Hb5 was next examined. The human and Xenopus b5 share ~55% identity with several conserved regions (Fig. 1A); thus, Hb5 was predicted to be a sufficient cofactor for XeCYP17. As anticipated, Hb5 improved XeCYP17-mediated lyase activity similarly to Xb5, raising the Vmax values by ~4-fold in both the Δ4 and the Δ5 pathways while having little effect on hydroxylase activities (Table I). These results suggest that the XeCYP17 is promiscuous in its ability to interact with b5 isoforms from other species.
Similar studies using the HuCYP17 in yeast microsomes revealed strikingly different results. First, as expected, HuCYP17 mediated very little b5-independent lyase activity in the Δ5 pathway (Vmax = 0.08 min−1) and none in the Δ4 pathway (Table II). Second, Hb5 enhanced the lyase activity of HuCYP17 significantly more than for the XeCYP17-mediated reaction, raising the Vmax value in the Δ5 pathway (17-hydroxypregnenolone to DHEA) by ~9-fold and raising the Vmax value in the Δ4 pathway (17-hydroxyprogesterone to androstenedione) by an even greater ratio (Table II). Together, these results indicate that, as described previously (10), the HuCYP17-mediated 17,20-lyase reaction is more dependent on and sensitive to cytochrome b5 than its Xenopus counterpart. Of note, the absolute values of lyase activity, as well as the magnitude of the b5-mediated enhancement of the HuCYP17- mediated 17,20-lyase reactions, were similar to published data, (10, 19), indicating that the model system used in these studies is appropriate. Finally, to our surprise, Xb5 did not enhance HuCYP17-mediated lyase activity in either the Δ4 or the Δ5 pathways (Table II), suggesting that HuCYP17 might have more stringent requirements for b5 interactions than XeCYP17.
Table II. Kinetic parameters of 17,20-lyase activity of HuCYP17 in yeast microsomes.
The kinetic parameters of HuCYP17 expressed in yeast microsomes were measured as described in the Experimental Procedures section. The 17, 20-lyase Km and Vmax values in both the Δ 4 and Ddelta; 5 pathways are indicated. All experiments were performed at least three times. Values are expressed as mean ± S.D. (n = 3). ND, activity too low to determine accurately.
| HuCYP17 | Km | Vmax |
|---|---|---|
| nM | min−1 | |
| Δ4 | ||
| −b5 | ND | < 0.003 |
| +Hb5 | 441 ± 132 | 0.13 ± 0.04 |
| +Xb5 | ND | < 0.003 |
| +H(1–69)Xb5 | 509 ± 99 | 0.13 ± 0.03 |
| +H(1–50)Xb5 | 660 ± 26 | 0.10 ± 0.01 |
| +H(1–11)Xb5 | 455 ± 27 | 0.04 ± 0.01 |
| +X(1–74)Hb5 | ND | < 0.003 |
| +X(1–55)Hb5 | 459 ± 6 | 0.05 ± 0.01 |
| +X(1–16)Hb5 | 322 ± 8 | 0.12 ± 0.01 |
| Δ5 | ||
| −b5 | 269 ± 75 | 0.08 ± 0.01 |
| +Hb5 | 338 ± 38 | 0.68 ± 0.05 |
| +Xb5 | 255 ± 55 | 0.10 ± 0.01 |
| +H(1–69)Xb5 | 312 ± 65 | 0.62 ± 0.07 |
| +H(1–50)Xb5 | 257 ± 9 | 0.40 ± 0.01 |
| +H(1–11)Xb5 | 378 ± 46 | 0.11 ± 0.01 |
| +X(1–74)Hb5 | 444 ± 140 | 0.10 ± 0.01 |
| +X(1–55)Hb5 | 191 ± 13 | 0.13 ± 0.01 |
| +X(1–16)Hb5 | 173 ± 16 | 0.29 ± 0.02 |
Residues 16–41 of the Hb5 Were Necessary for Its Specific Actions on the HuCYP17
The inability of Xb5 to enhance the HuCYP17-mediated lyase reaction was exploited to determine the regions of Hb5 that were important for its interactions with HuCYP17. Human/Xenopus b5 chimeras were made as represented schematically in Fig. 1C and then expressed in bacteria as described under “Materials and Methods.” Protein purity was qualitatively assessed by gel electrophoresis followed by Coomassie Blue staining, and protein concentration was determined using the BCA system (Pierce). Varying concentrations of human/Xenopus b5 chimeras, as well as the wild-type Xb5 and Hb5, were added to yeast microsomes containing HuCYP17, and the conversion from 17-hydroxyprogesterone to androstenedione was measured (Fig. 3, A and B). As expected, Xb5 did not enhance the lyase activity of HuCYP17 at any concentration, whereas Hb5 up-regulated HuCYP17-mediated lyase activity in a dose-dependent fashion that peaked between 1 and 5 μg/ml Hb5 (Fig. 3A). Substitution of the first 69 amino acids of Hb5 with the cognate 74 amino acids of Xb5 (X(1–74)Hb5) did not increase the lyase activity of HuCYP17 (Fig. 3A, closed squares). A similar chimera containing the first 55 amino acids of Xb5 in place of the cognate region in Hb5 (X(1– 55)Hb5) mildly enhanced HuCYP17-mediated lyase activity, although 100-fold more X(1–55)Hb5 relative to Hb5 was needed to reach a still suboptimal maximum level of activity (Fig. 3A, closed triangles). These data suggest that the amino-terminal half of Hb5 might be necessary for regulating HuCYP17-mediated lyase activity.
Fig. 3. Effects of wild-type and mutant cytochrome b5 proteins on 17,20-lyase activities.
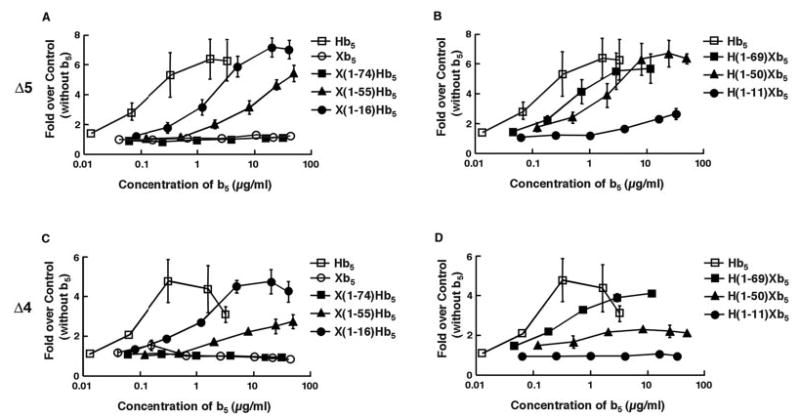
A and B, the production of DHEA from [1,2,6,7-3H]17α-hydroxypregnenolone (Δ5 pathway) was measured in yeast microsomes containing 8.7 pmol HuCYP17 (25 μg of microsomes). Reactions were incubated for 30 min at room temperature with increasing concentrations of the indicated b5 proteins. Steroids were then extracted, and radioactive spots representing 17-hydroxypregneolone and DHEA were counted by liquid scintillation. Activity is plotted as the fold over control (without b5) and was determined by cutting out the radioactive spots and counting them by liquid scintillation. C and D, similar studies examining the production of androstenedione from [1,2,6,7-3H(N)]17α-hydroxyprogesterone (Δ4 pathway). For all studies, each point represents the mean ± S.D. (n = 3). All experiments were performed at least three times with nearly identical results.
In contrast, substitution of the first 16 amino acids of Xb5 for the cognate region in Hb5 (X(1–16)Hb5) enhanced HuCYP17- mediated lyase activity to a maximum induction equal to that regulated by Hb5, although 5-fold more protein was necessary to do so (Fig. 3A, closed circles). Similar substitutions replacing Xb5 regions with the cognate Hb5 sequences revealed that the presence of the first 50 amino acids of Hb5 (H(1–50)Xb5) was sufficient to promote maximum HuCYP17-mediated lyase activity; however, again, 5-fold more protein was necessary to do so (Fig. 3B, closed triangles). In contrast, substitution of the first 11 amino acids of Hb5 into Xb5 (H(1–11)Xb5) did not enhance lyase activity (Fig. 3B, closed circles).
Similar results were obtained when examining the effects of the b5 chimeras on the substrate 17-hydroxyprogesterone (Fig. 3, C and D), although the effects were less dramatic due to the known slower rate of the huCYP17-mediated 17,20-lyase rate in the Δ4 pathway (10, 11). Furthermore, all of these results were confirmed quantitatively in Table II, using 2.5 μg/ml human, Xenopus, or chimeric b5 proteins. H(1–50)Xb5 enhanced HuCYP17-mediated 17,20-lyase activity similarly to Hb5 in both the Δ4 and the Δ5 pathways, whereas H(1–11)Xb5 only minimally enhanced the lyase reaction. Together, these results indicate that residues 12–50 in the Hb5 are necessary for regulating the lyase activity of HuCYP17. Since the first 4 and last 9 amino acids of this region are identical to those in Xb5, they are less likely to be important for this specificity; thus, the region of specificity can likely be narrowed down to resides 16–41 (Fig. 3A, boxed region).
Xb5 Did Not Block Hb5-mediated Lyase Activity of HuCYP17
The residues in region 16–41 of the Hb5 could be specifically regulating HuCYP17 by two possible mechanisms that are not mutually exclusive. First, residues 16–41 might be necessary for Hb5 binding to HuCYP17. Alternatively, residues 16–41 might be important for specifically activating HuCYP17 but not for binding. Competition studies were performed using increasing amounts of Xb5 in the face of a smaller, activating amount of Hb5 (0.2 μg/ml). If Xb5 was binding to the HuCYP17 enzyme but could not promote lyase activity, then the addition of excess Xb5 should have blocked the ability of Hb5 to bind to HuCYP17, thus reducing its activity. In fact, up to 100-fold excess of Xb5 had very little effect on Hb5-mediated lyase activity, confirming that the Xb5 molecule is likely unable to bind with high affinity to HuCYP17, at least at the same site as does Hb5 (Fig. 4). In contrast, the addition of excess Hb5 decreased HuCYP17-mediated lyase activity, a known phenomenon that is most likely due to b5-mediated competition with the cofactor oxidoreductase for electron transfer during the 17,20-lyase reaction (20).
Fig. 4. Xb5 does not block Hb5-induced lyase activity of HuCYP17.
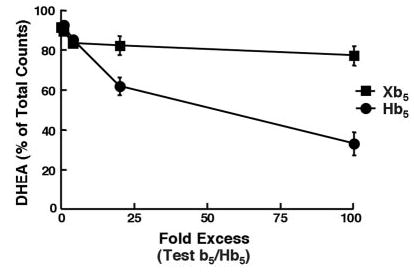
The 17,20-lyase reaction of HuCYP17 was performed by the addition of 0.2 μg/ml Hb5 (final concentration) and varying amounts of Xb5 or Hb5 (indicated on the x axis). Reactions were run at room temperature for 30 min, and then steroids were extracted and separated by TLC. The percentage of conversion to DHEA was determined by cutting out the radioactive spots and counting them by liquid scintillation. The production of DHEA was plotted as the percentage of total counts. Each point represents the mean ± S.D. (n = 3). This experiment was performed at least three times with similar results.
DISCUSSION
Most well characterized mammalian isoforms of CYP17 have little to no intrinsic 17,20-lyase relative to 17α-hydroxylase activity. Significant 17,20-lyase activity is only seen when the cofactor cytochrome b5 is added to the enzyme. Furthermore, even in the presence of cytochrome b5, the rate of the 17,20- lyase activity remains lower than that of the hydroxylase activity and is usually preferentially enhanced in either the Δ4 or the Δ5 pathway. For instance, in small animals, such as rats (21), mice (22), hamsters (23), and guinea pigs (24), CYP17 is more active in the Δ4 pathway. In contrast, human and primate CYP17s utilize the Δ5 pathway (10, 11).
The Xenopus laevis isoform of CYP17 (XeCYP17) serves as an unusual example of this enzyme. Ovarian expression of XeCYP17 appears to be almost exclusively within oocytes, whereas all other steroidogenic enzymes are found in the surrounding follicular cells (14). Thus, oocytes play a critical role in regulating ovarian androgen and estrogen production, which is in turn necessary for the regulation of oocyte maturation and vitellogenin production, respectively (14, 25). Furthermore, frog ovarian androgen production is extremely efficient, most likely due to the remarkably potent 17,20-lyase reaction mediated in the Xenopus oocyte. Here, characterization of XeCYP17 expressed in yeast microsomes revealed that XeCYP17 contained significantly higher 17,20-lyase activity than its human counterpart HuCYP17 (Tables I and II). Furthermore, unlike most mammalian isoforms of CYP17, the XeCYP17-mediated 17,20-lyase reaction was very potent without b5 in both the Δ4 and the Δ5 pathways. In fact, the rates of cytochrome b5- independent XeCYP17-mediated 17,20-lyase activity were similar to b5-dependent HuCYP17-induced 17,20-lyase activities (Tables I and II). Finally, although the addition of b5 enhanced XeCYp17-mediated lyase activity, the observed 2–4-fold increase was significantly lower than what was seen with HuCYP17 under similar conditions, in which Hb5 enhanced lyase activities by 9-fold or more.
In sum, these kinetic studies suggest that XeCYP17 does not rely on cytochrome b5 as much as its mammalian counterparts for normal androgen production. This difference might reflect the need of the frog to produce more androgens relative to cortisol. Interestingly, frogs, sharks, eels, and fish, all of which contain phylogenetically related CYP17 enzymes (14), generally utilize corticosterone or its metabolites as their primary glucocorticoids (26–28). Since these species do not require the accumulation of 17-hydroxyprogesterone or 17-hydroxypregnenolone for survival, the major purpose of CYP17 might therefore be to regulate androgen production. In contrast, human CYP17 is necessary for both androgen as well as cortisol production. Since cortisol production requires the accumulation of 17-hydroxlated steroids, mechanisms may have evolved that led to tighter regulation of 17,20-lyase activity in the adrenal glands. For example, 17,20-lyase activity would not be desired in the zona fasciculata, where cortisol production occurs. This could be accomplished in higher mammals simply by limiting b5 expression, favoring the production of 17-hydroxlyated steroids. In contrast, initiation of adrenarche involves a carefully timed increased in adrenal androgen production that requires up-regulation of the CYP17-mediated 17,20-lyase reaction in the zona reticularis. Although not completely understood, this increased lyase activity may be due in part to increased expression of cytochrome b5 in the zona reticularis (29, 30) as well as to post-translational modifications of CYP17 (18).
Curiously, XeCYP17 was relatively promiscuous in its interactions with cytochrome b5 as both Xb5 and Hb5 were equally potent enhancers of XeCYP17-mediated lyase activity. The similar activities of the two b5 isoforms with regard to Xe- CYP17 suggests that both are capable of interacting with the enzyme, as well as the cofactor oxidoreductase, in a meaningful and functional manner. In contrast, HuCYP17 responded only to Hb5, suggesting that the human enzyme could not appropriately interact with Xb5. We defined a region of Hb5 between residues 16 and 41 that, when exchanged into the cognate portion of Xb5, maximally enhanced HuCYP17-mediated 17,20- lyase activity. These results suggest that residues 16–41 of Hb5 might comprise a specific binding site for HuCYP17 that is not present in Xb5. Accordingly, Xb5 did not compete for Hb5- mediated 17,20-lyase activity in HuCYP17 at any concentration, indicating that the Xenopus cofactor might not bind to the HuCYP17 with significant affinity. To test whether this region was sufficient to bind to HuCYP17, a peptide encoding residues 12–69 was produced in bacteria but failed to block Hb5-mediated 17,20-lyase activity (data not shown). This result, combined with the data in Fig. 4 and Table II, suggests that region 16–41 is necessary, but not sufficient, for the interaction between Hb5 and HuCYP17. Interestingly, the most divergent region between resides 16 and 41 is located at an external surface of cytochrome b5 (Fig. 5, left, represented as green residues in the blue region) and therefore could serve as a possible docking site for CYP17. The decreased activity of Xb5 might be due to the dramatic charge differences in this region (Fig. 5, right). For example, the weakly positively charged histidine residues in Hb5 at positions 20 and 22 are replaced by a strongly positive arginine and a neutral threonine, respectively, in Xb5. In addition, the relatively neutral serine and glutamine residues at positions 25 and 18 of Hb5 are replaced by negatively charge glutamate and positively charge arginine residues, respectively.
Fig. 5. A model comparing region 16–41 from the Hb5 (A) to the cognate region of Xb5 (B).
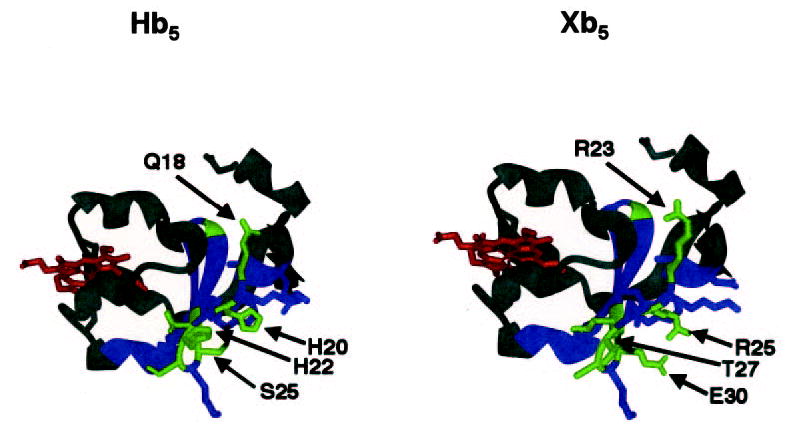
The heme-binding region is represented by red, region 16–41 is represented by blue, and the least homologous residues within 16–41 are represented by green. Note that these green residues are part of an external, less-confined region and that the side-chain charges of this region of Xb5 differ from those in the Hb5.
Although residues 16–41 of Hb5 appear to be important for its specific interactions with HuCYP17 during the 17,20-lyase reaction, the critical interacting domains within the human enzyme itself remain undefined. More studies are needed to answer these questions and to fully determine the nature of the interactions between CYP17 and b5. Given the unusual nature of XeCYP17-mediated 17,20-lyase activity, perhaps continued comparison of the human with Xenopus CYP17 will provide useful insight into these unresolved issues.
Supplementary Material
Acknowledgments
We are indebted deeply to Dr. Richard Auchus and Dr. Jacqueline Naffin for their valuable advice and input.
Footnotes
This work was supported by grants from the National Institutes of Health (Grant DK59913) and the Welch Foundation (Grant I-1506).
The on-line version of this article (available at http://www.jbc.org) contains a supplementary table.
The abbreviations used are: DHEA, dehydroepiandrosterone; b5, cytochrome b5; Xb5, Xenopus b5; Hb5, human b5; CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid; XeCYP17, Xenopus CYP17; human CYP17, HuCYP17.
The nucleotide sequence reported in this paper has been submitted to the DDBJ/GenBank™/EBI Data Bank with accession number(s) AY775052.
References
- 1.Heinlein CA, Chang C. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 2.Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Jones M, Davis S. Trends Endocrinol Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SG, Tetsuka M. Baillieres Clin Obstet Gynaecol. 1997;11:249–260. doi: 10.1016/s0950-3552(97)80036-3. [DOI] [PubMed] [Google Scholar]
- 4.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Proc Natl Acad Sci U S A. 2004;101:11209–11214. doi: 10.1073/pnas.0404372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speiser PW. J Endocrinol Investig. 2001;24:681–691. doi: 10.1007/BF03343913. [DOI] [PubMed] [Google Scholar]
- 6.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Fisher CR, Conte FA, Grumbach MM, Simpson ER. Proc Natl Acad Sci U S A. 1993;90:11673–11677. doi: 10.1073/pnas.90.24.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, Fauser BC. Histopathology (Oxf) 1991;19:445–452. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 9.Zuber MX, Simpson ER, Waterman MR. Science. 1986;234:1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]
- 10.Auchus RJ, Lee TC, Miller WL. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 11.Lin D, Black SM, Nagahama Y, Miller WL. Endocrinology. 1993;132:2498–2506. doi: 10.1210/endo.132.6.8504753. [DOI] [PubMed] [Google Scholar]
- 12.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Proc Natl Acad Sci U S A. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR. Mol Endocrinol. 2003;17:1106–1116. doi: 10.1210/me.2003-0032. [DOI] [PubMed] [Google Scholar]
- 14.Yang WH, Lutz LB, Hammes SR. J Biol Chem. 2003;278:9552–9559. doi: 10.1074/jbc.M212027200. [DOI] [PubMed] [Google Scholar]
- 15.Holmans PL, Shet MS, Martin-Wixtrom CA, Fisher CW, Estabrook RW. Arch Biochem Biophys. 1994;312:554–565. doi: 10.1006/abbi.1994.1345. [DOI] [PubMed] [Google Scholar]
- 16.Gupta MK, Geller DH, Auchus RJ. J Clin Endocrinol Metab. 2001;86:4416–4423. doi: 10.1210/jcem.86.9.7812. [DOI] [PubMed] [Google Scholar]
- 17.Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. J Biol Chem. 2003;278:48563–48569. doi: 10.1074/jbc.M307586200. [DOI] [PubMed] [Google Scholar]
- 18.Pandey AV, Mellon SH, Miller WL. J Biol Chem. 2003;278:2837–2844. doi: 10.1074/jbc.M209527200. [DOI] [PubMed] [Google Scholar]
- 19.Katagiri M, Kagawa N, Waterman MR. Arch Biochem Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 20.Geller DH, Auchus RJ, Miller WL. Mol Endocrinol. 1999;13:167–175. doi: 10.1210/mend.13.1.0219. [DOI] [PubMed] [Google Scholar]
- 21.Koh Y, Buczko E, Dufau ML. J Biol Chem. 1993;268:18267–18271. [PubMed] [Google Scholar]
- 22.Youngblood GL, Payne AH. Mol Endocrinol. 1992;6:927–934. doi: 10.1210/mend.6.6.1323057. [DOI] [PubMed] [Google Scholar]
- 23.Cloutier M, Fleury A, Courtemanche J, Ducharme L, Mason JI, Lehoux JG. DNA Cell Biol. 1997;16:357–368. doi: 10.1089/dna.1997.16.357. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay Y, Belanger A, Fleury A, Beaudoin C, Provost P, Martineau I. Endocr Res. 1995;21:495–507. doi: 10.3109/07435809509030467. [DOI] [PubMed] [Google Scholar]
- 25.Redshaw MR, Follett BK, Nichollis TJ. J Endocrinol. 1969;43:47–53. doi: 10.1677/joe.0.0430047. [DOI] [PubMed] [Google Scholar]
- 26.Sandor T, Chan SW, Phillips JG, Ensor D, Henderson IW, Jones IC. Can J Biochem. 1970;48:553–558. doi: 10.1139/o70-091. [DOI] [PubMed] [Google Scholar]
- 27.Simpson TH, Wright RS. J Endocrinol. 1970;46:261–268. doi: 10.1677/joe.0.0460261. [DOI] [PubMed] [Google Scholar]
- 28.Armour KJ, O’Toole LB, Hazon N. J Mol Endocrinol. 1993;10:235–244. doi: 10.1677/jme.0.0100235. [DOI] [PubMed] [Google Scholar]
- 29.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Trends Endocrinol Metab. 2002;13:234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]
- 30.Auchus RJ, Rainey WE. Clin Endocrinol. 2004;60:288–296. doi: 10.1046/j.1365-2265.2003.01858.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


