Abstract
Objective
To develop a model of the life cycle of clinical documents from inception to use in a person's medical record, including workflow requirements from clinical practice, local policy, and regulation.
Design
We propose a model for the life cycle of clinical documents as a framework for research on documentation within electronic medical record (EMR) systems. Our proposed model includes three axes: the stages of the document, the roles of those involved with the document, and the actions those involved may take on the document at each stage. The model includes the rules to describe who (in what role) can perform what actions on the document, and at what stages they can perform them. Rules are derived from needs of clinicians, and requirements of hospital bylaws and regulators.
Results
Our model encompasses current practices for paper medical records and workflow in some EMR systems. Commercial EMR systems include methods for implementing document workflow rules. Workflow rules that are part of this model mirror functionality in the Department of Veterans Affairs (VA) EMR system where the Authorization/ Subscription Utility permits document life cycle rules to be written in English-like fashion.
Conclusions
Creating a model of the life cycle of clinical documents serves as a framework for discussion of document workflow, how rules governing workflow can be implemented in EMR systems, and future research of electronic documentation.
Introduction
In some organizations, practitioners creating notes to document medical encounters are increasingly moving away from using handwritten or dictated notes placed in paper charts to using notes created and filed in electronic medical record (EMR) systems. Use of electronic notes may rise in response to growing national interest in EMR systems, 1,2 requirements of payors, 3 and other pressures. 4 As this transition to electronic documentation occurs, workflow of documentation may also change. For example, electronic documents can be created remotely and filed in the EMR system, eliminating the need to place a piece of paper in a chart. When practitioners create and file notes in an EMR system, other aspects of documentation workflow remain, such as the need to sign and cosign documents and to check the accuracy of documents before others view them.
One way to study issues regarding the workflow associated with medical documentation is to build a model of the life cycle of documents. We use the phrase document life cycle to describe the stages through which a medical document passes from its creation to its ultimate use as a component of a person's medical record. In some cases, the life cycle is very simple—for example, a note may be created electronically, signed, and viewed immediately through the EMR system. In other cases it can be more complex. Given the broad range of documents that can comprise a medical record and the complexity of modern health care, understanding and managing this life cycle is both important and difficult for implementers of EMR systems.
In this paper, we present a model of the life cycle of clinical documents, and describe how this life cycle can be managed by EMR systems. We also describe the approach used in one mature EMR system for assuring that important steps of the life cycle are preserved.
Background
A growing body of literature addresses challenges of electronic clinical documentation, including entry 5 and content 6,7 of notes, accuracy of directly entered text, 8 note copying, 9 naming conventions, 10,11 and other topics. Much of this literature is based on systems operating successfully within healthcare organizations. Each paper describes a portion of the process of creating, completing, and using electronic documents.
Models for clinical documents exist, including the Clinical Document Architecture (CDA) published by HL7. The CDA is a document markup standard that specifies the structure and semantics of clinical documents for the purpose of exchange. 12,13 As described in the CDA document, “the CDA does not specify the creation or management of documents, only their exchange markup. Document management is critically interdependent with the CDA specifications, but the specification of document management messages is outside the scope of the CDA.” 12 Models also exist for representations of specific types of data within an EMR system, such as organ donor data 14 and nursing information. 15 In addition to the CDA, HL7 describes methods for communicating documents, and data surrounding documents needed for their management within EMR systems. The Logical Observation Identifiers Names and Codes (LOINC) database contains codes for identifying clinical documents to facilitate the exchange and pooling of results. 16,17 It can be used to aid electronic communication of documents between organizations.
Formulation Process
This model is based on the experience of the authors, expert colleagues, and a review of published literature and publicly available documents. Published literature on clinical documentation models was collected using the Medline search phrase model∗ AND (document∗ OR note∗) AND (Medical Records Systems, Computerized), and from the references listed in papers identified using this search strategy. We drew from our experience using VA's Computerized Patient Record System (CPRS) and the VistA (Veterans Health Information Systems and Technical Architecture) utilities on which CPRS depends, as well as from our experiences using commercial electronic medical record software. Comments on current and desired functionality from clinician users of CPRS and commercial EMR systems contributed to elements of the model. Our focus is on the steps and workflow involved in creating, reviewing, editing, and using electronic clinical documents in the healthcare delivery setting.
The concept of rules that govern electronic documentation is based on the authors' experience implementing document workflow requirements using VistA and with commercial EMR systems.
Model Description
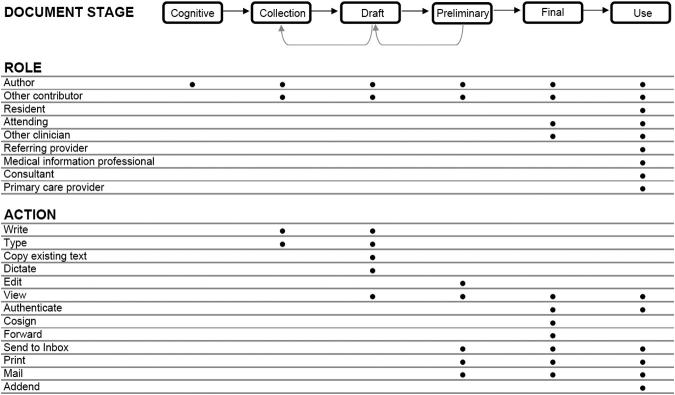
The first part of our model is composed of three axes (▶) that describe how a clinical document progresses from an idea to a component of a person's medical record used by those providing care to that person. The second part introduces the concept of rules that govern progression of documents through the document life cycle.
Figure 1.
Model of the life cycle of a clinical document. The life cycle model has 3 axes: Stage, role and action, as described in the text. This model can be applied to electronic documents in an EMR system, paper documents and hybrids such as electronically generated templates that are completed with handwriting. Rules (not shown) described in the text determine what actions can be performed by what role at each stage of the document's life cycle. Black dots indicate examples of roles and actions involved in each stage. Rules may pertain to certain document types (Progress note, discharge summary, operative note) and not to others. The lists of Roles and Actions contain examples and are not complete.
Part. 1. Model of the Document Life Cycle
The First Axis: Stages of Document Development
The first axis is the stage of the document. We have identified six document phases. The first is the cognitive phase, in which the author mentally formulates the content of the note to be written. This is followed (or may occur concurrently with) the collection phase, during which information to become part of the document is gathered and added to the note. In the draft phase, the document is created in a rough form, and is usually not viewed except by the author. The document may be written, dictated or typed; portions may be copied from an existing document, or created using a template. It may be written at one time or gradually over the course of a day; created by one person or the result of the contributions of many. Information stored in the EMR system may be automatically inserted (such as vital signs, allergies, or laboratory results), and the author may copy or read text from another source into the document.
In the next phase, the preliminary phase, the document is still in preliminary form, and may be edited. In this phase the author can view it, and in some institutions it is viewable by others, as discussed below. Next comes the finalization phase, where review (for example by the attending physician of the intern who composed the document) occurs, and the document is authenticated. The final phase is the use phase, at which point the document is broadly used by those involved in the patient's care and by others as permitted.
As it is created, the document may flow forward and backward through these phases, especially the cognitive, collection, and draft phases. For example, as results become available during the morning they may be added to a document, with the text extended to incorporate important new findings. A document in the final phase may have an addendum attached or words corrected using strike-through functionality or other mechanisms.
The Second Axis: Roles
The second axis of the model concerns the role of the individuals involved in creating and using the document. A single individual may play different roles in the development of different documents. Some roles pertain to creation of the document, others to contribution of content, and some pertain to review or use the document for care of a particular patient. Role is important to consider in the model because the actions individuals with different roles take may vary at different document phases.
The Third Axis: Actions Taken on Documents
The third axis incorporates actions taken as the document is created, revised and used. Examples of actions taken on documents include writing, typing, editing, cosigning, printing, viewing, sending to an electronic inbox, ∗ mailing to a referring physician, and many other activities that are an important part of every clinician's day. An important example of an action is authentication, which may include signatures, written initials, or computer entry 20 to indicate the identity of the individual who takes responsibility for the contents of the note.
Part 2. Rules Governing the Document Life Cycle
The next part of the model builds on this framework by introducing the concept of rules governing the timing and permissions of actions taken on documents. We use the term “rule” to refer to an explicit statement of which individuals can perform what action on a document and at what stage they can do so. Rules can be simple or complex, and may differ among organizations or by EMR system. They are not a separate axis used in the model, but instead describe permissions and policies held by healthcare organizations that govern how the three axes fit together. The dots in ▶ are a graphical representation of some rules. The abstraction of a rule is useful to express policies that come from many sources, as we describe below.
The rules we discuss in this paper focus on the later stages of the life cycle.
Numerous examples of workflow requirements that can be expressed as rules originate from local practice, organizational bylaws, and external regulatory organizations such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO). In many teaching hospitals, the resident is responsible for dictating the hospital discharge summary, while the attending physician reviews, optionally edits, and then cosigns it. The attending physician usually bears ultimate responsibility for the contents of the discharge summary. At the University of Washington, after a discharge summary has been transcribed, it may be viewed simultaneously by the resident and the attending through an electronic signature application. Some attending physicians wait until the resident has corrected and signed the discharge summary before they review and cosign the document, while others cosign the document before the resident has reviewed it. (After attending cosignature, the document is final can be addended but not changed.) These workflows require that the attending be able to edit and cosign a document that has been edited and possibly signed by the author, a resident. Each version of the document is retrievable. An important question is at what point other users may view this document: after transcription (preliminary phase), after the author has signed it, after the attending has signed it (final phase), or at all of those times? Within VA, current policy permits only the author and the expected cosigner to view unsigned documents, and does not allow the attending to edit after the author (resident) has signed the document. These VA policies are based on the fact that the VA EMR system does not have an audit trail of the state of an unsigned or uncosigned document that shows who has viewed the document.†
Rules governing who can view documents at which phases should consider a number of factors including the demands of emergency room physicians, who may need to see preliminary documents from all disciplines when they care for patients requiring emergency care. (Viewing preliminary documents carries a risk that they may contain errors not yet corrected by the author.) Though an organization may not send documents to referring physicians until they are in the final phase, as in Example 2 below, they may permit these preliminary documents to be viewed in the emergency room.
Other document workflow rules may originate in hospital bylaws and regulations. For example, at the University of Washington, bylaws stipulate that only an author or designated authenticators may edit or authenticate documents, 18 and that physicians must countersign all medical student signatures. 19 The Centers for Medicare and Medicaid Services, in its Conditions of Participation for Hospitals, requires that documents be authenticated by the person responsible for ordering, providing, or evaluating services, and that authors of record entries authenticate their own entries. 20 The Privacy Act of 1974 and the Health Insurance Portability and Accountability Act (HIPAA) of 1996 have requirements that govern who can view amendments to the electronic health record made in response to patient records. 21
JCAHO stipulates policies for some type of documents that do not necessarily apply to others. For example, Operative Notes and Discharge Summaries have completion timeliness requirements that are not applied to other types of documents. 22 Because management of different document types varies, workflow rules need to be able to distinguish, for example, an Operative Note from a Discharge Summary. Important questions include: Who can view, print, and edit documents at what stage of the document life cycle? Who is required to cosign documents? It is also important to recognize that documents may become unexpectedly “stuck” in one stage and not progress to the next, and therefore may not be viewed or mailed to referring physicians. At the University of Washington we have developed applications to identify such problems; other EMR system implementers should address this problem as well.
Expressing Rules Governing Document Life Cycle
One of the advantages of modeling document life cycle is the ability to express a set of rules governing who can perform actions on a type of document, and at what state in the document's life cycle the actions can be taken. Expressing document life cycle practices as rules has the advantage of clarifying expectations of clinicians, administrators, and regulators. Components of these rules include the role of the person taking action, such as an attending physician, intern, or medical information professional; the type (or class) of documents, such as discharge summary, progress note, and others; the actions that can be taken on documents include editing, amending, editing, signing, cosigning, viewing, printing, and mailing; and the stage of the document, such as draft, preliminary, or final. Examples of rules are given in ▶.
Table 1.
Table 1. Document Workflow Rules, Derived from Examples in the Text
| An unfinished admission note can be viewed only by the document author. |
| An oncology consultation note is mailed to the referring physician only when it has been cosigned by the attending. |
| A consultation note is mailed to a referring physician when it has been cosigned by the attending physician. |
| Preliminary phase (unauthenticated) documents are viewable within a in patient's electronic record if you make the effort to look in the patient's EMR system record (e.g. an ER physician), but are not delivered to the electronic inbox until authenticated. |
| The document author may edit an unsigned document. |
| Unsigned documents can be edited by the author and expected signers (but not by others). |
| A document must be signed by the author. |
Some rules address circumstances specific to particular document types, while others apply to classes of documents or to all documents. In ▶ is a listing of the document types, statuses, actions, and persons who take action as described in the rules in ▶.
Table 2.
Table 2. Document Types, Statuses, Actions, and Persons Who Take Action as Described in the Rules in ▶
| Document Type | Stage | Action | Role Taking Action |
|---|---|---|---|
| History and physical | Draft | Viewed | Document author |
| Any document | Preliminary | Viewed | ER physician |
| Any document | Draft, preliminary | Edit | Author |
| Discharge summary | Preliminary | Printed | Medical assistant |
| Any document | Preliminary | Signed | Author |
Validation Through Example
Three examples illustrate our model:
Example 1. An intern begins writing a hospital admission note on a piece of progress notes paper, adding to it during the day, and carrying it on her clipboard until it is completed. When she has completed the note she signs it and places it in the paper chart. The next morning the attending physician reviews it, writes comments in the margin to supplement additional history, writes a brief addendum, and cosigns the note. At the time of discharge, portions of the admission are read into the discharge summary. When the entire hospital record is photocopied and mailed to the patient's referring physician, the handwritten admission note is included.
In our model, the document passes through the cognitive and collection phase, and then is placed in the chart when it is in the preliminary (but not yet finalized) phase. It is converted to a finalized document by the attending physician's authentication. The roles involved in this model are the author (intern), the attending, others who view the document during the hospitalization, and the referring physician. The consequences of this workflow include the following: the unfinished note is viewable only by the intern as she adds to it during the workday; the signed, completed note is viewable by all who view the medical record; the attending can addend the note; and only the completed, cosigned note is viewable by the referring physician after discharge.
In some centers, rather than beginning with a blank piece of progress note paper, the resident begins with a template that includes recent vital signs, laboratory results, medication list, problem list, and other information. She writes history, review of systems, physical exam findings, newly available results, assessment, and plan on the printed template. 23
Example 2. An oncology fellow evaluates a patient in clinic, and reviews management with the oncology attending. The fellow dictates a document incorporating the recommended treatment, which is transcribed the next day. The attending reviews the transcribed document, makes several corrections, adds treatment recommendations, and includes the address of the referring physician to the document. The finished document is then printed, signed by the fellow and cosigned by the attending, placed in the patient's chart, and a copy is mailed to the referring physician.
In this example, the author is the fellow, the attending views and edits the note, and the referring physician receives it in its finalized form. Important workflow components include the following: The unsigned document in its preliminary phase is viewable by both the fellow and the attending but not by the referring physician; both the fellow and attending can make changes to the document before signature; the document is not printed or placed in the patient's chart until it is cosigned by the attending.
Example 3. A resident begins writing an electronic Intensive Care Unit (ICU) progress note in an EMR system by copying the contents of the prior day's progress note into a new note. The history, review of systems, and physical exam sections are rewritten in light of relevant new results, and yesterday's problem list and medication list are slightly edited to reflect changes during the prior 24 hours. The assessment and plan are also edited by the resident. The note is electronically signed by the resident and automatically routed to the attending for review, additional comments, and cosignature. The note is viewable by others immediately following the resident's electronic signature.
In this example from an EMR system, the draft document is viewable only by the resident until it is signed, but before and after the attending cosignature the note is viewable to others who view that patient's electronic record.
Example 4. A physician in a small, private office makes notes on a piece of paper as he sees a patient. At the conclusion of the visit, he uses those notes to dictate a progress note, indicating that a copy should be sent to the referring physician. The next day the transcription service mails a copy of the transcribed note to the referring physician, and sends a printed, transcribed note to the physician which he reviews, corrects, signs, and places it in a stack of notes to be filed in the patient's paper chart.
In this example, notes made in the cognitive phase of the document are not retained, but the preliminary, transcribed note is returned to the author for manual review, correction and signature. The corrected version is placed in the paper chart where it will be seen by those in the practice and by others who request copies of the patient's record. Another copy of the preliminary note was mailed to the referring physician prior to review and correction by the author.
Discussion
The examples above demonstrate that documents in the medical record pass through many stages from their inception to final form. In the world of paper records, incomplete documents can be viewed only by a few people, usually those writing them. Trainees create documents that are amended by supervising physicians and referring physicians see the finished product, but usually not early document versions. In the third example, the incomplete draft note in the EMR system is also viewable only by the author, but after the author has signed it (and before the attending cosigns) it is viewable by others. In the last example, the need to send a note copy to the referring physician in a timely manner has lead to mailing preliminary notes, while only finalized copies are included in the permanent record. As these examples demonstrate, the workflow to create and use documents has evolved over decades using paper medical records, and should be carefully considered when implementing electronic documentation in EMR systems.
These examples also demonstrate that despite differences in methods used to create notes—dictation, handwriting, direct entry into an EMR system—clinical documents progress through stages, and implicitly or explicitly there are rules brought to bear on the progression through these stages. By modeling the document life cycle, EMR system developers, implementers, and users may better determine whether their system operates as they expect it to. As methods for medical documentation change, we must assure that document workflow is preserved, where it is important to do so, and changed and improved where desired.
How Document Life Cycle Rules can be Implemented
In paper medical records, important document life cycle rules can be enforced by mechanisms developed and tailored after long experience. As we saw in the examples above, an unfinished document remained on the intern's clipboard until it was finished. With the advent of EMR systems, other strategies have been developed to meet workflow requirements.
Some rules are extremely important to the integrity and medicolegal standing of the medical record. For example, should it be possible to change the contents of a finalized, authenticated note? With rare exceptions, this is not permitted in most organizations. Other rules may vary broadly between organizations. For instance, should a preliminary, unauthenticated document be viewable by all clinicians caring for the patient? In some cases this is permitted, provided it is possible to later determine who viewed what version of the document, and that each version is preserved. In some organizations, preliminary discharge documents may be viewed by ER physicians when, for example, a patient returns unexpectedly after discharge.
There are a variety of ways to implement document life cycle rules, including human intervention, automation at the departmental computing system or EMR system level, or by devoting a system or utility to this purpose.
Enforce Rules Using Human Intervention
Paper medical record systems require multiple manual steps by staff members. Medical record room personnel may take charts along with discharge summaries to physician work areas in offices where the documents are then signed before being placed in the medical record. When implementing an electronic health record, it is possible to accommodate document life cycle rules by requiring people to intervene at various stages in order to prevent premature viewing of unfinished documents, or to permit documents to be shared before they are edited and completed. For example, a department secretary may respond to requests from the emergency room for a copy of preliminary reports by printing and faxing a document when a patient arrives unexpectedly.
Implement Rules in Departmental Applications
One solution is to incorporate rules within departmental system applications. A radiology information system, anatomic pathology system, and transcription system from different vendors may all contribute documents that are stored and viewed through an EMR system. Each of these systems may have rules about distributing preliminary, final, and amended reports, which have evolved in response to practices specific to their respective discipline. For example, an anatomic pathology system may not send a surgical pathology report to the EMR system through the interface until it has been reviewed and signed by the attending pathologist. In some departmental systems, sending a report to an EMR system is treated much as printing and mailing a report was before the advent of the EMR system. This strategy may give great flexibility to some departments, but not to others. If a department is large enough to justify a separate, tailored, departmental system the set of rules governing the reports it creates may meet all its needs. A smaller department that relies on the EMR system itself to distribute its reports may not have this flexibility.
Embed Rule Implementation Within the EMR System
In some cases, the rules governing document life cycle may be an intrinsic part of the EMR system application code; that is they may be “hard coded.” One site uses the interface engine to permit documents received by the transcription system to be routed to an electronic inbox for certain physicians, while other physicians review and sign printed paper copies of their transcribed documents. 24 Sets of privileges or ‘keys' can be assigned to groups of users and then specific code written to permit holders of those keys to perform certain tasks such as entering or authenticating documents.
At the University of Washington, where we use a commercial EMR system, we have implemented document workflow rules in two ways. We use collections of permissions (called positions) that roughly correspond to roles to determine which users can finalize documents with their signature, and which users require cosignature before their documents are finalized. We also extensively use special programs or scripts to enforce document life cycle rules. For example, each night a script finalizes outpatient progress notes that have been signed by residents at one medical center, but not outpatient progress notes written by residents at another medical center using the same EMR system. These approaches—privileges and scripts—may be employed by other sites using commercial EMR systems.
Such approaches can address needs of specific rules, but are difficult to generalize and to maintain. Difficulties may arise when different types of documents require different steps to be taken at various life cycle stages; for example if discharge summaries and progress notes differ in the need for cosignature. The number of rules may be large as ▶ shows, due to the need to determine which actions are taken at which phase, especially if the rules vary by document type. Using the approaches above becomes quite cumbersome as the number of rules grows.
A more general approach is to use a central repository for rules governing document life cycle. This approach permits rules to be created, viewed, and edited using a common set of tools applicable to many types of documents. This is the solution adopted by the VA EMR system, using the Authorization/Subscription Utility, which we will describe in more detail.
The Authorization/Subscription Utility (ASU) used in the Department of Veterans Affairs EMR System
VA's Computerized Patient Record System (CPRS) is one the most widely used EMR system in the United States. 25 CPRS has been in use since 1997, and is currently used by more than 1,300 sites-of-care across the United States. Its functions include results review, a wide variety of provider documentation tools, computerized practitioner order entry, imaging, and many other features. CPRS relies on a large collection of foundational software modules for the functionality used by clinicians. These modules are important parts of VistA, 26 and include department applications for pharmacy and radiology, and utilities such as the Text Integration Utility, which governs management of text documents. A subset of the Text Integration Utility is ASU, which provides the ability to create, edit, and use a hierarchy of document life cycle rules such as those described in this paper. 27 CPRS and its VistA foundation are highly integrated; many departmental systems such as laboratory, pharmacy, and radiology systems share the same underlying database and core functionality. This is in contrast to many non-governmental health care organizations, where laboratory and radiology systems may be from different vendors than the core EMR system. Patient and provider identification are handled in a central location within VistA, for example, eliminating the need for the pharmacy and laboratory system to keep separate lists synchronized.
Features of VistA follow the document life cycle model proposed in this paper in several ways: document stages are implemented as document status within CPRS. Roles correspond to the hierarchy of users within the Text Integration Utility. There is also a document hierarchy that permits extension of the model by adding another axis, document type. Most importantly, ASU permits implementation of a large number of workflow rules governing who can perform what actions on a document, and when they can do it.
ASU implements a large collection of business rules governing the handling and viewing of documents within CPRS. ASU was developed early in VA's EMR system development process, because the methods available then to manage rules were found to be inadequate to cover the growing complexity of document handling within the EMR system. Early attempts to use security keys to determine which users had permission to perform certain document tasks were abandoned when it became clear that a set of rules with a system to manage them was necessary. Characteristics of ASU rules include the following:
Rules are entered into ASU in English-like form, as in the following examples:
An UNSIGNED (CLASS) CLINICAL DOCUMENT may BE EDITED by an AUTHOR/DICTATOR.
An UNCOSIGNED RADIOLOGY NOTE may be COSIGNED by A STAFF RADIOLOGIST who is also AN EXPECTED COSIGNER
An UNSIGNED CLINICAL DOCUMENT may be COPIED by AN AUTHOR/DICTATOR
The familiarity of rules in the form of prose sentences permits those without extensive training to be able to review and understand document rules.
The rules are dependent on hierarchy of users and document types created in ASU. The user and document hierarchies representing those who use the EMR system and the documents they create greatly simplify the rule collection. The ASU rules themselves are organized in a hierarchy that mirrors the document hierarchy, which means that general rules, applying to all classes of documents, can be written only once instead of being repeated for specific document classes. The rule
An UNSIGNED (CLASS) CLINICAL DOCUMENT may BE EDITED by an AUTHOR/DICTATOR.
applies to all clinical documents, and does not need to be repeated for discharge summaries, consultation notes, and other specific document classes. This is an important point: if the rules were not hierarchical and there were 100 document types, five document statues, 10 user positions, and five actions, this could generate 100 × 5 × 10 × 5, or 25,000, independent rules. By organizing the rules in a hierarchy tied to classes of documents, the set of rules is much smaller. A copy of the 83 ASU rules used at VA Puget Sound Health Care System at the Clinical Document level, which is the highest level of the document hierarchy, is available as Data Supplement to this article. These rules apply to all more specific types of documents (discharge summaries, consultation notes, for example) unless a rule pertaining to a specific document type overrides them.
ASU rules apply to all documents as they are created and used within the EMR system, whether the documents are directly entered or dictated, though some rules pertain specifically to dictated records. This is an important feature, because many organizations offer a variety of methods for entering documents other than dictation.
The ability to use a set of general rules may in the future be applied to other CPRS functions, such as computerized practitioner order entry (CPOE). Rules pertinent to CPOE may require, for example, an order for a cancer chemotherapy drug to be cosigned by two physicians rather than one physician, whereas a prescription for a diuretic may require only one signature.
ASU has been used extensively within VA as a foundation for its broadly used EMR system. The flexibility ASU provides to organizations permits individual medical centers to follow VA-wide policies, and to tailor rules to specific requirements such as the presence of house staff, midlevel practitioners, and document workflow needed by clinical units within individual medical centers. Utilities such as ASU have played an important role in permitting VA to adopt an EMR system throughout its diverse healthcare delivery system. Most clinicians who use CPRS are not aware of ASU, but rely on the flexibility it provides to meet the document life cycle requirements of the medical record. Health information management CPRS support staff use ASU extensively to meet the changing clinical, regulatory, and quality improvement requirements of the EMR system.
Other Document Life Cycle Topics Worth Study
The subject of how clinical documentation occurs and who contributes is, in our judgment, ripe for further analysis. Who contributes to the progress note, and what does review by an attending physician mean? Is it appropriate for the attending physician to sign notes in batches without carefully reviewing and editing them, if this means more time can be spent in bedside care of critically ill patients? Is it clear in a completed document who contributed to what portion of the early draft? For example, if a preliminary document is not viewable by others in its early stages, does it matter that more than one member of the team caring for the patient contributed a portion of the note? Should inpatient notes be written at the end of the day, or built as events unfold to give an up-to-date summary that may help consultants? As documentation changes from paper, to paper templates, to electronic notes, these and many other issues are worth examining.
Conclusion
Clinical documents can be viewed as passing through a life cycle that we have grown to expect from paper records. We have modeled of this life cycle using three axes to describe what can be done with them at each stage in their ‘life,' and by whom. Portions of the life cycle of clinical documents are influenced by the needs of clinicians, and requirements of hospital bylaws and regulators. Each of these groups expects that electronic documents will be handled and viewed according to traditions and regulations derived from the world of paper medical records. Rules to enforce the life cycle must consider document stage, and who is permitted to take action on a document at each stage. An excellent model for handling many of these rules exists within VA's CPRS, where the ASU permits document life cycle rules to be written in English-like fashion. Other aspects of the clinical document life cycle provide fertile ground for further research.
Footnotes
The authors thank David Stone, Gordon Schiff, MD, and Steven Brown, MD for their thoughtful review and suggestions for improvements of early versions of this manuscript, and JAMIA reviewers for their contributions.
Portions of this paper were presented at the 2005 HIMSS Conference, in Dallas, Texas, February 15, 2005.
We use the term electronic inbox to refer to a place where documents and other information from many patients of interest to the clinician are brought together in one place for review. Similar capabilities exist in many EMR systems: View Alerts (CPRS), Inbox (Cerner), Inbasket (Epic).
When the audit trails become available in future systems, VA will revisit the policy.
References
- 1.Berner ES, Detmer DE, Simborg D. Will the wave finally break? A brief view of the adoption of electronic medical records in the United States J Am Med Inform Assoc 2005;12(1):3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush GW Jr. State of the union address. Feb 2, 2005. http://www.whitehouse.gov/stateoftheunion/2005/. Accessed Oct 2, 2005..
- 3. 1995 Documentation Guidelines for Evaluation & Management Services. Washington, DC: Centers for Medicare & Medicaid, Services; 1995.
- 4.Committee on Quality of Health Care in America, Using Information Technology Crossing the Quality Chasm. A New Health System for the 21st Century. Washington, D.C: Institute of Medicine; 2001.
- 5.Rosenbloom ST, Grande J, Geissbuhler A, Miller RA. Experience in implementing inpatient clinical note capture via a provider order entry system J Am Med Inform Assoc 2004;11(4):310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tornvall E, Wilhelmsson S, Wahren LK. Electronic nursing documentation in primary health care Scand J Caring Sci 2004;18(3):310-317. [DOI] [PubMed] [Google Scholar]
- 7.Payne TH, Hirschmann JV, Helbig S. The elements of electronic note style J AHIMA 2003;74(2):6870. [PubMed] [Google Scholar]
- 8.Weir CR, Hurdle JF, Felgar MA, Hoffman JM, Roth B, Nebeker JR. Direct text entry in electronic progress notes. An evaluation of input errors Methods Inf Med 2003;42(1):61-67. [PubMed] [Google Scholar]
- 9.Hammond KW, Helbig ST, Benson CC, Brathwaite-Sketoe BM. Are electronic medical records trustworthy? Observations on copying, pasting and duplication Proc. AMIA Annu Symp. 2003:269-273. [PMC free article] [PubMed]
- 10.Brown SH, Lincoln M, Hardenbrook S, Petukhova ON, Rosenbloom ST, Carpenter P, Elkin P. Derivation and evaluation of a document-naming nomenclature J Am Med Inform Assoc 2001;8(4):379-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne TH, Kalus R, Zehner J. Evolution and use of a note classification scheme in an electronic medical record Proc AMIA Symp 2005:599-603. [PMC free article] [PubMed]
- 12.In: Alschuler L, Dolin RH, Boyer S, et al. editors. Version 3 Standard. Clinical Document Architecture Framework, Release 1.0. Health Level Seven; 2000.
- 13.Dolin RH, Alschuler L, Boyer S, Beebe C, Behlen FM, Biron PV, Shabo Shvo A. HL7 Clinical Document Architecture, Release 2 J Am Med Inform Assoc 2006;13:30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staes CJ, Huff SM, Evans RS, et al. Development of an Information Model for Storing Organ Donor Data Within an Electronic Medical Record J Am Med Inform Assoc 2005;12:357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goosen WTF, Ozbolt JG, Coenen A, et al. Development of a Provisional Domain Model for the Nursing Process for Use within the Health Level 7 Reference Information Model J Am Med Inform Assoc 2004;11:186-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regenstrief Institute, Inc. Logical observation Identifiers Names and Codes (LOINC®). Available at http://www.regenstrief.org/loinc/. Accessed Jan 8, 2006..
- 17.McDonald CJ. The barriers to electronic medical record systems and how to overcome them J Am Med Inform Assoc 1997;4:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medical Staff Bylaws. University of Washington Medical Center, 2001..
- 19. Harborview Medical Center Medical Staff Organization Bylaws, Rules, and Regulations. Harborview Medical Center; 2002.
- 20.Code of Federal Regulations, Title 12, Volume 1, Revised as of January 1, 2003. From the U.S. Government Printing Office via GPO Access, 42CFR482, Page 484–95. Title 42-Public Health Chapter IV. Centers For Medicare & Medicaid Services, Department of Health and Human Services. Part 482. Conditions Of Participation For Hospitals. Table of Contents Subpart C. Basic Hospital Functions Sec. 482.24 Condition of participation: Medical record services. Available at: http://www.access.gpo.gov/nara/cfr/waisidx_04/42cfr482_04.html. Accessed Jan 15, 2006..
- 21.Health Insurance Portability and Accountability Act of 1996 45 CFR Part 164; Section 164.526..
- 22.Joint Commission on Accreditation of Healthcare Organizations. Critical Access Hospital 2006, Management of Information, Elements of Performance for IM.6.10 and 6.30. Available at: http://www.jcaho.org. Accessed Jan 29, 2006..
- 23.Van Eaton EG, Horvath KD, Lober WB, Rossini AJ, Pellegrini CA. A randomized, controlled trial evaluating the impact of a computerized rounding and sign-out system on continuity of care and resident work hours J Am Coll Surg 2005;200:538-545. [DOI] [PubMed] [Google Scholar]
- 24.Brazelton N. Cerner Health Conference 2004 presentation. (Personal communication)..
- 25.Hurdle JF, Weir CR, Roth B, Hoffman J, Nebeker JR. Critical gaps in the world's largest electronic medical recordAd Hoc nursing narratives and invisible adverse drug events. Proc AMIA Annu Symp. 2003:309-312. [PMC free article] [PubMed]
- 26.Kolodner RM. Computerizing Large Integrated Health Networks. The VA Success. New York: Springer-Verlag; 1997.
- 27.CPRS: Authorization Subscription Utility (ASU), Clinical Coordinator Manual and Technical Manual. Vista Documentation Library, VHA Office of Information. Available at: http://www.va.gov/vdl/Clinical.asp?appID=58. Accessed Jan 16, 2006..