Abstract
Invasive species pose serious threats to community structure and ecosystem function worldwide. The impacts of invasive species can be more pervasive than simple reduction of species numbers. By using 7 years of data in a biological preserve in northern California, we documented the disassembly of native ant communities during an invasion by the Argentine ant. In sites without the Argentine ant, native ant communities exhibit significant species segregation, consistent with competitive dynamics. In sites with the Argentine ant, native ant communities appear random or weakly aggregated in species co-occurrence. Comparisons of the same sites before and after invasion indicate that the shift from a structured to a random community is rapid and occurs within a year of invasion. Our results show that invasive species not only reduce biodiversity but rapidly disassemble communities and, as a result, alter community organization among the species that persist.
Keywords: community organization‖null model‖Linepithema humile‖invasive ants
By displacing native species or reducing their abundance, biological invaders have severely disrupted the organization and function of many native communities and ecosystems (1, 2). The loss of biodiversity in the face of invasive species reflects the breakdown of community assembly rules (3) that structure intact assemblages. However, the disruption of community assembly rules has not been documented during the course of a biological invasion. Studies of invasions as natural experiments (4) are usually snapshot analyses that often lack preinvasion data on community structure. In this study, we used 7 years of data from monitoring an invasion in progress to assess the impact of a widespread invasive species, the Argentine ant (Linepithema humile), on the organization and disassembly of native ant communities in northern California. We used null model analysis (5) to ask three related, but distinct, questions with these data: (i) Is there evidence of nonrandom co-occurrence of native species within intact or invaded regions? (ii) Do co-occurrence patterns differ between invaded and intact regions? (iii) Do co-occurrence patterns in intact regions change once L. humile invades?
Methods
The Argentine ant is native to South America and has been introduced to Mediterranean and subtropical climates worldwide. As of 2001, the Argentine ant occupied 335 counties in 21 states in the United States and six continents in the world (6). Perhaps because the Argentine ant locates resources efficiently and interferes with native species in its introduced range (7, 8), native ant (9–13) and arthropod (9, 14) species richness is drastically reduced in the presence of Argentine ants.
Survey Methods.
Since 1993, we have monitored the distribution of the ground-foraging native ant fauna and the invasive Argentine ant at Jasper Ridge Biological Preserve, a 481-hectare preserve in northern California. By using aerial photographs, we divided the preserve into 133 1-hectare quadrats. Each May and September since 1993, a Stanford graduate student (K. G. Human from 1993 to 1996, N.J.S. from 1996 to 2000, and N.E.H. from 2000 to present) surveyed the ant community in a permanent 20-m-radius circle located in the center of each quadrat, to which we refer as a sample plot. In each sample plot, we recorded the species present during 5 min of active searching. Although this method may not sample species that forage entirely under the leaf litter or in the canopy, it reliably indicates the presence of the conspicuous ground-foraging species (15). If no ants were found by active searching, we left two 40-ml vials partially filled with honey and collected them 24 h later. Two previous studies (8, 13) compared our general collecting and honey trap techniques to pitfall trapping and intensive searching for more than 5 min and found little difference in species number or composition. In May 1995 and 1996, native ant taxa were not identified, so we excluded those surveys in all subsequent data analysis.
For each sampling date, we organized the survey data as a presence–absence matrix, a fundamental unit of study in community ecology and biogeography (16). In such a matrix, each row represents a different native ant species, each column represents a different sample plot, and the entries in the matrix indicate the absence (0) or presence (1) of a particular ant species in a particular sample plot (17).
Quantifying Species Co-occurrence.
To compare the co-occurrence patterns of intact and invaded assemblages, we created at each sampling date separate matrices for sample plots with and without L. humile (for a total of 12 sampling dates). Although the presence of L. humile was used to classify the plots as invaded or intact, L. humile itself was not included in any of the analyses of species occurrence. Because the number of invaded sample plots increased as the invasion progressed (May 1994, n = 83; September 1994, n = 88; September 1995, n = 88; September 1996, n = 91; May 1997, n = 88; September 1997, n = 94; May 1998, n = 93; September 1998; n = 102; May 1999, n = 106; May 2000, n = 91; September 2000, n = 91), the sizes of the invaded and intact matrices also vary. To compare the structure of pre- and postinvasion assemblages, we analyzed the subset of sample plots that were newly invaded by L. humile each year and compared them with the same sample plots before invasion.
We used the C-score of Stone and Roberts (18) as a quantitative index of community organization. The index quantifies the number of “checkerboard units” that can be found for each species pair. A checkerboard unit is a 2 × 2 submatrix of the form 01/10 or 10/01. For each species pair, the number of checkerboard units is (Ri − S)(Rj − S), where Ri is the number of occurrences (equal to the row total) for species i, Rj is the number of occurrences for species j, and S is the number of sample plots in which both species occur. The C-score is the average number of checkerboard units for each unique species pair. If this index is unusually large compared with a null distribution, there is less pairwise species co-occurrence (segregation) than expected by chance. If the index is unusually small, there is more species co-occurrence (aggregation) than expected.
Null Model Algorithms.
To assign a probability value to an observed C-score, we used randomization to construct a null distribution for the C-score (19). An appropriate randomization for data collected from small sample plots of fixed area is a fixed, equiprobable algorithm in which row totals (species occurrences) are preserved but columns (sample plots) are treated as equiprobable (20). This model allows species number per sample plot to vary in the null assemblages and also takes into account “empty” sample plots, which did not contain species but which might have been occupied in a randomly assembled community. This algorithm behaves well in benchmark tests for type I and type II statistical errors (20). We obtained similar results from a parallel set of analyses in which we used only those plots that contained at least one native ant species. For each presence–absence matrix, we created 5,000 random matrices by reshuffling the elements of each row of the matrix. We then calculated the C-score for each random matrix and estimated the tail probability (one-tailed test) of the observed matrix by comparing it with the histogram of simulated values.
To test for differences in co-occurrence between intact and invaded sample plots and pre- and postinvaded plots, we used a simple partition test as a null model (21). We first organized the data from one time period as a single matrix in which each row is a species and each column is a sample plot (invaded or intact). Next, we reshuffled the plot labels of “invaded” and “intact” among the different columns. We then calculated the C-score for invaded and intact matrices that were created this way. Finally, we calculated the variance of the two C-score measurements for each random partition. The larger this variance, the more different the intact and invaded plots were in the pattern of species co-occurrence. The observed variance in C-scores between intact and invaded plots was compared with the histogram of 5,000 variances created through random partitions of the data set. If the observed variance was larger than expected by chance, the co-occurrence patterns in the two matrices were statistically distinct. We used a similar procedure to test for difference in pre- and postinvasion matrices. All null model analyses were conducted with ECOSIM 7.0 software (ref. 22; http://homepages.together.net/∼gentsmin/ecosim.htm).
Standardized Effect Size (SES).
To compare results across sampling periods, we calculated an SES (23) for each matrix. The SES measures the number of standard deviations that the observed index is above or below the mean index of the simulated communities. In meta-analysis, an effect size is calculated by standardizing the difference between control and treatment groups. In our analysis, the observed index (Iobs) corresponds to the treatment group. The mean of the 5,000 indices from the simulated communities (Isim) corresponds to the control group because it reflects the pattern expected in the absence of species interactions. We used the standard deviation of the 5,000 indices from the simulated communities (Ssim) to calculate the SES as follows: SES = (Iobs − Isim)/Ssim.
We used a single sample t test to test the null hypothesis that the SES did not differ from 0.0. Assuming a normal distribution of deviations, ≈95% of the SES values should fall between −2.0 and 2.0. For the within-group analyses, this test measured whether the C-scores for the set of 12 matrices (one measured at each sampling time) differed significantly from randomness. For the partition test, this analysis revealed whether the 12 pairs of intact and invaded matrices differed significantly from one another.
Results
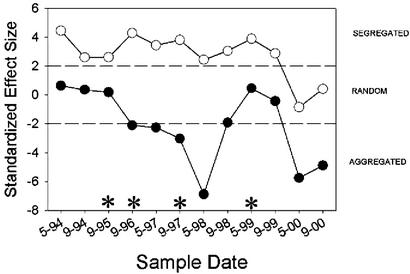
There was evidence for nonrandom co-occurrence patterns (segregation) of native species within intact regions but not within invaded regions. For the set of intact matrices, there was significantly less species co-occurrence than expected by chance (single sample t test, t = 6.087, df = 11, P < 0.001), and the null hypothesis was rejected on all sampling dates except the May and September 2000 surveys. For the set of invaded matrices, there was significantly more co-occurrence than expected by chance (single sample t test, t = −2.877, df = 11, P = 0.015), with statistically significant aggregation patterns occurring in May 1997, May 1998, May 2000, and September 2000.
There were also consistent differences in the co-occurrence patterns of intact and invaded regions sampled at the same time period (single sample t test, t = 2.285, df = 11, P = 0.043). Differences in the pattern were statistically significant on sampling dates of September 1995, September 1996, September 1997, and May 1999. On all sampling dates, the co-occurrence pattern always tended more toward species segregation in the intact sample plots and species aggregation in the invaded sample plots (Fig. 1).
Figure 1.
A comparison of native ant community organization in intact sample plots and sample plots invaded by L. humile. The standardized C-score is a measure of the extent to which species co-occur less frequently than expected by chance. Larger C-scores indicate less co-occurrence than in randomly assembled communities. The dotted lines represent 1.96 standard deviations, the approximate level of statistical significance (P < 0.05). *, Statistical differences in co-occurrence patterns of intact and invaded plots sampled during the same sampling period (partition test, P < 0.05). Paired symbols indicate invaded and intact plots sampled during the same survey. ●, Invaded plots; ○, uninvaded plots.
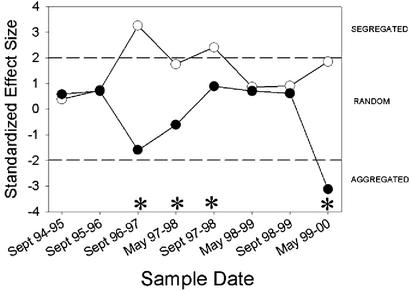
Co-occurrence patterns change once L. humile invades. For the set of preinvasion matrices, there was significantly less species co-occurrence than expected by chance (single sample t test, t = 4.394, df = 11, P = 0.003), and the null hypothesis was rejected for four of the eight matrices (September 1996, May 1997, September 1997, and May 1999). For the set of postinvasion matrices, co-occurrence patterns in aggregate did not differ significantly from random (single sample t test, t = −0.451, df = 11, P = 0.666), and none of the individual matrix tests was significant. Finally, the partition test was marginally nonsignificant (single sample t test, t = 1.438, df = 7, P = 0.194), although differences between pre- and postinvasion co-occurrence patterns were significant in four of the eight comparisons (September 1996 and 1997, May 1997 and 1998, September 1997 and 1998, and May 1999 and 2000). All of the statistically significant differences were in the direction of more species segregation before invasion and more random patterns after invasion (Fig. 2).
Figure 2.
A comparison of native ant community organization in sample plots immediately before and after invasion by L. humile. Paired symbols indicate the same plots sampled before and 1 year after invasion. ●, Plots sampled in the presence of L. humile; ○, the same plot sampled before invasion. Other symbols as in Fig. 1.
Discussion
Our results demonstrate that invasive species do more than reduce the number of native species: The presence of L. humile led to a reorganization of co-occurrence patterns of native species and a shift from species segregation in intact communities to species aggregation in invaded communities. Although previous studies have documented the effects of invasive species on native communities and pointed out the utility of invasions as natural experiments, such studies are retrospective analyses of circumstantial evidence (24, 25). For example, Gotelli and Arnett (26) documented similar effects of the red imported fire ant, Solenopsis invicta, on native communities. Like most studies of invasions, Gotelli and Arnett's study was a snapshot study. One problem with such snapshot studies is that invaded and intact sites may differ in some way, such as in the level of disturbance, that can promote the success of the invader but hinder native populations. Another problem is that snapshot studies lack preinvasion data on native communities, so it is impossible to determine whether sites that were subsequently invaded already differed from intact sites in native species richness or co-occurrence patterns. One part of our study, like others, compares invaded areas with uninvaded areas (Fig. 1). In contrast to most other studies of the impact of invasive species, a second part of our study compares organization in communities before invasion and immediately after invasion (Fig. 2).
Our study also documents dynamic changes in community organization that occurred over several consecutive years. Studies over several years of a S. invicta invasion in Texas have shown that native ant species richness is rapidly reduced (27), but that the impact of S. invicta may vary with time (28). Our comparison of pre- and postinvasion plots (Fig. 2) suggests that the reorganization of native ant communities is rapid, and the shift from segregated toward random or aggregated species occurrences begins within 1 year of the appearance of L. humile. Thus, our results suggest that this invasive species, by its ability to exploit resources and interfere with competitors, causes rapid and drastic changes in ant community organization.
Our results indicate that assembly rules act to organize these ant communities. The most influential and fiercely debated assembly rule comes from Diamond's work (29) on the distribution of bird species of the Bismarck Archipelago: “Some pairs of species never coexist, either by themselves or as part of a larger combination.” Stone and Roberts's (18) C-score is a related index. Substantial evidence supports Diamond's assembly rule (30). When distribution data are based on retrospective analyses, the processes underlying such patterns often remain elusive. For example, checkerboard distributions may arise because preferred habitat occurs in checkerboards or because of evolutionary or biogeographic history. By studying an invasion in progress and using pre- and postinvasion data as a natural experiment, our results clearly show that competition by a dominant species influences community co-occurrence patterns in this system. Our results thus confirm Diamond's assembly rule in these ant communities by showing how competition can disassemble a community.
Native ant communities before invasion by L. humile were highly structured. Among uninvaded plots and plots before invasion, native ant species co-occurred less often than expected based on chance alone, suggesting that communities may have been structured by competition. In plots invaded by L. humile or postinvasion plots, co-occurrence patterns tended toward randomness.
The shifts from segregated to random or aggregated co-occurrence patterns did not arise because L. humile preferentially invades sample plots with low native ant species richness or because habitat characteristics promote invasion by L. humile but negatively affect native ant populations in some sites. One of our analyses (Fig. 2) compared sites before invasion with sites after they had been invaded by L. humile. All of the sites were apparently equally susceptible to L. humile; all were invaded within 1 year. To check further that L. humile does not invade sample plots with lower native ant richness, we compared, with t tests, native ant richness in invaded and uninvaded plots in each year before invasion. We found that there were no statistical differences in native ant richness before invasion in any year (data not shown). In fact, there was a slight trend for invaded sample plots to have had more native species before invasion than uninvaded plots had.
The shifts from segregated to random or aggregated co-occurrence patterns did not arise because L. humile reduces native ant richness to essentially zero in many invaded plots, thus increasing the number of 0/0 matrices. We checked for this by repeating all of our analyses, using only those sites that contained at least one native ant species. Although there was less aggregation in the invaded sites, the pattern of differences between invaded and uninvaded sites did not differ qualitatively from the one we present in Figs. 1 and 2. Thus, our results strongly indicate that these shifts from highly structured co-occurrence patterns to random patterns were caused by the invasive Argentine ant.
There are at least three explanations for this shift from segregation to aggregation or randomness. First, L. humile may have differential impacts on species in the community (11–13), thereby altering species interactions. Previous work at Jasper Ridge and other sites in California has shown that not all native ant species are equally affected by L. humile invasions (11–13). For example, some habitat generalists such as Formica moki or Camponotus semitestaceus temporarily persisted when L. humile invaded at Jasper Ridge. Another native species, Prenolepis imparis (the winter ant), co-occurs in many sample plots with L. humile, probably because it is often active during the coldest months of the year, when L. humile activity is reduced. Other native species never or rarely co-occurred in the same sample plots with L. humile (e.g., Aphaenogaster occidentalis, Liometopum occidentale, and Neivamyrmex californicus), perhaps because they are habitat or resource specialists or are weak competitors (7, 8). Because some species persist while others do not, interactions among the surviving species are altered. This seems to be what alters co-occurrence patterns among surviving species.
Second, the density of L. humile varies spatially, so its effect also varies spatially (ref. 11; N.E.H., unpublished data). This variation could result in the co-occurrence of more native species in plots where both the density and impact of L. humile are low if L. humile reduces population densities of competing species so that competition among them is reduced. For example, near the edge of Jasper Ridge, where L. humile is well established, we rarely detected native ant taxa. In some areas at the invasion front, where L. humile may not be well established, native ant species co-occurred more often with L. humile (9, 13).
Third, rates of L. humile invasion vary through time, perhaps because of climatic differences in abiotic factors among sites or climatic variation over years (13). Although we compared co-occurrence patterns of native communities within years, L. humile may not be well established in some plots because the invasion is progressing more slowly in some areas than others. Thus, its impact on native community organization may vary because rate of spread varies.
Recent studies have shown that, over the course of many years, invasion by Argentine ants can disrupt tightly evolved interactions such as those between specialist predators and their prey (31) or between plants and their seed dispersers (32). Interspecific competition is a major force structuring intact ant communities (33–35), and species co-occurrence patterns may reflect a shared evolutionary history resulting from competition among native ant species (36). Our study reveals the subtle nature of community disassembly as native species are lost and interactions among surviving species are altered.
Acknowledgments
We thank D. Simberloff and D. Vázquez for helpful discussions and comments on the manuscript. The work was supported by U.S. Department of Agriculture Grants 95-37302-1885 and 2001-35302-09981 (to D.M.G.). ecosim software development was supported by National Science Foundation Grant DEB 01-07403 (to N.J.G.).
Abbreviation
- SES
standardized effect size
References
- 1.Vitousek P M, D'Antonio C M, Loope L L, Westbrooks R. Am Sci. 1996;84:468–478. [Google Scholar]
- 2.Mack R N, Simberloff D, Lonsdale W M, Evans H, Clout M, Bazzaz F A. Ecol Appl. 2000;10:689–710. [Google Scholar]
- 3.Weiher E, Keddy P. Ecological Assembly Rules: Perspectives, Advances, Retreats. Cambridge, U.K.: Cambridge Univ. Press; 1999. [Google Scholar]
- 4.Diamond J M. In: Community Ecology. Diamond J M, Case T J, editors. New York: Harper & Row; 1986. pp. 3–22. [Google Scholar]
- 5.Gotelli N J, Graves G R. Null Models in Ecology. Washington, DC: Smithsonian Institution Press; 1996. [Google Scholar]
- 6.Suarez A V, Holway D A, Case T J. Proc Natl Acad Sci USA. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holway D A. Ecology. 1999;80:238–251. [Google Scholar]
- 8.Human K G, Gordon D M. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 9.Human K G, Gordon D M. Conserv Biol. 1997;11:1242–1248. [Google Scholar]
- 10.Holway D A. Oecologia. 1998;116:252–258. doi: 10.1007/s004420050586. [DOI] [PubMed] [Google Scholar]
- 11.Suarez A V, Bolger D T, Case T J. Ecology. 1998;79:2041–2056. [Google Scholar]
- 12.Ward P S. Hilgardia. 1987;55:1–16. [Google Scholar]
- 13.Sanders N J, Barton K E, Gordon D M. Oecologia. 2001;127:123–130. doi: 10.1007/s004420000572. [DOI] [PubMed] [Google Scholar]
- 14.Cole F R, Medeiros A C, Loope L L, Zuehlke W W. Ecology. 1992;73:1313–1322. [Google Scholar]
- 15.Bestelmeyer B T, Agosti D, Alonso L E, Brandão C R F, Brown W L, Jr, Delabie J H C, Silvestre R. In: Ants: Standard Methods for Measuring and Monitoring Biodiversity. Agosti D, Majer J D, Alonso L E, Schultz T R, editors. Washington, DC: Smithsonian Institution Press; 2000. pp. 122–144. [Google Scholar]
- 16.McCoy E D, Heck K L. J Biogeogr. 1987;14:79–87. [Google Scholar]
- 17.Connor E F, Simberloff D. Ecology. 1979;60:1132–1140. [Google Scholar]
- 18.Stone L, Roberts A. Oecologia. 1990;85:74–79. doi: 10.1007/BF00317345. [DOI] [PubMed] [Google Scholar]
- 19.Manly B F J. Randomization and Monte Carlo Methods in Biology. London: Chapman & Hall; 1991. [Google Scholar]
- 20.Gotelli N J. Ecology. 2000;81:2606–2621. [Google Scholar]
- 21.Stone L, Dayan T, Simberloff D. Am Nat. 2000;156:322–328. doi: 10.1086/303384. [DOI] [PubMed] [Google Scholar]
- 22.Gotelli N J, Entsminger G L. ecosim, Null Models Software for Ecology (Acquired Intelligence, Inc. & Kesey-Bear, Burlington, VT), Version 7.0. 2002. [Google Scholar]
- 23.Gurevitch J, Morrow L L, Wallace A, Walsh J S. Am Nat. 1992;140:539–572. [Google Scholar]
- 24.Brown J H. Macroecology. Chicago: Univ. of Chicago Press; 1995. [Google Scholar]
- 25.Sagarin R D, Barry J P, Gilman S E, Baxter C H. Ecol Monogr. 1999;69:465–490. [Google Scholar]
- 26.Gotelli N J, Arnett A E. Ecol Lett. 2000;3:257–261. [Google Scholar]
- 27.Porter S D, Savignano D A. Ecology. 1990;71:2095–2106. [Google Scholar]
- 28.Morrison L W. Ecology. 2002;83:2337–2345. [Google Scholar]
- 29.Diamond J M. In: Ecology and Evolution of Communities. Cody M L, Diamond J M, editors. Cambridge, MA: Harvard Univ. Press; 1975. pp. 342–444. [Google Scholar]
- 30.Gotelli N J, McCabe D J. Ecology. 2002;83:2091–2096. [Google Scholar]
- 31.Suarez A V, Richmond J Q, Case T J. Ecol Appl. 2000;10:711–725. [Google Scholar]
- 32.Christian C E. Nature. 2001;413:635–639. doi: 10.1038/35098093. [DOI] [PubMed] [Google Scholar]
- 33.Andersen A N. Am Nat. 1992;140:401–420. doi: 10.1086/285419. [DOI] [PubMed] [Google Scholar]
- 34.Andersen A N, Patel A D. Oecologia. 1994;98:15–24. doi: 10.1007/BF00326085. [DOI] [PubMed] [Google Scholar]
- 35.Savolainen R, Vepsäläinen K. Oikos. 1988;51:135–155. [Google Scholar]
- 36.Hölldobler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]