Abstract
Objective
Many hospitals utilize antimicrobial management teams (AMTs) to improve patient care. However, most function with minimal computer support. We evaluated the effectiveness and cost-effectiveness of a computerized clinical decision support system for the management of antimicrobial utilization.
Design
A randomized controlled trial in adult inpatients between May 10 and August 3, 2004. Antimicrobial utilization was managed by an existing AMT using the system in the intervention arm and without the system in the control arm. The system was developed to alert the AMT of potentially inadequate antimicrobial therapy.
Measurements
Outcomes assessed were hospital antimicrobial expenditures, mortality, length of hospitalization, and time spent managing antimicrobial utilization.
Results
The AMT intervened on 359 (16%) of 2,237 patients in the intervention arm and 180 (8%) of 2,270 in the control arm, while spending approximately one hour less each day on the intervention arm. Hospital antimicrobial expenditures were $285,812 in the intervention arm and $370,006 in the control arm, for a savings of $84,194 (23%), or $37.64 per patient. No significant difference was observed in mortality (3.26% vs. 2.95%, p = 0.55) or length of hospitalization (3.84 vs. 3.99 days, p = 0.38).
Conclusion
Use of the system facilitated the management of antimicrobial utilization by allowing the AMT to intervene on more patients receiving inadequate antimicrobial therapy and to achieve substantial time and cost savings for the hospital. This is the first study that demonstrates in a patient-randomized controlled trial that computerized clinical decision support systems can improve existing antimicrobial management programs.
Introduction
Many hospitals are currently utilizing antimicrobial management teams to control and manage the growing problem of antimicrobial resistance and ensure a high quality of patient care by optimizing antimicrobial utilization. Such teams represent one method for controlling the use of antimicrobials, which is one of the actions recommended by the Society for Healthcare Epidemiology (SHEA) and the Infectious Disease Society of America (IDSA) for the prevention of antimicrobial resistance in hospitals. 1 Antimicrobial management teams typically consist of pharmacists and physicians who round daily on patients in order to optimize antimicrobial choice, dosing, and delivery. 2–4 This approach to controlling antimicrobial utilization is sometimes referred to as a “back-end” approach, in that antimicrobial utilization is reviewed post-prescription. 2,5 Post-prescription review has previously been shown to reduce antimicrobial expenditures and improve the appropriateness of antimicrobial utilization in the hospital. 2,5,6
These programs for managing antimicrobial use are labor and time intensive and many teams function with minimal computer support. 7,8 Computerized clinical decision support systems are designed to assist healthcare staff by allowing them to more efficiently and accurately complete their work and improve upon the quality of patient care. When the intended purpose is to improve upon antimicrobial utilization, such systems can act by facilitating appropriate treatment choice, dosing, and duration. 9–13 These systems can be either independent or function as part of a computerized physician order-entry system. While in general computerized clinical decision support systems are believed to be valuable tools, evaluations of previous systems have not always found this to be the case. 14,15 The effectiveness of any given system is dependent on the system's design, implementation, the user(s) of the system, and the setting into which the system is being introduced. This randomized controlled trial evaluated a new web-based application designed to assist existing antimicrobial management teams in their efforts to optimize patient antimicrobial therapy and minimize inappropriate and inadequate antimicrobial use.
Methods
This trial was conducted at the University of Maryland Medical Center, a 648-bed, tertiary-care referral center in Baltimore, Maryland. Since 2001, the University of Maryland Medical Center has utilized an antimicrobial management team to actively monitor and intervene on restricted antimicrobial treatments on all inpatient wards, with the exception of shock trauma, pediatrics and cancer wards. The team consists of one infectious disease attending physician (50% FTE) and one clinical pharmacist (80% FTE).
The following describes the standard care provided by the antimicrobial management team prior to this trial and in the control arm during the trial. Each weekday the team received a list of all patients who had received any antimicrobial within the past 24 hours (on Mondays, the list also included patients who had received an antimicrobial the prior weekend). The list was generated by University of Maryland Medical Center's information technology group by querying the hospital's Cerner pharmacy database. The list was provided to the team as a Microsoft Excel worksheet. The clinical pharmacist would then reduce the list to patients who had received any of the hospital's 23 restricted antimicrobials. The team would then review the patients' charts and, if necessary, recommend changes to the patients' current antimicrobial therapy. The team would only intervene on patients who were receiving a restricted antimicrobial, however they were not limited to make changes to only restricted antimicrobials. It should be noted that the University of Maryland Medical Center did not possess a computerized physician order entry system or electronic medical records during the time-period of the randomized trial.
This study was a randomized controlled trial; patients admitted to wards managed by the antimicrobial management team (all wards except shock trauma, cancer, and pediatric wards) between May 10 and August 3, 2004 were randomized to one arm of the trial according to their medical record number (MRN). Patients with an even MRN were assigned to the control arm and received the standard care as provided by the team prior to start of this trial (described above). Patients with an odd MRN were assigned to the intervention arm and received the standard care provided by the team but supplemented with the web-based clinical decision support system (PharmWatch™, Cereplex Inc.) designed to assist in the management of antimicrobial utilization (hereafter referred to as ‘system'). Even and odd MRNs are, in effect, randomly distributed in the patient population since patients are assigned their MRNs consecutively at the time of their first visit to a University of Maryland Medical System institution.
Each weekday, for the intervention arm, the team would access the system via a secure internet connection and view a list of alerts for patients who may potentially require a change in their current antimicrobial therapy. The criteria for the alerts were created with the collaboration of the team and are based on the patient's antimicrobial use and microbiological laboratory results. The alerts were designed to detect all scenarios of potentially inappropriate or inadequate antimicrobial use that the team detected when providing the standard of care. Thirty-two alerts were created; examples of alert types include: 1) equivalent oral antimicrobial possibly indicated for a patient receiving an intravenous antimicrobial; 2) potentially unnecessary double coverage of antimicrobial therapy; 3) organism potentially resistant to current antimicrobial therapy. After accessing the alerts, the team could then view each patient's microbiologic laboratory results, medications, admission, discharge, and transfer information within the system. Patient data were automatically uploaded to the system from the hospital information systems nightly. If additional information were still needed to assess the appropriateness of the antimicrobial therapy, the team would then obtain this additional data from the patient charts. While the frequency that the antimicrobial management team referred to the patient charts was not recorded, the team estimates that the chart was reviewed for approximately 20% of those patients receiving alerts.
If, based on these data, the team decided to recommend a change in a patient's current antimicrobial therapy, they could complete and print an intervention form within the system that allowed them to describe the problem with the current therapy and make recommendations for more appropriate therapy. In the event that the antimicrobial management team was not able to verbally relay the message to a member of the admitting or primary team, the form, which included the antimicrobial management team's contact information, would then be temporarily placed within the patient's chart.
Note that all interventions were made every weekday in both arms of the study and that these interventions were made through the antimicrobial management team, just as was done both prior to the start of this study and in the control arm. Again, in both arms of the study, the primary treating team was responsible for making changes in patient antimicrobial therapy. Interventions consisted of therapy recommendations made by the antimicrobial management team. It should be noted that the system evaluated here differs from similar systems that have previously been evaluated in this field, in that the intended user is the antimicrobial management team and not all infectious disease physicians or all treating physicians.
The antimicrobial management team was blinded from receiving system alerts on patients assigned to the control arm of the trial. This blinding was accomplished by programming the system not to display alerts on control arm patients to the antimicrobial management team when they logged in to the system. However, these alerts were saved for subsequent data analyses. Patients and their healthcare providers did not have access to the system and were blinded as to the randomization status. The team could not be blinded as to the randomization status of patients, as their recommendations were the mode through which interventions were administered to patients thus making the team a component of both the intervention under study and the standard of care.
For each study patient, we collected the following demographic data: sex, age, and the chronic disease score (CDS). The CDS is a measure of patient comorbidity that utilizes patient medications as indicators for comorbid conditions. 16 The CDS includes seventeen different comorbid conditions such as diabetes, respiratory illness, cancer, and hypertension. Each condition contributes between one and five points to the total score, and the potential range of values for each patient's CDS is 0–35. The CDS was calculated using the medications ordered for each patient within the first 24 hours of admission.
The primary outcome of interest in this trial was hospital antimicrobial costs. The cost data were measured using the hospital's pharmacy purchase prices, which are assessed per unit dose. These data were obtained using the hospital's Cerner pharmacy database. Additional outcomes of interest were patient mortality, length of hospitalization, frequency of testing for Clostridium difficile (an indicator for the presence of diarrhea and adverse effect of antimicrobial therapy), and time spent by the team managing antimicrobial utilization. The time spent was measured during one week during the last full calendar week of the study period. The time spent by the antimicrobial management team was recorded on study timesheets and were separately recorded by both members of the team. The latter were compared using the Fisher's exact test, t-test, and Wilcoxon rank-sum test, where appropriate. Statistical significance was defined as p < 0.05. All data analyses were performed using SAS v. 8.02 (SAS Institute, Inc., Cary, North Carolina).
This study was approved by the University of Maryland, Baltimore Institutional Review Board. The primary purpose of this study was the evaluation of the system by the antimicrobial management team and by University of Maryland Medical Center infection control. Because antimicrobial management by the team is part of the patients' standard care, and because of the minimal risk to patients, the University of Maryland, Baltimore Institutional Review Board waived the requirement for individual consent. Initial power calculations for this study led to a targeted study duration of four months, however an interim data analysis was planned for two months post-study implementation. The results of the interim data analysis were evaluated by the study investigators and by the Medical Director for Infection Control and Antimicrobial Effectiveness at the University of Maryland Medical Center.
Results
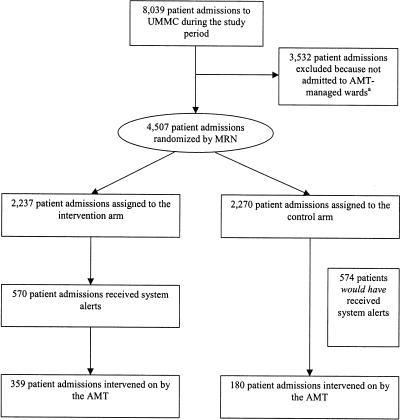
This randomized trial lasted from May 10, 2004 to August 3, 2004. The study was stopped after evaluation of the results of the interim analysis, after which the Medical Director for Infection Control and Antimicrobial Effectiveness decided to implement the use of the system in all patient wards managed by the antimicrobial management team. During the study period, there were 4,507 patient admissions to team-managed wards: 2,237 were assigned to the intervention arm and 2,270 to the control arm (see ▶). Of the 2,237 patient admissions assigned to the intervention arm, 1,315 (58.8%) received an antimicrobial and of the 2,270 patient admissions assigned to the control arm, 1,325 (58.4%) received an antimicrobial during the admission. No statistically significant difference was found between the two arms in the distribution of sex, age, CDS, or admit service (▶). During the study period, 117 different antimicrobials were ordered for patients included in this trial. Because 65 of these antimicrobials were ordered for twenty or fewer patients, a valid statistical comparison could not be made between the frequency of the use of each drug between the intervention and control arms of the trial. However, a statistical comparison of only those antimicrobials that were prescribed to twenty or more patients indicated no statistically significant difference in the frequency of individual antimicrobial orders between the two trial arms (chi-square test p = 0.08).
Figure 1.
Profile of Study Participants
Abbreviations: UMMC, University of Maryland Medical Center; AMT, antimicrobial management team; MRN, medical record number
Definition: system refers to the intervention, a web-based, computerized clinical decision support system
aAMT-managed wards include all hospital wards except shock trauma, cancer, pediatric wards
Table 1.
Table 1. Characteristics of Study Patients
| Intervention Arm n = 2,237 a | Control Arm n = 2,270 a | p-value | |||
|---|---|---|---|---|---|
| All randomized patients | |||||
| No. Female (%) | 1189 | 53.15 | 1216 | 53.57 | 0.79 b |
| Age, mean in years (SD) | 50.36 | 17.54 | 49.55 | 17.57 | 0.13 c |
| Chronic Disease Score, median score (IQR d ) | 6.00 | 2.00–9.00 | 5.00 | 2.00–9.00 | 0.06 e |
| Admit Service (%) | 0.93 f | ||||
| Medicine | 992 | 44.35 | 1005 | 44.27 | |
| Surgery | 944 | 42.20 | 951 | 41.89 | |
| Other | 301 | 13.46 | 314 | 13.83 | |
| Patients who received system alerts g | |||||
| No. Female (%) | 272 | 47.39 | 277 | 48.60 | 0.72 b |
| Age, mean in years (SD) | 52.38 | 15.99 | 51.20 | 15.75 | 0.21 c |
| Chronic Disease Score, median score (IQR) | 8.00 | 4.00–10.00 | 7.00 | 4.00–10.00 | 0.32 e |
| Admit Service (%) | 0.83 f | ||||
| Medicine | 304 | 52.96 | 312 | 54.74 | |
| Surgery | 255 | 44.43 | 243 | 42.63 | |
| Other | 15 | 2.61 | 15 | 2.63 | |
a 574 patients in the intervention arm received system alerts, and 570 patients in the control arm would have received system alerts had the system been utilized in this group.
b Fisher's exact test.
c Pooled t-test.
d IQR, interquartile range.
e Wilcoxon rank-sum test.
f Chi-square test.
g The antimicrobial management team was blinded from receiving alerts from the computerized clinical decision support system on control arm patients, but the alerts were saved for data analysis.
The team received system alerts on 570 (25.5%) intervention arm patients and intervened on the antimicrobial therapy of 359 (16.0%) of these patients. In the control arm, the team intervened on 180 (7.9%) patients. Note that not all alerts resulted in interventions because the system was designed to have high sensitivity but not perfect specificity. Thus, the team was still responsible for reviewing the portions of the charts of every patient with an alert before determining the need for a change in therapy. Note that patients may have had multiple alerts and that not all alerts resulted in interventions. For example, if a patient was receiving intravenous azithromycin for community-acquired pneumonia and was switched to oral gatifloxacin, the double coverage alert would have been triggered, but because the patient had a medication switch and was not simultaneously receiving both antimicrobials, no intervention was necessary. As a second example, if a non-ICU patient was receiving pipercillin-tazobactam but had no cultures positive for microbial growth, then the alert for potentially inappropriate use of pipercillin-tazobactam in a non-ICU would have been triggered. However in some circumstances, after reviewing the patient's chart, the choice of this antimicrobial for empiric therapy may have still been warranted and no intervention would be necessary. Had the system been used in the control arm, the team would have received alerts on 574 (25.3%) of these patients. The frequency of occurrence of each alert is shown in ▶ . Note that of the 1,717 alerts that occurred among patients in the intervention arm, 1,092 (61%) were triggered by antimicrobials that were restricted at the University of Maryland Medical Center. Of the 1,795 alerts that would have occurred among the patients in the control arm had the system been used in this group, 1,717 (59%) would have been triggered by restricted antimicrobials. No significant difference was observed in the distribution of alert types between the intervention and control arm of the trial (chi-square test, p = 0.72). We also compared the demographics of patients with system alerts in the two arms of the trial. No significant differences were observed between the subset of patients with alerts in the intervention and control arms in the distribution of sex, age, or CDS (▶).
Table 2.
Table 2. Frequency of System Alerts by Trial Arm ∗
| System Alerts | Frequency of Alerts n (%) |
|
|---|---|---|
| Intervention Arm | Control Arm | |
| Use of restricted antimicrobial | 501 (29.2) | 546 (30.4) |
| Use of restricted IV agent | 400 (23.3) | 459 (25.6) |
| Use of restricted oral agents | 101 (5.9) | 87 (4.8) |
| Use of a specific antimicrobial | 391 (22.8) | 418 (23.3) |
| Piperacillin/tazobactam in non-ICUs | 219 (12.8) | 209 (11.6) |
| 5 days of ampicillin/sulbactam, ceftazidime, piperacillin/tazobactam, or ticarcillin/clavulanate | 156 (9.1) | 185 (10.3) |
| Aminoglycosides | 16 (1.0) | 24 (1.3) |
| Double coverage of antimicrobial therapy† | 348 (20.3) | 338 (18.8) |
| Gram negative double coverage (5 alerts) | 154 (9.0) | 158 (8.8) |
| Anaerobe double coverage (8 alerts) | 118 (6.9) | 106 (5.9) |
| Gram positive double coverage (4 alerts) | 48 (2.8) | 22 (1.2) |
| Other double coverage (1 alert) | 20 (1.2) | 34 (1.9) |
| Fungal double coverage (3 alerts) | 8 (0.5) | 16 (0.9) |
| Antiviral double coverage (1 alert) | 0 | 2 (0.1) |
| Use of a specific antimicrobial without specified organism | 232 (13.5) | 252 (14.0) |
| Vancomycin use without MRSA | 192 (11.2) | 199 (11.1) |
| Cefepime without Pseudomonas present | 20 (1.2) | 28 (1.6) |
| Linezolid use without MRSA or VRE | 20 (1.2) | 25 (1.4) |
| Oral equivalent indicated | 140 (8.2) | 147 (8.2) |
| Coverage mismatch between antimicrobial and organism susceptibility | 105 (6.1) | 94 (5.2) |
| Total | 1,717 (100.0) | 1,795 (100.0) |
∗ No significant difference was observed in the distribution of alert types between the intervention and control arm of the trial (chi-square test, p = 0.72).
† The numbers in parentheses represent the number of distinct alerts that fall into each category.
During the 3-month study period, the University of Maryland Medical Center spent $285,812 on antimicrobials in the intervention arm and $370,006 in the control arm, for a savings of $84,194 (22.8%). Antimicrobial cost savings can also be calculated per patient for an average savings of $37.64 per patient in the intervention arm. We also calculated the hospital's costs for just the restricted antimicrobials. During the trial, the hospital spent $131,660 on restricted antimicrobials in the intervention arm and $191,948 in the control arm, for a savings of $60,288 (31%) in just the restricted antimicrobial costs. For the purposes of generalizability, we also calculated the wholesale (2004 Red Book) costs for the antimicrobials. 17 The Red Book wholesale prices are commonly used to create comparable cost estimates between healthcare institutions. The total wholesale cost of antimicrobials was $4,841,474 in the intervention arm and $5,758,788 in the control arm, for a savings of $917,314 (15.9%).
We observed no significant difference in the in-hospital mortality between patients assigned to the intervention and the control arms (p = 0.55), or between patients in the intervention arm with system alerts and patients in the control arm who would have received alerts (p = 0.52; ▶). Also, no significant difference was observed in the length of hospitalization between the two study arms (p = 0.38). For patients who received, or in the control arm would have received a system alert, we also compared the length of hospitalization from the time of the first system alert to discharge but still observed no significant difference (p = 0.64).
Table 3.
Table 3. Comparison of In-hospital Mortality and Length of Stay Between the Intervention and Control Arms of the Trial
| Outcome | Patients | n(%) or Median(IQR a ) | p-value | |
|---|---|---|---|---|
| Intervention Arm | Control Arm | |||
| In-hospital mortality | All randomized patients | 73 (3.26%) | 67 (2.95%) | 0.55 b |
| Patients with alerts | 45 (7.84%) | 51 (8.19%) | 0.52 b | |
| Length of stay (days) | All randomized patients c | 3.84 (2.12–7.57) | 3.99 (2.19–7.57) | 0.38 d |
| Patients with alerts e | 4 (2–10) | 5 (2–10) | 0.64 d | |
a interquartile range.
b Fisher's exact test.
c Length of stay is from admission to discharge.
d Wilcoxon rank-sum test.
e Length of stay is from day of first alert to discharge; fractions of days could not be measured because no exact time was associated with system alerts.
During the trial, fewer patients in the intervention than the control arm experienced diarrhea as a side effect of antimicrobial use as indicated by testing for C. difficile, though the difference was not statistically significant (127/2,237 (5.7%) vs. 150/2,270 (6.6%) patients, respectively; p = 0.21). No significant difference was observed in the number of positive C. difficile tests between the intervention and control arm of the study (20 versus 19 positive tests, respectively; p = 0.49).
The antimicrobial management team spent an average of 4.1 person-hours per day making interventions on the control arm and 3.2 person-hours per day on the intervention arm. Thus the team spent roughly one hour less each day intervening on the intervention arm than the control arm of the trial. The majority of time savings occurred in the identification of patients needing interventions.
Discussion
To our knowledge, this study was the first patient-randomized controlled trial to evaluate the effectiveness of a computerized clinical decision support system for the management of antimicrobial utilization. 15 This system was designed to assist the antimicrobial management team in the post-prescription review of inpatient antimicrobial utilization. A key difference between the system evaluated in this study, and previously evaluated systems for the management of antimicrobial utilization, is that this system was designed such that the antimicrobial management team is the intended user as opposed to the primary or treating physician. Because at our institution, the antimicrobial management team was already in existence and worked well with the providers, use of the system by the antimicrobial management team allowed for improved post-prescription review without any transition or training period for the providers—who were already accustomed to receiving therapy recommendations from the antimicrobial management team. That said, it is also entirely possible that a system designed for use directly by the providers might either be equivalent or preferable. The preferred choice is likely institution-specific and depends on the current standard procedures in place at an institution, the willingness and ability of the providers to adopt new technologies, and the capability of the institution/system-developer to provide support for a provider-utilized system. In this study, we observed that a computerized clinical decision support system was effective in alerting the antimicrobial management team thereby allowing them to intervene on more patients and reduce hospital antimicrobial expenditures without otherwise negatively impacting patient healthcare and safety. These results are consistent with previous studies evaluating post-prescription review policies and the utility of computerized clinical decision support systems for the management of antimicrobial utilization. 2,5,6,9–12
The two endpoints most affected by the use of the system were the number of patients intervened upon and the hospital's antimicrobial expenditures. With the assistance of the system in the intervention arm, the antimicrobial management team intervened on nearly twice as many patients as the control arm (359 vs. 180 patients, respectively). More patients were identified as having received inappropriate or inadequate therapy in the intervention arm than the control arm because in the control arm, the patients received the standard of care that they would have received prior to this study, and this standard of care involved only reviewing the appropriateness of antimicrobial therapy for patients receiving an antimicrobial on the hospital's restricted antimicrobial list. Antimicrobial utilization was monitored in this way (both before the study and during the study, in the case of the control arm) because the team could not manually review all patient antimicrobial therapies due to time limitations. Use of the system allowed the team to more efficiently identify patients potentially receiving inadequate or inappropriate antimicrobial therapy (i.e., greater specificity) thereby allowing the team to intervene on more patients.
During this 3-month study, the University of Maryland Medical Center spent roughly $84,000 dollars less on antimicrobials in the intervention group than the control group. Had the system also been utilized in the control arm during this study period, the total projected cost savings for the hospital would be approximately $168,000. If the observed savings are consistent across time, the yearly savings could be as great as $672,000. While the differences in patient outcomes measured in this trial were not significantly different between the two arms, trends generally favored better outcomes in the intervention arm, especially among those patients who received system alerts.
Also noteworthy was the ability of the antimicrobial management team to intervene on more patients in the intervention arm while still spending roughly 1 hour less each day. This time saving was largely due to the increased efficiency in identifying patients needing interventions. This occurred because, while the system alerts were not perfectly specific, their specificity was far greater than the list of all patients receiving antibiotics, which was the starting point for identifying patients needing interventions in the control arm.
A limitation to the generalizability of this study was that it was conducted at a single institution and the effectiveness of an informatics intervention, such as the computerized clinical decision support system we have evaluated, may vary between institutions since the system cannot be evaluated independently of the system's users. Thus variations in the users of the system, in terms of computer-associated skills, consistent use of the system, etc., may affect the generalizability of these findings to other institutions. Another limitation to this study is that the antimicrobial management team could not be blinded as to the randomization status of the patients and thus there remains the potential for bias. Further evaluation of the system at additional institutions is still needed.
Through the use of a new web-based, computerized clinical decision support system, the University of Maryland Medical Center's antimicrobial management team intervened on the antimicrobial therapy of nearly twice as many patients in the intervention arm than the control arm of this 3-month trial, while spending roughly one hour less each day on the intervention arm, resulting in a savings of approximately $84,000 in the hospital's antimicrobial expenditures. This study demonstrates that computerized clinical decision support systems may be useful tools for increasing the efficiency and effectiveness of hospital antimicrobial management programs.
Footnotes
This research was supported by National Institutes of Health grant R44NR008958A and by a Maryland Industrial Partnerships grant.
The authors thank Colleen Reilly and Jingkun Zhu, who assisted in data transfer and extraction, and Kathleen Flannery, PharmD for her assistance with pharmaceutical cost data.
Portions of this research were presented at the annual meeting of the Society of Healthcare Epidemiology of America, Los Angeles, CA, April 2005; and at the annual meeting of the Infectious Diseases Society of America, San Francisco, CA, October 2005.
The authors have no financial or commercial relationships regarding the product evaluated in this research and have not received any consulting fees.
The University of Maryland Medical Center served as a beta-testing site for the product evaluated in this study and purchased the product after the study's conclusion.
References
- 1.Shlaes DM, Gerding DN, John Jr JF, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistanceguidelines for the prevention of antimicrobial resistance in hospitals. Infect Control Hosp Epidemiol 1997;18(4):275-291Apr. [DOI] [PubMed] [Google Scholar]
- 2.Paskovaty A, Pflomm JM, Myke N, Seo SK. A multidisciplinary approach to antimicrobial stewardshipevolution into the 21st century. Int J Antimicrob Agents 2005;25(1):1-10Jan. [DOI] [PubMed] [Google Scholar]
- 3.Minooee A, Rickman LS. Expanding the role of the infection control professional in the cost-effective use of antibiotics Am J Infect Control 2000;28(1):57-65Feb. [DOI] [PubMed] [Google Scholar]
- 4.Bearden DT, Allen GP. Impact of Antimicrobial Control Programs on Patient Outcomes Dis Manag Health Outcomes 2003;11(11):723-736. [Google Scholar]
- 5.Paterson DL. The role of antimicrobial management programs in optimizing antibiotic prescribing within hospitals Clin Infect Dis 2006;42(Suppl 2):S90-S95Jan 15. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Srinivasan A, Seo SK, et al. Evaluation of Post-Prescription Review as a Method of Promoting Rational Antimicrobial Use: A Multi-Center Study. Presented at: 15th Annual Scientific Meeting of the Society for Healthcare Epidemiology of America, 2005; Los Angeles, CA..
- 7.Fraser GL, Stogsdill P, Owens RCJ. Antimicrobial Stewardship InitiativesA Programmatic Approach to Optimizing Antimicrobial Use. In: Owens RC, Ambrose PG, Nightingale CH, editors. Antibiotic optimization. concepts and strategies in clinical practice. Vol 33. Boca Raton, FL: Marcel Dekker; 2005. pp. 261-326.
- 8.Giblin TB, Sinkowitz-Cochran RL, Harris PL, Jacobs S, Liberatore K, Palfreyman MA, et al. Clinicians' perceptions of the problem of antimicrobial resistance in health care facilities Arch Intern Med 2004;164(15):1662-1668Aug 9–23. [DOI] [PubMed] [Google Scholar]
- 9.Samore MH, Bateman K, Alder SC, et al. Clinical decision support and appropriateness of antimicrobial prescribinga randomized trial. Jama 2005;294(18):2305-2314Nov 9. [DOI] [PubMed] [Google Scholar]
- 10.Sintchenko V, Iredell JR, Gilbert GL, Coiera E. Handheld computer-based decision support reduces patient length of stay and antibiotic prescribing in critical care J Am Med Inform Assoc 2005;12(4):398-402Jul–Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents N Engl J Med 1998;338(4):232-238Jan 22. [DOI] [PubMed] [Google Scholar]
- 12.Pestotnik SL, Classen DC, Evans RS, Burke JP. Implementing antibiotic practice guidelines through computer-assisted decision supportclinical and financial outcomes. Ann Intern Med 1996;124(10):884-890May 15. [DOI] [PubMed] [Google Scholar]
- 13.Glowacki RC, Schwartz DN, Itokazu GS, Wisniewski MF, Kieszkowski P, Weinstein RA. Antibiotic combinations with redundant antimicrobial spectraclinical epidemiology and pilot intervention of computer-assisted surveillance. Clin Infect Dis 2003;37(1):59-64Aug 1. [DOI] [PubMed] [Google Scholar]
- 14.Johnston ME, Langton KB, Haynes RB, Mathieu A. Effects of computer-based clinical decision support systems on clinician performance and patient outcomeA critical appraisal of research. Ann Intern Med 1994;120(2):135-142Jan 15. [DOI] [PubMed] [Google Scholar]
- 15.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomesa systematic review. JAMA 2005;293(10):1223-1238Mar 9. [DOI] [PubMed] [Google Scholar]
- 16.Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data J Clin Epidemiol 1992;45(2):197-203Feb. [DOI] [PubMed] [Google Scholar]
- 17. 2004 Drug Topics Red Book. 108th ed.. Montvale, NJ: Thomson Healthcare; 2004.