Abstract
Predictions concerning development, interrelations, and possible independence of working memory, inhibition, and cognitive flexibility were tested in 325 participants (roughly 30 per age from 4 to 13 years and young adults; 50% female). All were tested on the same computerized battery, designed to manipulate memory and inhibition independently and together, in steady state (single-task blocks) and during task-switching, and to be appropriate over the lifespan and for neuroimaging (fMRI). This is one of the first studies, in children or adults, to explore: (a) how memory requirements interact with spatial compatibility and (b) spatial incompatibility effects both with stimulus-specific rules (Simon task) and with higher-level, conceptual rules. Even the youngest children could hold information in mind, inhibit a dominant response, and combine those as long as the inhibition required was steady-state and the rules remained constant. Cognitive flexibility (switching between rules), even with memory demands minimized, showed a longer developmental progression, with 13-year-olds still not at adult levels. Effects elicited only in Mixed blocks with adults were found in young children even in single-task blocks; while young children could exercise inhibition in steady state it exacted a cost not seen in adults, who (unlike young children) seemed to re-set their default response when inhibition of the same tendency was required throughout a block. The costs associated with manipulations of inhibition were greater in young children while the costs associated with increasing memory demands were greater in adults. Effects seen only in RT in adults were seen primarily in accuracy in young children. Adults slowed down on difficult trials to preserve accuracy; but the youngest children were impulsive; their RT remained more constant but at an accuracy cost on difficult trials. Contrary to our predictions of independence between memory and inhibition, when matched for difficulty RT correlations between these were as high as 0.8, although accuracy correlations were less than half that. Spatial incompatibility effects and global and local switch costs were evident in children and adults, differing only in size. Other effects (e.g., asymmetric switch costs and the interaction of switching rules and switching response-sites) differed fundamentally over age.
Keywords: Task switching, Inhibition, Working memory, Simon effect, Asymmetric switch costs, Global and local switch costs, Stimulus-response compatibility, Development, Children, Frontal lobe
Mature cognition is characterized by abilities that include being able: (a) to hold information in mind, including complicated representational structures, to mentally manipulate that information, and to act on the basis of it, (b) to act on the basis of choice rather than impulse, exercising self-control (or self-regulation) by resisting inappropriate behaviors and responding appropriately, and (c) to quickly and flexibly adapt behavior to changing situations. These abilities are referred to respectively as working memory, inhibition, and cognitive flexibility. Together they are key components of both “cognitive control” and “executive functions” and have been studied in a wide variety of experimental paradigms with diverse subject groups.
Our battery of interrelated tasks enabled us to independently and systematically vary demands on these abilities and to track their development across a wider age range than heretofore investigated using the same measures at all ages. Having measures that span a wide age range is important given the protracted developmental progressions of many executive function and cognitive control skills. While some cognitive abilities develop early, executive functions do not reach their peak until early adulthood (DeLuca et al., 2003; Diamond, 2002; Fischer, Biscaldi, & Gezeck, 1997; Harnishfeger & Pope, 1996; Kail, 1991a, b, c; Kail & Salthouse, 1994; Luciana & Nelson, 2002; Luciana, Conklin, Hooper, & Yarger, 2005; Luna, Garver, Urban, Lazar, & Sweeney, 2004; Lyons-Warren, Lillie, & Hershey, 2004; Munoz, Broughton, Goldring, & Armstrong, 1998; Zelazo, Craik, & Booth, 2004). Each test in our battery can be performed by children as young as 4 years; yet adults still find many of them challenging. The entire battery takes less than 30 min to complete. These tests are also designed to be appropriate for testing nonhuman primates and for neuroimaging research using functional magnetic resonance imaging (fMRI) (Diamond, O’Craven, & Savoy, 1998; O’Craven, Savoy, & Diamond, 1998).
Across this wide age span, our battery provides within-subject measures of two classic paradigms in cognitive psychology, the Simon task and task switching. In the Simon task paradigm, a non-spatial aspect of the stimulus (such as its color or identity) is relevant and its spatial location is irrelevant. Nevertheless, the well-replicated finding in adults is that responses are faster and more often correct when the stimulus and response are on the same side than when they are on opposite sides (the Simon effect, also called spatial incompatibility or stimulus–response compatibility; e.g., Craft & Simon, 1970; Fitts and Seger, 1953; Hommel, 1995; Hommel, Proctor, & Vu, 2004; Lu & Proctor, 1995; Simon & Small, 1969; Simon, 1990; Simon & Berbaum, 1990). This effect indicates: (a) the influence of an irrelevant stimulus attribute on performance and (b) a prepotent tendency to respond on the same side as the stimulus (confirmed at the neuronal level [see Georgopoulos, 1994; Georgopoulos, Lurito, Petrides, Schwartz, & Massey, 1989] and with lateralized readiness potentials [Valle-Inclán, 1996]) which must be inhibited when the locations of stimulus and response are incompatible. It thus provides insight into an aspect of inhibitory control. A finding that the Simon effect decreases over a certain age range provides evidence for when developmental improvement in that aspect of inhibition occurs and insight into when maturational changes in the neural system underlying that might be occurring. That neural system overlaps substantially with the neural system activated during Stroop interference and other cognitive control paradigms. It includes the anterior cingulate, lateral prefrontal cortex (dorsolateral and ventrolateral), pre-SMA, premotor cortex, posterior and superior parietal cortex, inferior temporal cortex, the insula, and precuneus (Bush, Shin, Holmes, Rosen, & Vogt, 2003; Dassonville et al., 2001; Fan, Flombaum, McCandliss, Thomas, & Posner, 2003; Iacoboni, Woods, & Mazziotta, 1998; Liu, Banich, Jacobson, & Tanabe, 2004; Maclin Gratton, & Fabiani, 2001; Peterson et al., 2002; Thomas et al., 1999; Wager & Smith, 2003).
We investigated spatial incompatibility effects both decreasing and increasing the working memory requirements traditionally required for Simon tasks. We decreased it in one case by providing icons depicting stimuli A and B over their respective response-sites so that which response goes with which stimulus did not have to be held in mind and in another case by using stimuli (Arrows) that pointed to where to respond. We increased the working memory requirements by introducing conceptual rules, where the correct response required mental manipulation. Instead of a rule being “for A press left,” a rule was “for A press on the side opposite A.” Thus, in addition to activating the rules associated with the two stimuli (the memory requirement in standard Simon tasks), participants had to instantiate the appropriate rule for the particular spatial location of the stimulus on each trial.
Task-switching paradigms target the ability to flexibly shift from one mindset to another, often times acting according to rules that would be incompatible with the other mindset. This has been studied extensively in adults (e.g., Allport, Styles, & Hsieh, 1994; Jersild, 1927; Meiran, Gotler, & Perlman, 1996; Monsell & Driver, 2000; Rogers & Monsell, 1995; Meiran et al., 2000a,b; Meiran, 2005; Spector & Biederman, 1976; Sudevan & Taylor, 1987), including the elderly (e.g., Kramer, Hahn, & Gopher, 1999; Mayr, 1996; Meiran, Gotler, & Perlman, 2001), and in various clinical groups (e.g., Aron, Sahakian, & Robbins, 2003; Brown & Marsden, 1988; Downes et al., 1989; Flowers & Robertson, 1985; Hayes, Davidson, Rafal & Keele, 1998; Mecklinger, von Cramon, Springer, & Matthes-von Cramon, 1999; Rogers et al., 1998). However, to date, only a handful of studies have looked at task switching in children (Cepeda, Kramer, & Gonzalez de Sather, 2001; Cohen, Bixenman, Meiran, & Diamond, 2001; Crone, Bunge, Van der Molen, & Ridderinkhof, in press; Crone, Ridderinkhof, Worm, Somsen, & van der Molen, 2004; Reimers & Maylor, 2005; Zelazo, Craik, & Booth, 2004).
Switching is fundamentally difficult and a paradigmatic instance of when top–down executive control is required because generally it cannot be done “on automatic.” It taxes both working memory and inhibition (the newly-relevant rules and stimulus–response relations must be activated and the previously-relevant ones suppressed). One cannot get in the “groove” of repeatedly doing the same thing or staying in the same mindset because periodically one will have to change that. A groove is a good analogy because it takes effort to climb over the banks of the groove (the mindset) one is in and settle, however temporarily, into another grove. Neuroimaging studies confirm that task-switching (as opposed to continuing to do the same task) activates the neural system associated with executive function and top–down cognitive control, that is lateral prefrontal cortex (dorsolateral and ventrolateral), inferior frontal junction (IFJ) and premotor cortex, pre-SMA and the anterior cingulate, and the insula and cerebellum (Brass et al., 2003; Brass, Derrfuss, Forstmann, & von Cramon, 2005; Braver, Reynolds, & Donaldson, 2003; Crone, Wendelken, Donohue, & Bunge, 2005; DiGirolamo et al., 2001; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000; Dreher & Berman, 2002; Dreher & Grafman, 2003; Kimberg, Aguirre, & D’Esposito, 2000; Meyer et al., 1998; Omori et al., 1999; Pollmann, 2001; Sohn, Ursu, Anderson, Stenger, & Carter, 2000; Sylvester et al., 2003; Wager, Reading, & Jonides, 2004). Consistent with this, patients with frontal cortex damage are impaired at switching between tasks (Aron, Monsell, Sahakian, & Robbins, 2004; Diedrichsen, Mayr, Dhaliwal, Keele, & Ivry, 2000; Keele & Rafal, 2000; Owen et al., 1993; Rogers et al., 1998; Shallice & Burgess, 1991).
We report here on the developmental progression in almost 300 children from 4 to 13 years of age and the performance of young adults for comparison, all tested on the same test battery. Various manipulations exploited task switching and spatial incompatibility effects, with and without an added memory component, or taxed memory without taxing inhibition or task switching, enabling us to test predictions concerning interrelations, independence, and the developmental progressions of working memory (how much information you must hold in mind and how many steps must be mentally executed using that information), inhibition (resisting an incorrect response you are inclined to make in order to make the correct response), and cognitive flexibility (switching between tasks or rules). The predictions we tested were generated from hypotheses concerning inhibition and working memory and hypotheses concerning cognitive flexibility and task switching.
1. Hypotheses relevant to inhibition and working memory
We hypothesized that inhibition would exact a greater relative cost for young children than for older children or young adults, and thus predicted that inhibitory demands would account for a greater proportion of the variance in children’s performance than in adults, and the more so the younger the child. In young adults, in whom inhibitory control is more mature, we hypothesized that memory demands would exact a greater cost than inhibitory demands.
Because we hypothesized that inhibitory control is extremely problematic for very young children, we predicted they would perform poorly on all trials requiring inhibition (Incongruent trials and switch trials) and that those effects would be additive. We predicted that older children and adults would show the same “asymmetric switch costs” (a greater relative switch cost for switching to the easier [Congruent] condition) previously reported in adults (Allport & Wylie, 2000; Allport et al., 1994; De Jong, 1995; Kleinsorge & Heuer, 1999; Los, 1996; Stoffels, 1996; Wylie & Allport, 2000). Further, for slightly older children, who are beginning to exercise better inhibitory control, doing so should require greater effort than in older participants. Hence, we predicted that undoing that inhibition (switching back to making a dominant response) should exact a greater cost in those children than in adults. Thus, we predicted that beginning after 6 or 7 years, asymmetric switch costs would be larger in younger than older participants, but that the youngest children would show an opposite pattern of asymmetry.
The ability to simply hold items in mind (without any added requirement to manipulate that information or exercise inhibition) develops early, is robust even in preschoolers, and shows little improvement with age (Brown, 1975; Dempster, 1985; Diamond, 1995). Given the early maturation of the ability to hold items in mind, we predicted that although it would be harder for everyone to hold more items in mind than fewer, the relative difficulty of that would not change over age.
Finally, Diamond (1991, 2002) and others (Anderson & Spellman, 1995; Gernsbacher & Faust, 1991; Hasher, Stoltzfus, Zacks, & Rypma, 1991) have hypothesized that working memory and inhibition are separable functions. This is consistent with the results of the factor analyses of Miyake et al. (2000) that found working memory and inhibition to be moderately correlated but clearly separable. Many scholars, however, have argued that there is no need to postulate an inhibitory function separate from working memory and have produced neural network models consistent with that (Cohen, Dunbar, & McClelland, 1990; Kimberg & Farah, 1993; Miller & Cohen, 2001; Morton & Munakata, 2002; Munakata, 2000). Given that we hypothesized that working memory and inhibition are independent, we predicted that performance on tasks that tax primarily memory or primarily inhibition would not be highly correlated, and tested this for relatively easy tasks and for relatively difficult tasks requiring primarily memory or primarily inhibition, matched on difficulty.
2. Hypotheses relevant to cognitive flexibility and task switching
Diamond (1990, 1991, 2002) has long maintained that it is the conjunction of simultaneous demands on holding information in mind and inhibition that is truly difficult, especially if one’s mental settings have to be continually re-set because the task changes. We thus predicted that the most difficult condition at all ages would be the one that taxes inhibition and memory in a switching context, where top–down executive control is continually required, and that that would be even more difficult than having to hold three times as much information in mind but with no inhibition or switching component. Further, since we hypothesized that switching is so difficult, we predicted that having to switch between task sets would show a long developmental progression even when memory demands are minimized.
Diamond has recently theorized that several seemingly independent findings in cognitive psychology can be integrated under the hypothesis that the brain and mind tend to work at a grosser level, and only with effort, or more optimal functioning, work in a more selective manner (a theory Diamond has called “all or none” (Diamond, 2005, in preparation)). For example, it is easier to take into account all salient aspects of a stimulus than only some of its properties. Indeed, it is difficult to ignore irrelevant properties of an attended stimulus, as the Simon effect and children’s difficulties on card sorting tasks so amply demonstrate (Diamond, Carlson, & Beck, 2005; Kirkham, Cruess, & Diamond, 2003).
Another finding that fits under the all or none rubric is that it is easier to inhibit a dominant response all the time than only some of the time. One of the most demanding cognitive requirements is to switch back and forth, to overcome inertial tendencies favoring staying in the “groove” one is in (Kirkham et al., 2003). Once in a “groove,” even if it was a difficult one to settle into (because it required resisting a tendency to act otherwise, for example) it is not that difficult to continue along that path. It is re-mapping stimulus–response associations, changing mind-sets, that is quite difficult (Brass et al., 2003; Fagot 1994; Los, 1996, 1999; Schuch & Koch, 2003, 2004; Waszak, Hommel, & Allport, 2003). We thus predicted that performance at all ages would be better in Incongruent-only blocks (where inhibition is consistently required on all trials) than in Mixed blocks (where inhibition is only required on the 50% of trials that are Incongruent), and that this difference would be greater the younger the children. This might seem obvious, but most studies of the classic Stroop effect still tend to administer the conditions in single-task blocks (read all the words or state the ink color of all the words), missing the most difficult condition (switching between having to read the words and having to state the ink color).
A further seemingly independent finding that provides another example of the all or none principle is that it is easier to switch everything, or nothing, than to switch one thing (e.g., the rule or the response) but not the other (Hommel et al., 2001; Kleinsorge, 1999; Meiran, 2000a,b; Rogers & Monsell, 1995; Schuch & Koch, 2004). Similarly, if you are supposed to press the color opposite to a stimulus it is easier to also press the button on the side opposite to the stimulus (rather than the typical bias to respond on the same side as the stimulus; Hedge & Marsh, 1975). Issuing a global “change” or “opposite” command to all systems appears to be preferred by our neural machinery over a more selective command to just the action system or to just one aspect of cognition. This has been demonstrated not only in young adults, but also in older adults (Mayr, 2001) and children (Crone et al., in press). We predicted that we would demonstrate these effects, heretofore documented only in adults and older children, even in young children. Thus, we predicted that throughout our age span, participants would do better at switching tasks if the response-site also changed and would be slower and less accurate on switch trials when the response-site remained the same as on the previous trial.
Another way of putting some of the above points is that context matters. For example, even “easy” trials do not seem so easy when they are presented in the context of switching between those and “harder” trials. Knowing that sometimes you will have to switch can cause you to slow down (and perhaps err more) on trials where you do not have to switch. Local context matters; it matters what trials came before a particular trial. For example, was the rule on the preceding trial the same as on the current trial? Performance is better on nonswitch than on switch trials. Was the response-site on the preceding trial the same as on the current trial? Studies in adults have shown that performance is better on nonswitch, same-response-site trials than on nonswitch, response-site-switch trials and on rule-switch, response-site-switch trials than on rule-switch, same-response-site trials. We predicted a different pattern in the youngest children and a more exaggerated version of the adult pattern in slightly older children (see above).
Global context also matters; it matters what kind of trial block a given trial occurs in. Performance on the same type of trial (e.g., Congruent, Incongruent) in the same type of local context (e.g., nonswitch) varies depending on the larger context (e.g., a single-task block or Mixed block). Performance even on “easy” nonswitch trials (where the rule on the present trial is the same as on the previous trial) is usually slower and less accurate when they are presented in the context of having to periodically switch between rules than in a block of all nonswitch trials. Such global switch costs (the difference in performance on nonswitch trials in a Mixed block versus in a single-task block; Fagot 1994, Mayr, 2000) have been shown to be greater for elders than for younger adults (Kray, Eber, & Lindenberger, 2004; Kray & Lindenberger, 2000; Mayr, 2000; van Asselen & Ridderinkhof, 2000) and higher for children than for young adults (Cepeda et al., 2001; Cohen et al., 2001; Reimers & Maylor, 2005), though this has not been investigated in children as young as the youngest tested here and though some studies have not found an age difference in global switch costs (Crone et al., in press; Kray, Li, & Lindenberger, 2002). We predicted that global switch costs would not only be found in our youngest participants but would be more exaggerated the younger the child.
Because of floor effects (subjects should already be slower and more error-prone in the Incongruent-only block), the effect of context (the Mixed block versus single-task block) should be greater on Congruent than Incongruent trials. We predicted that this would be more evident the younger the child. Thus, performance on “easy” (Congruent, nonswitch) trials should fall closer and closer to the level of “harder” trials in the context of sometimes having to switch back and forth the younger the child.
3. Methods
3.1. Participants
A total of 325 individuals participated, ranging in age from 4 to 45 years. Of these, 11 children were excluded from the analyses for failing to press any button or consistently pressing both. Of the remaining 314 participants, 50% were female (157 female, 157 male). Table 1 shows the number and gender of participants in each of the age groups. Children were recruited through local preschool and elementary school programs in the suburban Boston area. Adults were recruited from within the Eunice Kennedy Shriver Center in Waltham, MA. The majority of participants were Caucasian and from middle to upper middle class families. Informed consent was obtained from all adult participants and from a parent of each child participant; assent was obtained from the younger children and consent from the older ones. All participants received a small, token present for their participation.
Table 1.
Number of participants within each age and gender group
| Gender
|
|||||
|---|---|---|---|---|---|
| Age groupa (years) | Mean age (years) | S.D. | N | Female | Male |
| 4 | 4.43 | 0.25 | 30 | 14 | 16 |
| 5 | 5.19 | 0.17 | 30 | 14 | 16 |
| 6b | 6.01 | 0.40 | 30 | 15 | 15 |
| 6b | 6.22 | 0.35 | 30 | 12 | 18 |
| 7 | 7.12 | 0.20 | 30 | 13 | 17 |
| 8 | 7.97 | 0.28 | 30 | 10 | 10 |
| 9 | 9.07 | 0.30 | 30 | 17 | 13 |
| 10 | 9.92 | 0.30 | 30 | 13 | 17 |
| 11 | 11.01 | 0.32 | 28 | 11 | 17 |
| 13 | 12.89 | 1.21 | 26 | 17 | 9 |
| Adults | 26.30 | 5.40 | 20 | 14 | 6 |
| Total number of participants | 314 | 157 | 157 | ||
The age groups were used for illustrative purposes when preparing graphs. All regression analyses used the actual ages of participants and treated age as a continuous variable.
Two groups of 6-year-old children were tested to study the effects of short vs. long presentation time at this intermediate age. For one group, stimulus presentation time was 2500 ms, the slower version used with younger children. For the second group, stimulus presentation time was 750 ms, the faster version used with older children and adults.
3.2. Procedures common to all tests in our battery
All tasks were presented on a Macintosh computer using MacStim to present the stimuli and record responses. Participants held a button box (10 cm × 14 cm × 3 cm) with both hands and used their thumbs to press the two response buttons. For each task a horizontal rectangle (6 cm × 18 cm) with a central fixation cross was presented on the computer screen (25 cm × 33 cm). Only one stimulus was presented per trial and participants were positioned approximately 50 cm from the screen.
Participants completed a set of four related tests designed to manipulate demands on working memory and inhibitory control (see Fig. 1). For adults and older children (≥7 years), stimulus presentation time was 750 ms. For younger children (4–6 years), stimulus presentation time was 2500 ms. In all cases the interstimulus interval was 500 ms, resulting in total trial durations of 1250 and 3000 ms, respectively. An additional group of 6-year-old children was tested with the short (adult) presentation time (750 ms) to study the effects of presentation time at this intermediate age.
Fig. 1.
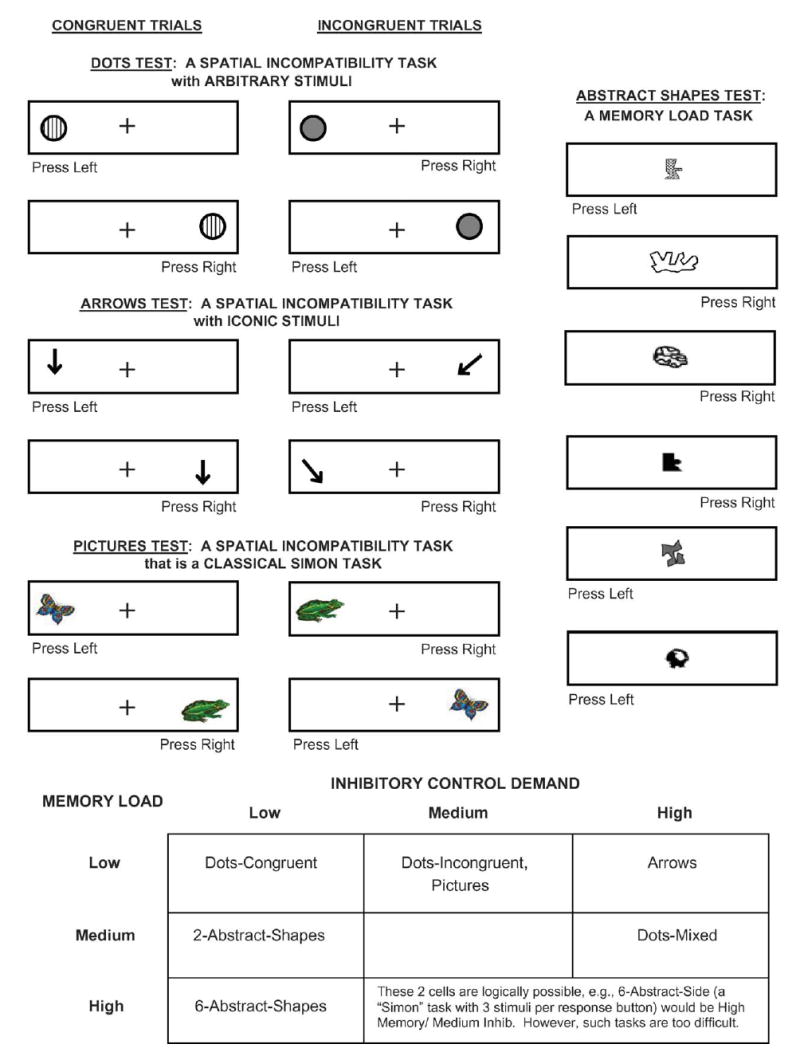
Illustration of the tasks in our battery with a table summarizing the demands of each on memory and inhibition.
Each task began with condition-specific instructions and a short practice block consisting of four or six trials. Different numbers of trials were used to allow presentation of all relevant trial types within each practice block. Participants could repeat the practice trials if needed to demonstrate learning of the requirements for a given task. Most children learned the task requirements with one practice block and no participant needed more than two practice blocks. The criterion for demonstrating learning was 75–80% correct on the practice trials and to be able to verbally tell the experimenter the rules. Testing blocks contained 20 trials and each participant completed 1 block for each condition of each task, except for the 2 conditions of the Abstract Shapes task, each of which contained 2 blocks (with a short break in between) for a total of 40 trials for each condition. The set of tests was administered with Arrows first, then Dots, Abstract Shapes (two then six shapes), and finally Pictures. A subset of participants were tested with Arrows presented last and Pictures presented first to check for order effects, but this did not affect performance, so results for both orders of presentation are collapsed together in the results reported here.
3.3. Procedures specific to individual tests
3.3.1. Pictures
This test is a classic Simon task. Here, a color picture of either a frog or butterfly was presented on the left or right side of the computer screen (see Fig. 1). Each stimulus had an associated right or left response. The exact instructions given participants were: “If you see a butterfly, press the button on the left, whether the butterfly appears on the left or right; if you see a frog, press the button on to the right, whether the frog appears on the left of right.” Small versions of the stimuli were attached next to the correct buttons on the response box to minimize the need to remember which stimulus was associated with which button. The stimuli were presented randomly on the left or right of the screen over the block of 20 trials, yielding Congruent (compatible) and Incongruent (incompatible) trials.
3.3.2. Arrows
Here, a single large arrow was presented at the left or right of the computer screen. The arrow pointed either straight down (toward the response button on the same side as the arrow) or toward the opposite side at a 45° angle (toward the response button on the opposite side; see Fig. 1). On Congruent trials, the arrow pointed straight down and participants were to respond on the same side as the arrow. On Incongruent trials, the arrow pointed diagonally toward the opposite side and participants were to respond on the side opposite the arrow. The precise instructions participants were told were, “I want you to push the button the arrow is pointing toward. If the arrow is on the side of the box pointing down like this [E demonstrated] to this button, press this button. If the arrow is on the other side pointing down like this [E demonstrated] to this button, press this button. If the arrow is on this side, pointing down across the screen like this [E demonstrated] to this button, press this button. If the arrow is on the other side, pointing down across the screen like this [E demonstrated] to this button, press this button.” Congruent and Incongruent trials were presented in a randomized Mixed block of 20 trials. This requires inhibiting the tendency to respond on the same side as the stimulus when a diagonal arrow appears, but it requires little or no working memory, as the arrow points directly to the correct response button on all trials.
3.3.3. Dots
The Dots test was designed to tax both working memory and inhibition, while the other tests were designed to tax primarily either working memory or inhibition, not both. Here, a large dot (diameter = 2.5 cm), was presented either at the left or right on each trial (see Fig. 1). Two types of Dots (striped or solid) were used. Striped Dots contained vertical black and white stripes, while solid Dots were a uniform gray color. These Dots were equated for visual characteristics such as size and luminance. For half of the participants a striped dot indicated they should make a response on the same side as the dot while a gray dot indicated they should respond on the side opposite the dot. These rules were reversed for the other half of the participants. An initial block of 20 Congruent trials (with all responses on the same side as the dot) was followed by a block of 20 Incongruent trials (with all responses on the side opposite the dot), and then by a Mixed block of 20 trials where Congruent and opposite trials were randomly intermixed. Instructions and practice were given before the Congruent and Incongruent blocks. Instructions alone were given before the Mixed block, e.g., “Remember, gray same side; striped opposite.” Memory is required on all trials of the Dots test to remember the rules (respond on the same or opposite side as the dot). Inhibition is required on Incongruent trials to inhibit the prepotent response to respond on the same side as the visual stimulus. This task is similar to one used by Shaffer (1965) though there each subject received only one type of trial block (Congruent, Incongruent, or Mixed) and therefore subjects did not have the benefit of testing with the two easier trial blocks before receiving the Mixed block. The Dots task is also similar to a task used by Vu and Proctor (2004) but the rules for their single-task blocks did not refer to stimulus appearance and so the memory demand in their Mixed condition might have been greater than in ours.
3.3.4. Abstract Shapes
In the Abstract Shapes test, unlike all other tests, each stimulus was presented in the center of the rectangle. Participants were taught a rule for each stimulus (“for this one press the left button”; “for this one press right”) during short practice blocks before each testing condition. There were two conditions involving two- or six-Abstract-Shapes. Participants first completed the two-shapes condition (2 blocks of 20 trials) and were then taught 4 additional rules, for a total of 6 shapes, and were then tested on another two blocks of 20 trials. The six-Abstract-Shapes condition taxes memory heavily (participants must hold six rules in mind), but it requires little or no inhibition (as the stimuli appear at the center of the screen and do not preferentially activate the right or left hand).
4. Results: general comments
The three dependent measures were percentage of correct responses (accuracy), speed (reaction time [RT]), and percentage of anticipatory responses (AR). Linear regressions were used for all analyses involving age and each subject’s exact age was entered, not simply the person’s age grouping. Within-subject ANOVAs were used for analyses comparing tasks, conditions within task, or trial types. All binary comparisons included Tukey corrections for multiple comparisons. Whenever the variance structure did not conform to the requirements for parametric analyses, logarithmic or arc sine transformations of the data were used to obtain the required conformity. All tables and figures present the raw, untransformed data.
A response time faster than 200 ms was considered anticipatory (too fast to be in response to the stimulus). Those responses were excluded from analyses of accuracy or speed, but were included in analyses of anticipatory responses (ARs). ARs occurred when a participant was either too eager and failed to wait for the stimulus on the current trial or failed to release the button following the previous trial. These anticipatory responses indicate inhibitory failures and are reported as a percentage of all possible responses where appropriate. A trial was considered correct if: (a) the first response following a stimulus was correct and (b) RT was >200 ms following stimulus onset.
The percentage of correct responses was calculated by dividing the number of correct responses by the sum of correct plus incorrect responses. Anticipatory responses were excluded from that calculation. The median RT for correct responses only was calculated for each participant. The median value, rather than the mean value, was used to reduce the effect of outlying RTs.
The youngest children received a slower version of our tasks than the rest of the children and adults. The stimuli were presented to the 4- and 5-year-olds and one group of 6-year-olds for much longer than they were presented to the rest of the children and adults (trial durations of 3000 and 1250 ms, respectively). Analyses over all ages might exaggerate RT differences over age (since children given longer to respond will naturally take longer) and might underestimate accuracy differences (since children given longer to respond are likely to make fewer errors). Hence, analyses of age differences are reported separately for the youngest children tested with a presentation time of 2500 ms and for all other participants tested with a presentation time of 750 ms.
The effects of gender, and interactions of gender with age, were tested in all analyses. Significant effects were not found. Independent age-related regressions for male and female participants showed comparable R2 values across the three dependent measures for all tests.
5. Results: basic level results for the tasks that included an inhibitory component (Pictures, Arrows, and Dots)
5.1. Pictures
The Pictures test was designed to provide a measure of the Simon effect in children. It tests the effect of an inhibitory demand (resisting the impulse to respond on the same side as the stimulus) with little or no working memory demand since small icons were attached above the appropriate response keys to indicate the correct response for each stimulus. The older the subjects, the better their performance (see Table 2). This was highly significant when all ages were included in the analyses (p < 0.0001 for each of the three dependent variables) and for ages 6 years through adults tested with the brief presentation time (accuracy: F(1,222) = 17.93, p < 0.0001; RT: F(1,222) = 35.36, p < 0.0001; anticipatory responses: F(1,222) = 10.8, p < 0.001), the effect of age being particularly marked on speed of responding. The youngest children (4–6 years of age) improved in speed and reduced anticipatory responses on the task over age, but given a long time to respond showed no difference over age in accuracy (RT: F(1,88) = 4.58, p < 0.04; anticipatory responses: F(1,88) = 6.07, p < 0.02).
Table 2.
Table of means for each of the task conditions by age of the participants
| Age in years
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task condition | 4 | 5 | 6 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 26 | Average | Tukey results | |
| Accuracy (percentage of correct responses) | ||||||||||||||
| Pictures | 91.67 | 87.59 | 93.92 | 88.07 | 85.26 | 88.03 | 86.14 | 91.45 | 91.08 | 92.25 | 100.00 | 98.23 | A | |
| Arrows | 83.36 | 79.11 | 90.22 | 77.87 | 73.67 | 77.97 | 80.22 | 78.38 | 84.63 | 88.19 | 95.19 | 90.06 | B | |
| Dots | Congruent | 96.33 | 94.96 | 98.61 | 96.00 | 96.86 | 99.65 | 99.30 | 99.67 | 99.62 | 99.55 | 98.50 | 91.47 | B |
| Incongruent | 86.93 | 86.12 | 92.62 | 88.05 | 88.77 | 90.46 | 89.51 | 93.83 | 96.38 | 95.02 | 89.73 | 76.37 | D | |
| Mixed | 68.57 | 68.03 | 77.37 | 71.24 | 71.97 | 73.70 | 74.94 | 76.96 | 81.71 | 85.81 | 96.22 | 82.71 | C | |
| Abstract Shapes | Two-shapes | 88.95 | 88.96 | 90.34 | 87.69 | 84.82 | 88.00 | 87.38 | 88.65 | 89.91 | 94.95 | 96.80 | 89.68 | B |
| Six-shapes | 76.87 | 77.16 | 87.39 | 73.29 | 72.60 | 73.97 | 81.34 | 78.42 | 79.16 | 86.46 | 89.92 | 79.69 | C | |
| Average | 84.67 | 83.13 | 90.07 | 83.17 | 81.99 | 84.54 | 85.55 | 86.77 | 88.93 | 91.75 | 95.19 | |||
| Reaction time (in ms) | ||||||||||||||
| Pictures | 1037.48 | 952.67 | 881.20 | 665.55 | 602.25 | 563.40 | 513.95 | 523.65 | 471.02 | 473.79 | 422.08 | 646.09 | C | |
| Arrows | 1121.28 | 1150.60 | 1090.42 | 797.73 | 725.57 | 683.07 | 613.12 | 651.77 | 578.04 | 555.46 | 465.25 | 766.57 | B E | |
| Dots | Congruent | 775.37 | 684.58 | 677.37 | 474.53 | 412.12 | 395.05 | 356.87 | 341.13 | 331.46 | 323.87 | 271.30 | 458.51 | A |
| Incongruent | 1023.12 | 905.75 | 875.02 | 619.87 | 546.27 | 501.63 | 444.02 | 451.83 | 398.09 | 402.87 | 321.28 | 589.98 | C D | |
| Mixed | 1172.32 | 1195.47 | 1177.00 | 787.10 | 728.18 | 725.98 | 644.72 | 654.15 | 597.36 | 593.85 | 562.98 | 803.55 | E | |
| Abstract Shapes | Two-shapes | 892.80 | 853.88 | 795.17 | 608.15 | 552.03 | 520.58 | 478.38 | 463.13 | 436.23 | 434.56 | 371.40 | 582.39 | D |
| Six-shapes | 1121.20 | 1038.10 | 987.53 | 726.72 | 694.15 | 662.98 | 640.55 | 612.53 | 592.23 | 568.29 | 532.93 | 743.38 | B | |
| Average | 1020.51 | 968.72 | 926.24 | 668.52 | 608.65 | 578.96 | 527.37 | 528.31 | 486.35 | 478.95 | 421.03 | |||
| Percentage of anticipatory responses | ||||||||||||||
| Pictures | 12.17 | 12.17 | 6.67 | 6.83 | 8.33 | 4.00 | 2.17 | 0.67 | 1.79 | 0.77 | 0.50 | 5.10 | E | |
| Arrows | 19.33 | 17.00 | 9.00 | 21.33 | 23.17 | 18.67 | 10.83 | 9.00 | 6.96 | 2.31 | 0.50 | 12.56 | B | |
| Dots | Congruent | 13.83 | 10.67 | 2.67 | 6.33 | 7.17 | 6.83 | 5.50 | 6.83 | 6.25 | 5.38 | 2.75 | 6.75 | A |
| Incongruent | 21.17 | 15.33 | 8.33 | 11.50 | 10.33 | 8.33 | 8.83 | 5.50 | 2.68 | 3.85 | 0.25 | 8.74 | A | |
| Mixed | 28.67 | 21.00 | 15.00 | 23.00 | 25.50 | 26.67 | 16.17 | 11.33 | 5.18 | 4.42 | 3.50 | 16.40 | D | |
| Abstract Shapes | Two-shapes | 18.00 | 11.50 | 7.75 | 8.42 | 7.08 | 6.58 | 3.67 | 4.42 | 2.50 | 1.35 | 0.88 | 6.56 | A E |
| Six-shapes | 18.00 | 17.83 | 11.58 | 15.33 | 15.33 | 13.83 | 6.08 | 5.83 | 4.73 | 2.98 | 2.75 | 10.39 | B C | |
| Average | 18.74 | 15.07 | 8.71 | 13.25 | 13.85 | 12.13 | 7.61 | 6.23 | 4.30 | 3.01 | 1.59 | |||
5.2. Arrows
The Arrows test was designed to require inhibitory control when a response was required on the side opposite the stimulus but to require little or no working memory as the stimuli themselves point to the correct response button. Performance was better as a function of age, with increased accuracy, increased speed, and reduced anticipatory responses (Table 2). This was highly significant for accuracy and anticipatory responses when all ages were included in the analyses but not significant for speed of responding (accuracy: F(1,312) = 57.06; p < 0.0001; AR: F(1,312) = 35.73, p < 0.0001). When the youngest children, tested with a long presentation time, were removed from the analyses, the age-related improvements in speed, as well as accuracy and reduced incidence of anticipatory responses, were significant at p < 0.0001 (F(1,222) = 76.88 [%correct]; 36.07 [RT], 38.56 [AR]). The youngest children (4–6 years of age) showed a steady reduction in anticipatory responses, and 6-year-olds responded correctly significantly more often than children of 4 or 5 years, but there was no difference over the age range of 4–6 years in response speed (accuracy: F(1,88) = 10.69; p < 0.005; AR: F(1,88) = 6.5, p < 0.02).
5.3. Dots
In the Dots test there were three conditions (Congruent, Incongruent, and Mixed). Performance in each condition improved significantly as a function of age, with increased accuracy and speed, and reduced anticipatory responses the older the participants (see Fig. 2). Unless otherwise noted, all results in the next three paragraphs for improvement over age are significant at p < 0.0001.
Fig. 2.
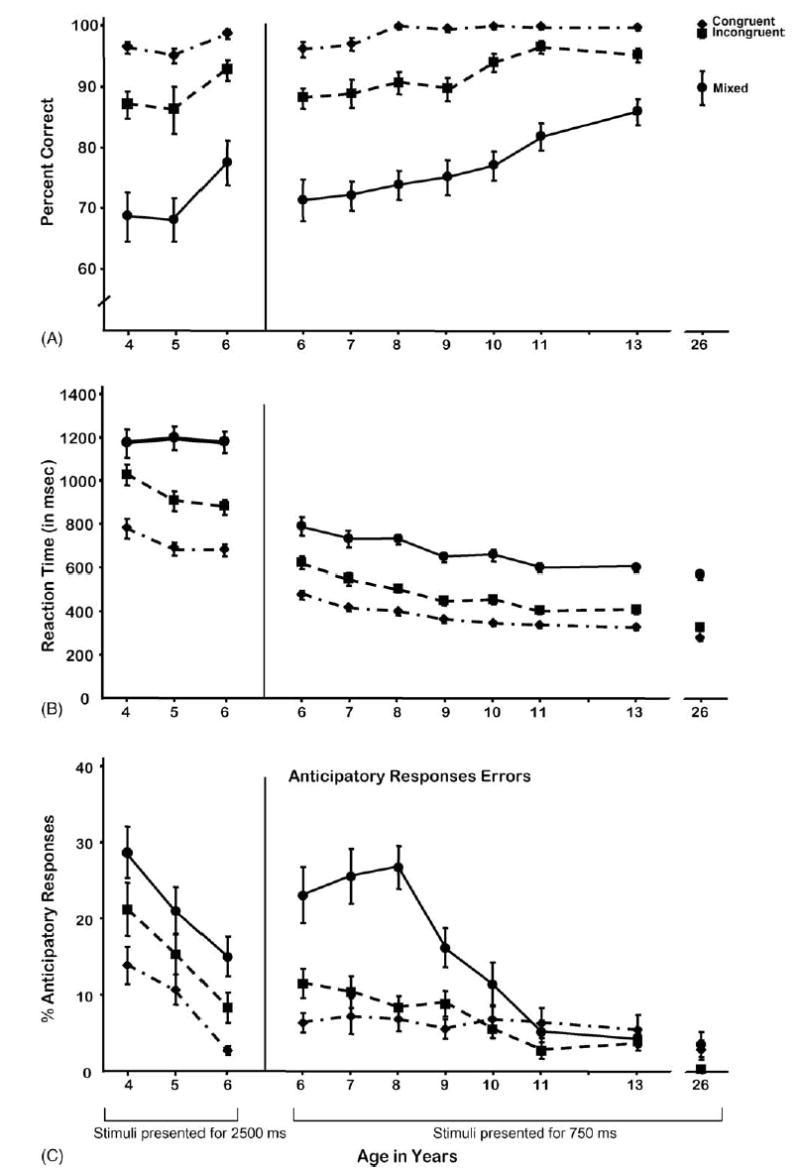
Dots conditions: (A) accuracy, (B) reaction time and (C) anticipatory response errors.
For the Congruent condition, performance improved over age in the percentage of correct responses, RT, and reduced anticipatory responses (F(1,312) = 34.68, 116.97, and 8.42 (p < 0.05 for AR), respectively with all subjects in the analyses). The corresponding results for only those tested with the 750-ms stimulus presentation time (6-year-olds through adults) are F(1,222) = 14.33 (p < 0.001), 55.05, and 2.59 (NS for AR). The corresponding results for only those tested with the 2500-ms presentation time (children of 4–6 years) are F(1,88) = 18.19 and 8.54 (p < 0.005), and 18.52.
For the Incongruent condition, with all subjects included, performance improved over age in accuracy (F(1,312) = 46.60), speed (F(1,312) = 110.76), and reduced anticipatory responses (F(1,312) = 39.77). The corresponding results for those ≥6 years of age are F(1,222) = 33.09, 47.21, and 24.33. The corresponding results for those 4–6 years of age are F(1,88) = 7.76 (p < 0.005), <1 (NS), and 15.07.
For the Mixed condition, the results for improvement over age with all subjects included in the analyses are F(1,312) = 66.65 (%correct), 62.15 (RT), and 42.84 (AR). For only those ≥6 years of age, the corresponding results are F(1,222) = 61.95, 10.68, and 31.51. For only those 4–6 years of age, the corresponding results are F(1,88) = 6.24 (p < 0.05), <1 (NS), and 11.21 (p < 0.005).
When the stimuli were presented for only 750 ms, 6-year-olds performed at a level of accuracy roughly comparable to that of 4–5-year-old children shown each stimulus for 2500 ms. While children of 4 or 5 years could perform well in the single-task blocks, even the Incongruent one, their average accuracy dipped below 70% in the Mixed block, even on Congruent trials. At the fast stimulus presentation rate (750 ms), it was not until the age of 11 years that children began responding at ≥80% correct on average in the Mixed block. Even our oldest children (13 years old) were not yet correct on 90% of the items in the Mixed block.
6. Results: spatial compatibility effects
6.1. Spatial compatibility effects: Pictures task
The Pictures test contained two intermixed trial types, Congruent and Incongruent, with spatial incompatibility present on the Incongruent trials. Participants made fewer errors and responded faster on Congruent than Incongruent trials (t[313] = 10.1 [accuracy], 8.38 [RT], both p < 0.0001; anticipatory responses NS; see Fig. 3). These comparisons indicate that the presence of spatial incompatibility affected performance. This effect was present at all ages and particularly pronounced in the younger children (t(89) = 5.35 [accuracy], 4.49 [RT], both p < 0.0001; ARs, NS). It was present, though smaller, in older children and adults (t(223) = 8.55 [accuracy], 10.41 [RT], both p < 0.0001 ARs, NS) decreasing from the age of 6 years onward (accuracy: F(1,222) = 7.46, p < 0.01; speed: F(1,222) = 5.23, p < 0.02; see Fig. 3). Children of 4–6 years, allowed a long time to respond, showed no change in the absolute size of the effect over age. However taking into account their baseline speed on Congruent trials, the percentage increase in RT on Incongruent trials decreased significantly over these ages (children 4–6 years old: t(89) = 4.23, p < 0.0001).
Fig. 3.
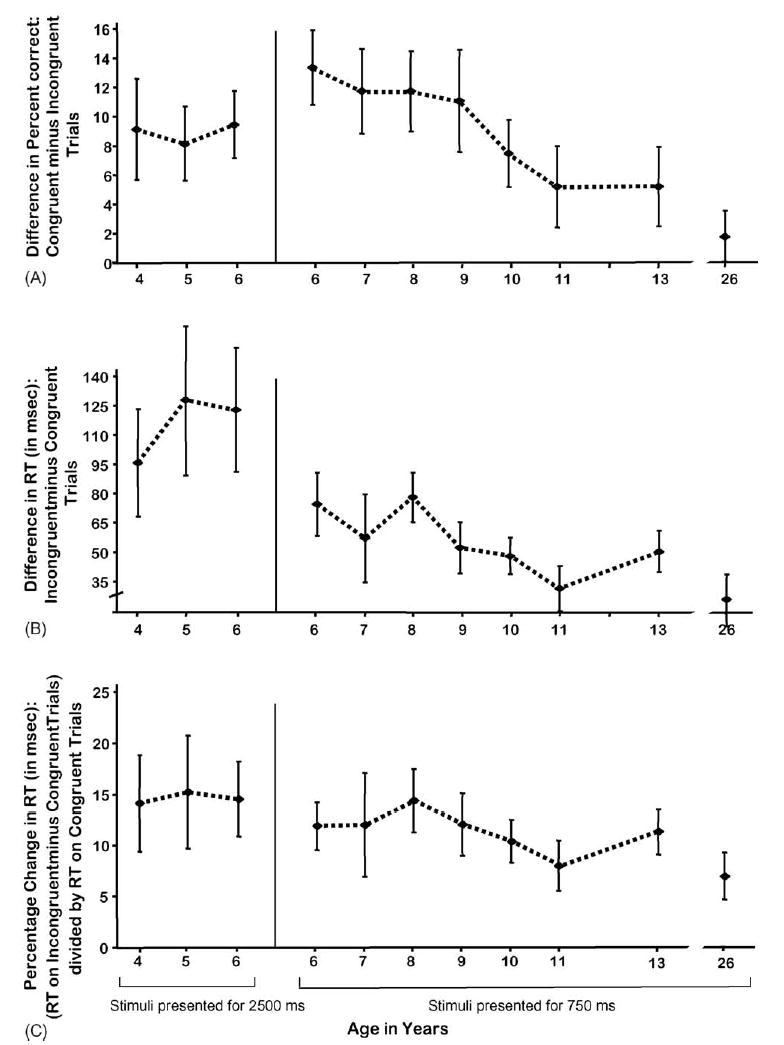
Simon effect on the Pictures task. (A) Difference in percent correct: Congruent minus Incongruent trials, (B) difference in reaction time: Incongruent minus Congruent trials and (C) percentage change in reaction time: (reaction time on Incongruent minus Congruent trials) divided by reaction time on Congruent trials.
Inhibition was required on only half the trials in the Pictures task (the Incongruent ones). Although children of 4–5 years were able to perform correctly on 90% of the Congruent trials, they were correct on only 80% of the Incongruent trials. Only the older subjects, and the 6-year-olds given a long time to respond, were able to perform at ≥85% on Incongruent trials in the Pictures task (88%, 88%, 89%, 94%, and 85%, at ages 10, 11 and 13 years, young adult, and 6 years allowed a long time to respond, respectively). Accuracy at ages 6–9 years, given a short time to respond, was comparable to that seen at 4–5 years with the longer response window.
6.2. Spatial compatibility effects: Arrows task
The Arrows test also presented Congruent and Incongruent trials randomly intermixed. The youngest participants (4–6 years of age, tested with the 2500-ms presentation time) were both more accurate (t[89] = 7.25, p < 0.0001) and faster (t[89] = 3.44, p < 0.001) on Congruent than Incongruent trials (showing interference similar to the Simon effect). Similarly, participants 6 years and older, tested with the 750-ms presentation time, were also more accurate and faster on Congruent than Incongruent trials (accuracy: t[223] = 8.76, p < 0.0001; RT: t[223] = 7.91, p < 0.0001). Among those ≥6 years, the difference in accuracy (but not speed) on Congruent versus Incongruent trials decreased as a function of age (accuracy: F(1,222) = 13.51, p < 0.0003).
6.3. Spatial compatibility effects: Dots task
There was a significant spatial incompatibility effect in the Mixed condition of the Dots task (where Congruent and Incongruent trials were again randomly intermixed). Participants were significantly faster on Congruent (spatially compatible) trials than on Incongruent (spatially incompatible) trials: t(223) = 2.09, p < 0.04 (all subjects included); t(217) = 2.49, p < 0.01 (subjects ≥6 years old); NS for the youngest children. This effect of spatial incompatibility on speed did not change significantly over age. There was no significant effect of spatial incompatibility for accuracy or anticipatory responses on this task.
7. Discussion: compatibility effects
Based on our hypothesis that even very young children can perform well when inhibition alone is taxed, we predicted they would perform well even on the Incongruent trials of the Pictures task, where memory demands were minimized. Since we hypothesized that inhibitory control shows a long developmental progression we predicted that the spatial incompatibility effect would decrease in size with age over an extended period, despite some findings in the literature to the contrary. For example, Band, van der Molen, Overtoom, and Verbaten (2000), using auditory stimuli and including neutral trials as well as compatible and incompatible ones, found an inverse relation between the size of the Simon effect and age. The effect on response speed was smaller in 5-year-olds than in subjects of 8, 11, and 21 years and the effect on accuracy was smaller in children of 5 and 8 years than in the two older groups. They did, however, find that the effect of the stimulus’s irrelevant spatial location persisted longer for the younger children. On the other hand, consistent with our prediction of a reduced compatibility effect over age, Gerardi-Coulton (2000) found evidence that even 2-year-old children show a propensity to respond on the same side as the stimulus, with the size of the effect seeming to decrease over the next 6–12 months. Because of problems with working with children so young, however, most of the 24-month-olds in that study did not provide useable data, and the few who did may not have been representative.
The youngest children we tested (4-year-olds) showed evidence of being able to inhibit a dominant response. Certainly they performed significantly better than chance even on Incongruent trials on the Pictures test where memory demands were minimized. Despite that, they still performed significantly better on Congruent than Incongruent trials. The Simon effect (faster and more accurate responses on spatially compatible than incompatible trials) was evident on the Pictures task at all ages. However, age differences in the Simon effect (the cost of inhibiting the pull to respond on the same side as the stimulus) on the Pictures task provide evidence that exercising this inhibition was disproportionately harder for younger children. Consistent with our prediction, the Simon effect showed a decrease in size from 6 years of age onward and a possible decrease in size between 4 and 6 years of age.
We also looked at spatial incompatibility effects in the context of higher-order rules and different memory loads in the Arrows and Dots tests, where the rules were more abstract, no icons were provided to remind subjects of the stimulus–response mappings, and where both the identity and the spatial location of the stimulus were relevant to determining the correct response. Although the rules for the Arrows task were more abstract, the memory demands were minimal because subjects needed only to look at the stimulus to see where to respond. On the Dots task, the abstract rules were arbitrary and memory demands were greater. On the Dots task, and to a lesser extent on the Arrows test, the rules had to be instantiated on each trial by mentally integrating the rule for the appearance of the stimulus with the location of the stimulus (e.g., “since the dot is striped, I should press on the opposite side, and since the dot is on the left that means I should press on the right”).
On the Arrows test, the spatial incompatibility effect in both speed and accuracy was significant throughout our age range and decreased from age 6 onward in accuracy but not in speed. On the harder Dots test, the spatial incompatibility effect on RT was significant throughout, beginning at age 6, but did not change over age and was not significant for accuracy. The lack of an accuracy cost on spatially incompatible (Incongruent) trials in the Mixed block of the Dots task is in sharp contrast to the results when comparing separate blocks of Congruent and Incongruent trials on the task (see below where results for the different conditions of the Dots task are presented and discussed).
8. Results: local switch costs
8.1. Local switch costs: Arrows task
The Arrows test contained nonswitch and switch trials, depending on whether the rule on the present trial was the same as on the previous trial (nonswitch trials) or different (switch trials). The difference between performance on nonswitch and switch trials administered in the same block is known as the “local switch cost.” Subjects were faster and more accurate on nonswitch trials relative to switch trials (all subjects: t[313] = 8.54 [%correct] and 8.33 [RT]; subjects ≤6 years: t[89] = 1.36, NS [%correct] and 5.92 [RT]; subjects ≥6 years: t[223] = 3.91 [%correct] and 9.80 [RT]; all p < 0.0001 except the one place noted; no differences in AR).
Among subjects 6 years old through young adults, tested with the briefer 750 ms stimulus presentation time, the accuracy cost of switching showed a marked quadratic trend, with the inverted U-shape peaking for accuracy switch cost at 9–10 years (F(1,222) = 5.65, p < 0.02; see Fig. 4). The youngest children (4–6 years), given a longer time to respond (2500 ms stimulus presentation time), showed a significantly smaller switch cost than did the older children of 6–13 years given less time to respond (F(1,292) = 9.39, p < 0.003). The youngest children achieved that small accuracy cost by using their allotted time to slow down on the harder trials (i.e., the switch trials), and their RT switch costs were over twice those at any age from 6 years through young adults (F(1,312) = 16.52, p < 0.0001).
Fig. 4.
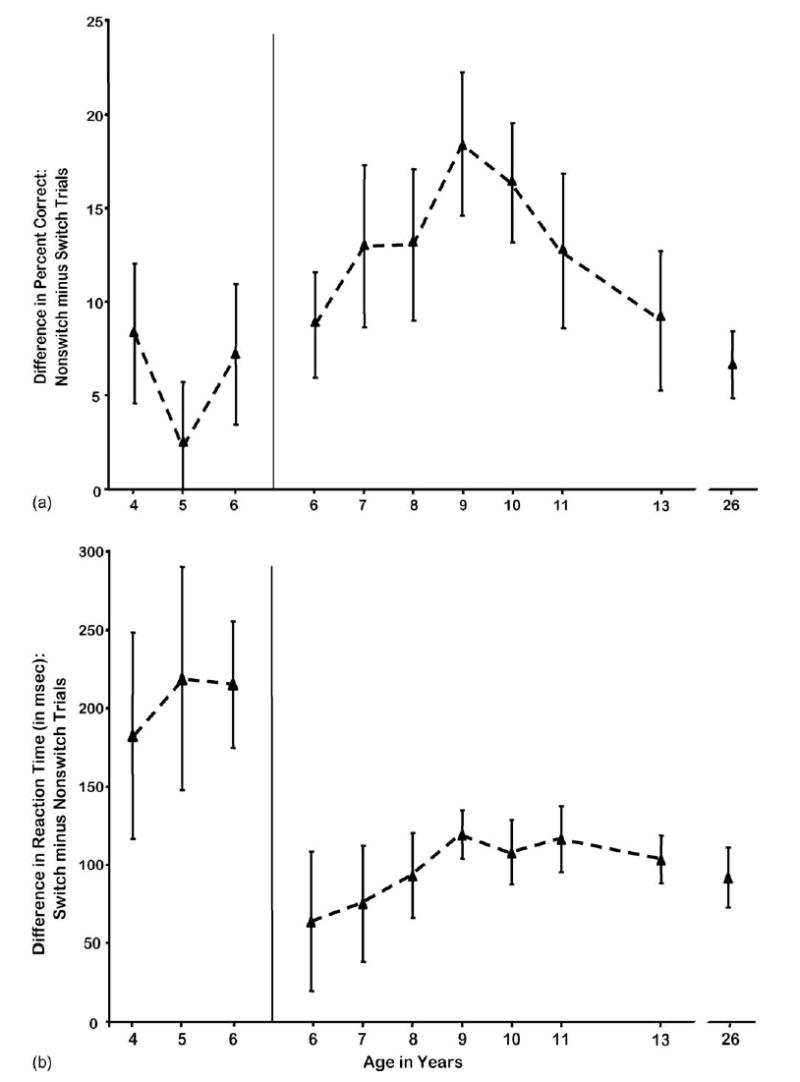
Local switch costs on the Arrows task. (a) Local switch costs in Accuracy and (b) local switch costs in reaction time.
8.2. Local switch costs: Dots task
Performance in the Mixed block of the Dots task was significantly slower and less accurate on switch than nonswitch trials. This local switch cost was significant for both accuracy and speed (all subjects: t[313] = 8.94, p < 0.0001 [%correct]; 8.56, p < 0.0001 [RT]; subjects ≥6 years: t[222] = 9.27, p < 0.0001 [%correct]; 9.02, p < 0.0001 [RT]; subjects of 4–6 years: t[89] = 2.36, p < 0.03 [%correct]; 4.31, p < 0.0001 [RT]; see Fig. 5). The local switch cost was evident on both Congruent and Incongruent trials in both accuracy and speed (Congruent trials: t[313] = 8.22, p < 0.0001 [accuracy]; 6.60, p < 0.0001 [RT]; Incongruent trials: t[313] = 4.41, p < 0.0001 [accuracy]; 5.76, p < 0.0001 [RT]).
Fig. 5.
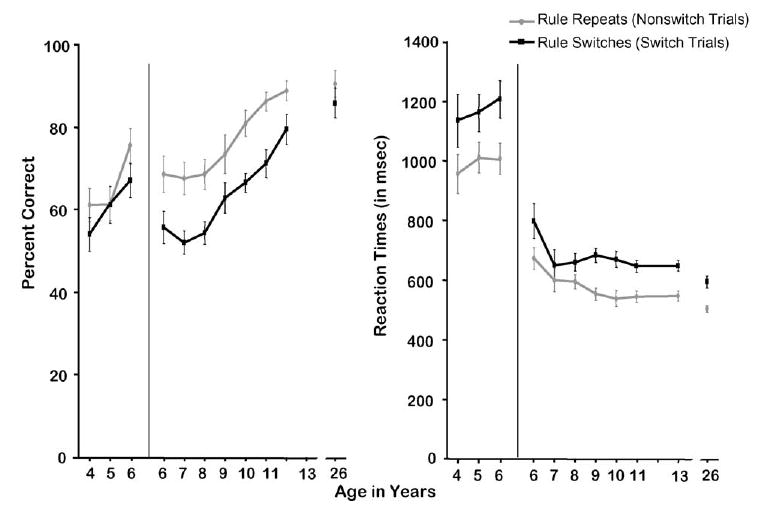
Difference between switch and nonswitch trials in the Mixed block of Dots task. (A) Percent correct and (B) reaction time.
The magnitude of the local switch cost on accuracy in the Dots-Mixed condition was greatest at 6–13 years of age and showed little change over that age range. The accuracy switch cost at 6–13 years was greater than that for adults (F(1,222) = 6.33, p < 0.01) and greater than that for the youngest children (4–6 years old: F(1,292) = 9.39, p < 0.003). Children of 4 years and children of 7–8 years performed near chance on switch trials; the 4-year-olds showed a smaller accuracy switch cost because they also made many errors on nonswitch trials. As on the Arrows test, but to a lesser extent, the youngest children benefited from the long time allotted to them for preparing their responses and their RT switch costs were larger than those for older children and adults (F(1,312) = 5.98, p < 0.02). The difference in speed of responding on switch and nonswitch trials tended to be smallest among subjects 6–8 years of age, presumably because the response window was sufficiently tight for them that they had little room to show differential RTs.
9. Discussion: local switch costs
As expected, performance was slower and less accurate on switch than nonswitch trials in both the Arrows task and the Dots-Mixed condition. For both Arrows and Dots-Mixed, local switch costs in accuracy were smaller in adults than in children 6–13 years of age tested under the same conditions as adults. Local switch costs on speed of responding, on the other hand, showed no differences between children of 6–13 years and adults and remained fairly constant from 6 years through young adulthood on both Arrows and Dots-Mixed.
Children of 4–6 years also showed smaller local switch costs in accuracy than did children of 6–13 years on both the Arrows task and the Dots-Mixed condition. Presumably children of 4–6 years were able to use the considerable time allowed for them to respond to slow down on switch trials to preserve their accuracy. Their local switch costs in RT were greater than those for participants at any older age, especially on the Arrows test.
Studies where the switches between tasks are unpredictable have tended to find larger local switch costs in older versus younger adults, in contrast to the lack of difference in global switch costs over age (Kray et al., 2002; van Asselen & Ridderinkhof, 2000). Studies with predictable switches, on the other hand, have generally found that local switch costs either did not change over age or are smaller in older adults, in contrast to the larger global RT switch costs found in those studies (Kray & Lindenberger, 2000; Mayr & Kliegl, 2000a,b; Mayr & Liebscher, 2001; Salthouse, Firstoe, Lineweaver, & Coon, 1995; Verhaeghen & Salthouse, 1997; Verhaeghen & De Meersman, 1998). It is not because older adults are performing well that they show smaller, or equivalent, local switch costs compared to young adults in predictable-switch studies. It is because their RT is elevated across the board in the Mixed block (on both nonswitch and switch trials) that they show no further disproportionate increase in RT on switch trials.
Results comparing children and young adults mirror those comparing older versus younger adults. The one study that used predictable switches found that local switch costs remained stable from age 10 through middle adulthood, though global switch costs were larger in children (Reimers & Maylor, 2005). All the studies that have used unpredictable switches report greater local switch costs in children than adults. Cohen et al. (2001) found greater local switch costs in accuracy, but not in RT, in children 5–11 years of age compared to adults using Meiran’s task-switching paradigm adapted for children. Crone et al. (in press), using a paradigm similar to our Dots test, found that local switch costs decreased with age from 8 to 11 to 23 years. Cepeda et al. (2001), who studied subjects aged 7 through 82 years of age, asking them the number of the digits displayed or the value of the digits, report larger local switch costs for both young children and older adults than for young adults. Similar results are reported by Kray et al. (2004).
There are two differences between our results and those of most studies. First, most studies find little or no difference in local switch costs in accuracy; the differences they find are in RT. We found only accuracy differences between children and adults and no RT differences. Second, we found that local switch costs in accuracy were greater and local switch costs in RT were smaller among children in our age range approximating the ages included in other studies (6–13 years of age) than in younger children rarely investigated previously in task-switching studies. Two differences in our design may account for our relatively large accuracy differences and small RT differences. One is the size of the window provided for subjects to compute their responses. Children find task-switching harder than adults. When given a large enough response window so they can slow down on switch trials, and when that window does not exceed young children’s ability to inhibit responding sufficiently long to compute the correct answer, children show larger RT switch costs than adults. When given a narrower response window, or the time needed to compute the answer is longer than young children are willing to delay their response, children show larger switch costs in accuracy than adults.
Two, unlike the vast majority of task switching studies, our stimuli were “univalent.” Each stimulus was unique to a task or rule. No stimulus had one meaning for the Congruent rule and a different meaning for the Incongruent rule; different stimuli were used for the different rule sets. Meiran’s model predicts, and Meiran reports results showing, that switch costs largely disappear if the stimuli are relevant to only one task (i.e., univalent; Meiran, 2000a,b). We clearly found robust switch costs with our univalent stimuli, but some differences in what we found versus what others have reported might be due to this characteristic of our stimuli.
10. Results: comparisons across the different conditions of the Dots task (Congruent Single-Task Block, Incongruent Single-Task Block, and the Mixed block)
Comparisons of performance among these three blocks show significant differences in the percentage of correct responses, RTs, and number of anticipatory responses (F(2,939) = 278.03 [%correct], 134.55 [RT], 49.86 [AR], all p < 0.0001). As can be seen in Fig. 2, performance was best in the Congruent condition, intermediate in the Incongruent one, and worst in the Mixed condition. Planned comparisons show that performance in each of the conditions was significantly different from performance in the other two conditions in accuracy, speed, and anticipatory responses (with the single exception of percentage of anticipatory responses in the Congruent and Incongruent blocks among subjects ≥6 years; see Table 3). Although performance was better for Dots-Congruent than Dots-Incongruent, that difference pales in comparison with the difference between performance in either of those conditions and Dots-Mixed (difference between Mixed and Incongruent versus the difference between Incongruent and Congruent: t[313] = 18.51 [accuracy], 18.06 [RT], and 5.45 [AR], all p < 0.0001; see Fig. 2).
Table 3.
T values for planned comparisons between trial blocks within the Dots test
| Percentage of correct responses | Response speed | Anticipatory responses | |
|---|---|---|---|
| All subjects (d.f. = 313) | |||
| Congruent vs. Incongruent blocks | 13.78 | 14.46 | 7.46 |
| Congruent vs. Mixed blocks | 28.55 | 24.49 | 14.58 |
| Incongruent vs. Mixed blocks | 18.81 | 15.66 | 10.51 |
| Younger subjects (4–6 years old; d.f. = 89) | |||
| Congruent vs. Incongruent blocks | 7.1 | 7.12 | 4.49 |
| Congruent vs. Mixed blocks | 15.32 | 10.51 | 8.27 |
| Incongruent vs. Mixed blocks | 8.83 | 5.99 | 4.82 |
| Older subjects (6–26 years old; d.f. = 223) | |||
| Congruent vs. Incongruent blocks | 12.24 | 17.06 | 6.24 |
| Congruent vs. Mixed blocks | 24.15 | 30.36 | 12.1 |
| Incongruent vs. Mixed blocks | 16.91 | 18.74 | 9.43 |
All significant at p < 0.0001.
Performance in the Incongruent and Mixed conditions can also be viewed as a percentage change from performance in the Congruent condition ([I or M minus C] divided by C), thus taking into account baseline performance. The percentage change was far greater for performance in the Mixed condition than the Incongruent one (see Fig. 6). The difference between accuracy in the Congruent and Incongruent conditions decreased significantly over age (F(1,312) = 14.95, p < 0.0001), but the decrease over age in the difference between how accurately participants performed the Congruent and Mixed conditions was far greater (F(1,312) = 43.81, p < 0.0001; see Fig. 6). Thus, the accuracy difference between the Mixed and Congruent conditions decreased more sharply over age than did the accuracy difference between the Incongruent and Congruent conditions (difference between accuracy difference scores: (F(1,312) = 12.02, p < 0.001)). Despite the marked improvement over age in accuracy in the Mixed condition, even for 13-year-olds the difference in accuracy in the Mixed condition versus the Congruent one was larger than for adults (F(1,88) = 7.47, p < 0.01).
Fig. 6.
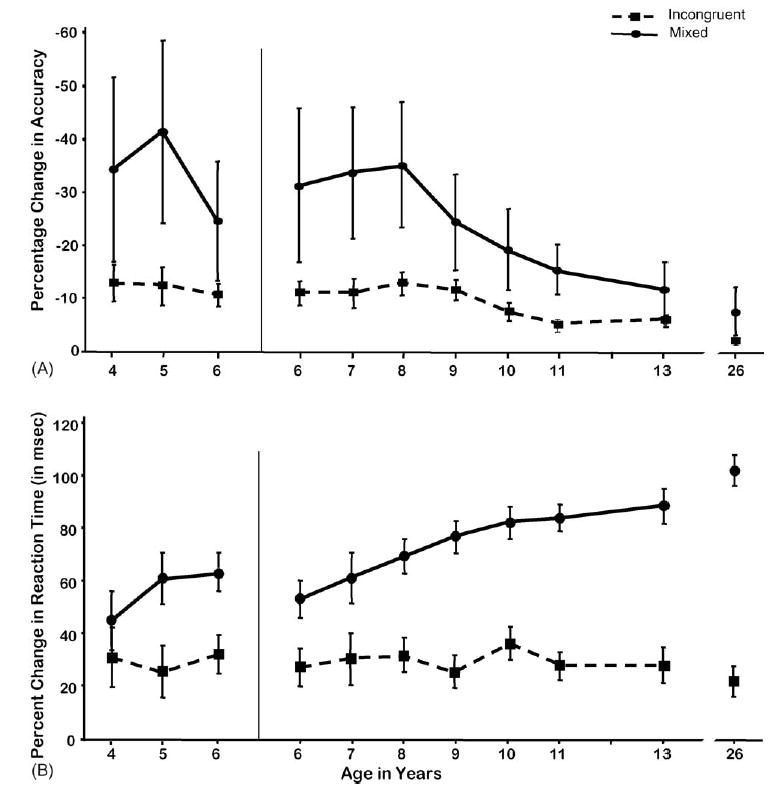
The Incongruent and Mixed conditions as percentage change from the Congruent condition of the Dots task. (A) Percentage change in accuracy and (B) percentage change in reaction time.
For neither the Incongruent nor Mixed conditions was there a significant linear trend for reduced percentage-change scores in any dependent measure between the ages of 4–6 years, except for percentage-change in RT for the Mixed condition (F(1,312) = 4.95, p < 0.04). The percentage change in speed of responding in the Incongruent condition compared with the Congruent one remained quite stable over age. The percentage change in RT in the Mixed condition compared with the Congruent condition was greater and increased significantly over age (F(1,312) = 28.75, p < 0.0001). Thus, with age participants were better able to modulate their performance speed, slowing down in the more difficult Mixed condition to minimize any reduction in accuracy; whereas younger subjects (even those given a very long response window) tended to keep their response speed more constant across conditions at the cost of accuracy in the more difficult Mixed condition.
The difference in response speed between the Mixed and Congruent conditions increased over age while the RT difference between the Incongruent and Congruent conditions remained constant. Hence the difference between RT in the Mixed and Congruent conditions showed a greater change over age than the difference between the Incongruent and Congruent conditions (difference between RT difference scores: F(1,312) = 42.09, p < 0.0001).
We had predicted that cognitive flexibility would improve with age and that therefore the difference in performance between Dots-Incongruent and Dots-Mixed would decrease over age. That was strongly confirmed for subjects 6 years and older tested with the 750 ms stimulus presentation time. The difference between their performance on the Incongruent and Mixed conditions steadily decreased in both speed and accuracy (F(1,222) = 12.9, p < 0.0005 [%correct]; 3.72, p < 0.05 [RT]). For children 4–6 years of age however, tested with the 2500 ms stimulus presentation time, the difference between performance in the Incongruent and Mixed conditions did not change consistently over age in either speed or accuracy.
11. Discussion: comparisons across the different conditions of the Dots task
We had predicted that inhibitory demands would account for a greater proportion of the variance in children’s performance than in adults, and the more so the younger the child. The Congruent and Incongruent blocks of the Dots test each contained the same memory load (one higher-order rule, with two embedded rules). The two blocks differed only in that the Incongruent block required inhibition while the Congruent Block did not. The prepotent tendency to respond on the same side as the stimulus had to be inhibited in the Incongruent block but should have facilitated performance in the Congruent Block. We predicted that the Dots-Incongruent block would be more difficult than Dots-Congruent Block, but more important, that the difference in performance between those two conditions would decrease over age as inhibitory control improved.
That prediction was confirmed. Dots-Incongruent was more difficult than Dots-Congruent, and the more so the younger the children. Accuracy and impulsivity differences between these two conditions decreased over age. (The larger spatial incompatibility effect we had found the younger the children [with memory demands minimized] is also consistent with this prediction). Indeed, accuracy differences between these two conditions must continue to decrease after 13 years of age since the difference in accuracy in Dots-Congruent and Dots-Incongruent was still greater in 13-year-olds than in young adults.
Since it is harder to switch back and forth between inhibiting a dominant response and making it, we predicted that performance at all ages would be better in the Incongruent-only block of the Dots test (where the tendency to respond on the same side as the stimulus must be inhibited all the time) than in the Mixed block of the task (where that tendency must be inhibited on only half the trials as the other half are Congruent trials), and that this difference would be greater the younger the children. Indeed, performance differences between the Dots-Incongruent and Dots-Mixed conditions were large at all ages, and especially large the younger the children, as predicted.
12. Results: global switch costs
The cost of knowing that on some trials you will have to switch rules can be evaluated by comparing (a) performance on Congruent trials following Congruent trials within a block of only Congruent trials to (b) performance on Congruent trials following Congruent trials within the Mixed block, and similarly by comparing Incongruent trials in the Incongruent block with Incongruent nonswitch trials in the Mixed block. In both cases on all dependent measures the difference is clear. Although the local context of all these trials is similar (all follow a trial of the same type), when these occurred in the context of a Mixed block, participants were significantly slower, less accurate, and more inclined to make anticipatory responses (see Fig. 7; nonswitch Congruent Dots-Mixed trials versus Dots-Congruent single-task block [t(313) = 13.48, p < 0.0001 (accuracy); 18.21, p < 0.0001 (RT); 2.85, p < 0.01]; nonswitch Incongruent Dots-Mixed trials versus Dots-Incongruent single-task block [t(313) = 9.44, p < 0.0001 (accuracy); 10.07, p < 0.0001 (RT); 3.74, p < 0.0003 (AR)]).
Fig. 7.
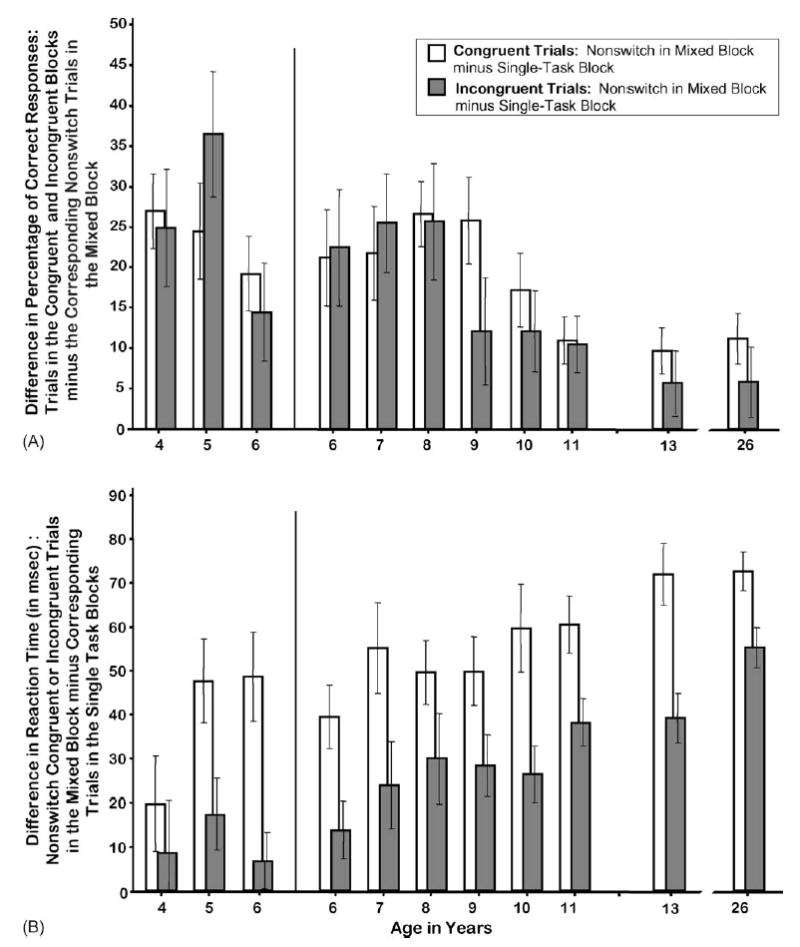
Mixing costs on the Dots task: performance on trials in the single-task blocks compared with performance on comparable nonswitch trials in the Mixed-task block. (A) Difference in percentage of correct responses: trials in the Congruent and Incongruent blocks minus the corresponding nonswitch trials in the Mixed block and (B) difference in reaction time: nonswitch Congruent or Incongruent trials in the Mixed block minus corresponding trials in the single-task blocks.
The accuracy cost of this difference in global (i.e., trial-block) context was roughly equal for Congruent and Incongruent trials especially among the younger subjects. From age 9 onwards there was a trend for the accuracy cost to be greater for Congruent trials (see Fig. 7). The cost in speed of this difference in global context was significantly greater for Congruent than Incongruent trials (only nonswitch trials: all subjects: t[313] = 4.46, p < 0.0001; subjects ≤6 years, 3000-ms trial duration: t[89] = 2.73, p < 0.01; subjects ≥6 years, 1250-ms trial duration: t[223] = 4.18, p < 0.0001; see Fig. 7). The difference in this RT cost for Congruent versus Incongruent trials was greater for the children 4–6 years old than for the older subjects (F(1,312) = 6.28, p < 0.01).
The mixing cost (the cost of Congruent [Incongruent] trials being Mixed in with Incongruent [Congruent] ones) for accuracy was much greater for subjects under 10 years of age than for those 10 years and older (reduction of the cost in accuracy over age [all subjects]: F(1,312) = 30.6, p < 0.0001; see Fig. 7). The mixing cost as assessed by response speed, however, increased over age (F(1,312) = 60.10, p < 0.0001; see Fig. 7), again showing that older subjects were better able (or more likely) to modulate their speed to preserve their accuracy. These findings are true for Congruent and for Incongruent trials.
13. Discussion: global switch costs
Global switch costs (worse performance on nonswitch trials in a Mixed block versus in a single-task block) were found here, as predicted. We had predicted they would be greater the younger the children. Indeed, global switch costs on accuracy were greater for participants <10 years old than for those older than 10 years. Global switch costs on accuracy declined from 9 to 13 years. However, global switch costs on RT showed the opposite pattern. They increased from age 6 to early adulthood. Adults adjusted their speed to preserve their accuracy; younger children did that less, resulting in a difference in the speed-accuracy trade-off with age.
We had also predicted that, because of floor effects for Incongruent trials, the effect of context (the Mixed block versus single-task block) would be greater on Congruent than Incongruent trials, and that this would be more evident the younger the child. However, contrary to the portion of our prediction concerning development, the size of the greater effect of context on Congruent versus Incongruent trials did not change over age.
It may well be that difficulty undoing inhibition of the prepotent response accounts for why switching back to making a response consistent with that tendency shows a greater cost than switching back to inhibiting that tendency, as Allport and others have suggested (Allport et al., 1994; Allport & Wylie, 2000). However, it is also true that the easier condition provides more room to find an effect because performance is so good on that condition on nonswitch trials. It is not that subjects are worse at switching to the easier rule than to the harder rule. It is that the floor is so much lower for the easier than the harder condition on nonswitch trials that there is more room for an effect to be found for switching to the easier condition.
As noted above in the discussion of spatial incompatibility effects, the lack of an accuracy difference on spatially compatible (Congruent) and spatially incompatible (Incongruent) trials in the Mixed block is in sharp contrast to the result of comparing separate blocks of Congruent and Incongruent trials on the task. The latter shows a significant incompatibility effect for children of all ages in both speed and accuracy, though not for adults. The cost in accuracy on the spatially incompatible block compared to the compatible block of the Dots task was greater than the cost in speed, and the accuracy cost decreased over age from 8 years onward (see Fig. 6). Our results for adults are consistent with a wealth of studies where adults have shown no cost (or greatly reduced cost) of inhibiting in steady-state the urge to make the spatially incompatible response in single-task blocks (Praamstra, Kleine, & Schnitzler, 1999; Ridderinkhof, 2002; Stürmer, Leuthold, Soetens, Schröter, & Sommer, 2002; Valle-Inclán, Hackley, & de Labra, 2002; Verbruggen, Liefooghe, Notebaert, & Vandierendonck, 2005; Wühr, 2004, 2005).
14. Results: interaction of local switch costs with prepotent response or its inhibition
14.1. Arrows test: interaction of rule switching with prepotent response or its inhibition
For younger children, there was barely any accuracy switch cost in the Arrows test. Their accuracy was much worse on Incongruent trials whether or not they were switch trials. For 7–10-year-olds, the cost to accuracy of switching was greater on Congruent trials (t[119] = 6.41, p < 0.0001). The difference in the accuracy cost of switching to Congruent versus Incongruent trials followed an inverted U-shaped function over age (see Fig. 8). It was negative at 6 and 11–13 years of age, showing a greater accuracy cost in switching to the Incongruent rule. It was largest at 8 years of age and intermediate at 7 and 9–10 years of age. For adults, there was no effect of spatial incompatibility on accuracy. Adults made more errors on switch than nonswitch trials in the Arrows test and it made no difference whether a Congruent or Incongruent response was required.
Fig. 8.
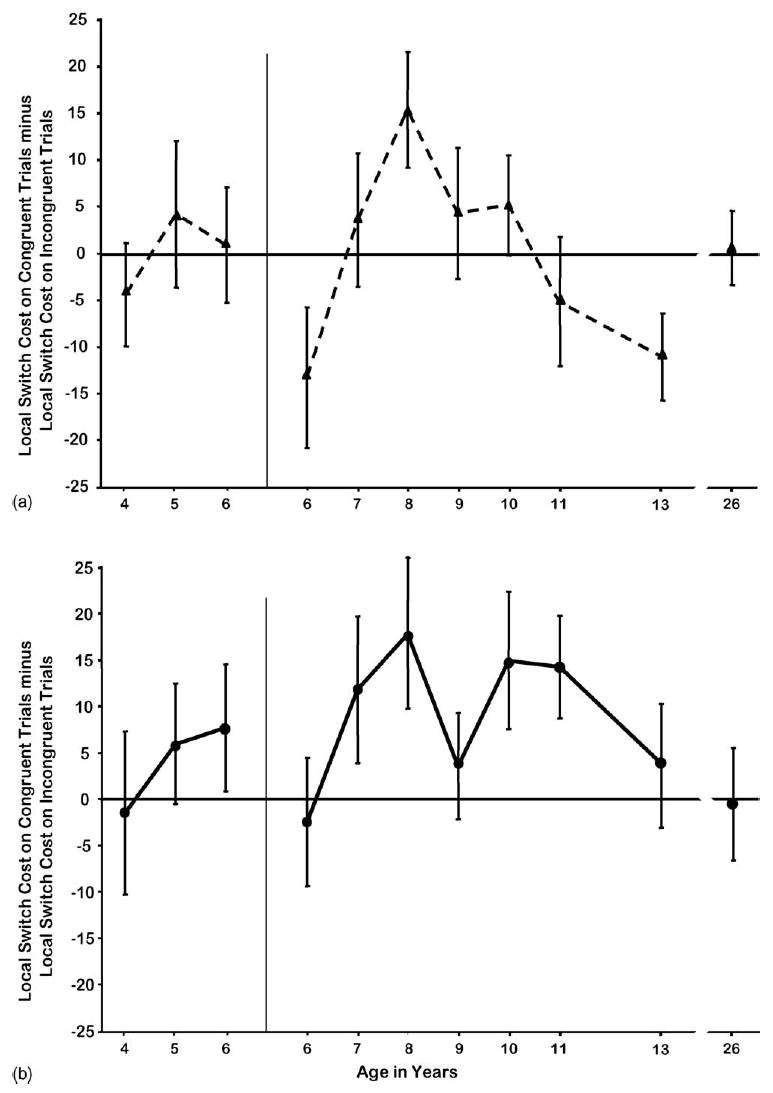
Differential accuracy cost of switching to the Congruent rule rather than the Incongruent rule. (a) Arrows test and (b) Dots-Mixed condition.
The effect of switching on RT in the Arrows test, depending on whether the rule on the switch trial was Congruent or Incongruent, showed a different pattern. Switching took a greater toll on the speed with which the younger children responded when the response rule on the switch trial was Incongruent rather than Congruent (difference for children 4–6 years old: [RT on Incongruent switch minus nonswitch trials] versus [RT on Incongruent switch minus nonswitch trials] (t[89] = 2.6, p < 0.03) with a similar difference for children 6–7 years old: (t[59] = 2.8, p < 0.01). For children 8–13 years of age, the RT cost of switching was equivalent on Congruent and Incongruent trials. For young adults, the difference seen in the youngest children reversed and the RT cost of switching was greater on Congruent trials (t[19] = 2.75, p < 0.01), consistent with reports in the literature for adults (e.g., Allport et al., 1994; Allport & Wylie, 2000). The progression over age was from an opposite pattern in the youngest children to no difference in the older children to finally seeing a greater RT switch cost on Congruent than on Incongruent trials for young adults.
14.2. Dots test: interaction of rule switching with prepotent response or its inhibition
The difference between accuracy on switch and nonswitch trials in the Mixed block of the Dots task was significantly greater for Congruent than for Incongruent trials [all subjects: t(313) = 2.96, p < 0.003; children ≤6 years: t(89) = 2.36, p < 0.02; children ≥6 years: t(223) = 3.03, p < 0.004]. The greater cost in accuracy of switching to the Congruent condition was evident at 7 through 11 years of age (see Fig. 8b). The children for whom the Dots task was most difficult (those 4–5 years old even though given a large response window and those 6 years old given a shorter response window) showed no greater accuracy switch cost for Congruent or Incongruent trials, nor did those who found the task easiest, 13-year-olds and young adults.
Beginning at 8 years of age there was also a greater switch cost in RT for Congruent than Incongruent trials, replicating the pattern previously reported for adults (that the RT cost of switching to the rule consistent with one’s prepotent inclination is greater than the cost of switching to the rule that requires resisting that inclination [e.g., Allport & Wylie, 2000; Allport et al., 1994]). The difference in speed of responding comparing Congruent switch and nonswitch trials was greater than the difference in speed of responding comparing Incongruent switch and nonswitch trials for children ≥8 years and for adults (children 8–13 years old: t[143] = 2.18, p < 0.001; young adults: t[19] = 2.75, p < 0.01) but not for children <8 years. Indeed, for the youngest children (4–6 years of age) the opposite was found: The RT cost of switching to an Incongruent trial was greater for them than the RT cost of switching to a Congruent trial (t[89] = 4.31, p < 0.0001); mirroring a similar finding on the Arrows task. For 6-year-olds performing the faster version of the task, the RT cost of switching was equivalent on Congruent and Incongruent trials. Hence, the age progression was from a greater RT switch cost for Incongruent trials (at 4–6 years), to no difference, to a greater RT switch cost for Congruent trials (from 8 years onward; see Fig. 8).
15. Discussion: interaction of local switch costs with prepotent response or its inhibition
“Asymmetric switch costs” refer to a greater relative cost in switching to the rule consistent with your prepotent tendency (Congruent trials in our study) than in switching to the rule that requires inhibiting that tendency (Allport & Wylie, 2000; Allport et al., 1994; De Jong, 1995; Kleinsorge & Heuer, 1999; Los, 1996; Stoffels, 1996; Wylie & Allport, 2000). One explanation for this pattern is that greater inhibition is required of the easier rule when responding according to the harder rule than vice versa, and that going back to responding according to easier rule requires undoing that inhibition. Hence, for example, Allport and Wylie (2000) looked at switching between reading color words and saying the color of the ink in the Stroop task. To report the ink color requires inhibiting the tendency to read the word; to switch back to reading the word presumably requires undoing that inhibition. To read the word requires minimal inhibition of reporting the ink color; hence there is minimal inhibition to undo when switching back to reporting the ink color (but see also Yeung & Monsell, 2003).
In the present experiment, to respond on the side opposite the stimulus should require inhibiting the tendency to respond on the same side as the stimulus. Switching back to responding on the same side as the stimulus should require undoing that inhibition. We had predicted we would replicate the effect previously reported in adults (greater RT costs for switching to the rule consistent with subjects’ inclinations than for switching to the rule requiring inhibition of that) but also predicted that the very youngest children, who have poor inhibitory control, would perform poorly on all trials requiring inhibition (Incongruent trials and switch trials) and that the effects would be additive. Thus, we predicted that the youngest children, unlike adults, would perform worse when switching to the Incongruent rule rather than to the Congruent one. Further, we predicted that intermediate-age children, who are beginning to exercise better inhibitory control, would require greater effort to do so than older participants. Hence, we predicted that undoing that inhibition (switching back to making the Congruent response) should exact a greater cost in intermediate-age children than in older participants. Thus, we predicted that beginning after 6 or 7 years, “asymmetric switch costs” would be larger in younger versus older participants. These predictions were confirmed.
In both the Arrows test and the Dots-Mixed condition, adults showed a greater RT cost (though no accuracy difference) for switching to the Congruent than the Incongruent rule. Those results replicate those of Allport and Wylie. However, a different pattern was found in children. On the Arrows test, the youngest children were slower to switch to the Incongruent rule than the Congruent one. Similarly in the Dots-Mixed condition, for the youngest children (4–6 years old) the RT cost of switching to the Incongruent rule was greater than the RT cost of switching to the Congruent one. For older children of 8–13 years on the Arrows test, the RT cost of switching was equivalent on Congruent and Incongruent trials. Only for young adults did the difference seen in the youngest children reverse. The progression over age on the Arrows test was from a greater RT switch cost on Incongruent than Congruent trials for the younger children (4–7 years old) to no difference in the older children (8–13 years old) to finally seeing a greater RT switch cost on Congruent trials for young adults. On the Dots test, beginning at 8 years of age and for older ages, the adult pattern was evident—greater RT switch costs on Congruent than Incongruent trials.
The pattern we report in children also differs from previous reports for adults (and our own findings for adults) in that children showed differences in the size of the switch costs on accuracy as well as RT. On the Arrows test, the youngest children made more errors on Incongruent trials, whether they were switch trials or not, and showed little difference in accuracy on switching to Congruent or Incongruent trials. Children 7–10 years of age, however, showed greater costs in accuracy when switching to Congruent than to Incongruent trials. The greater accuracy cost in switching to the Congruent versus Incongruent rule was largest in magnitude at 8 years and next largest at 7 and 9–10 years of age. For children 11–13 years of age, the cost in accuracy of switching was slightly greater on Incongruent than Congruent trials. For adults there was no difference in the accuracy cost.
In the Dots-Mixed condition, the difference between accuracy on switch and nonswitch trials was greater for Congruent than for Incongruent trials overall and at all individual ages except 4 and 5 years of age, age 6 when the shorter presentation time was used, and of course young adults. The children for whom the task was most difficult (those 4–5 years old even though given a large response window and those 6 years old given a shorter response window) showed no greater accuracy switch cost for Congruent or Incongruent trials. The size of their accuracy cost for switching to the Incongruent rule was limited by their relatively poor performance on even non-switch Incongruent trials. At all other ages, children showed a greater accuracy cost when switching to the Congruent rule in the Dots task. This was largest at the intermediate ages of 7–11 years.
The only other study to examine this in children (Crone et al., in press), using a task similar to our Dots task, found greater costs in both speed and accuracy for switching to Congruent versus Incongruent trials, as did we. However, unlike us, they did not find differences in this over age.
It may be that the difficulty of undoing inhibition of the prepotent response accounts for why switching back to making a response consistent with that tendency shows a greater cost than switching back to inhibiting that tendency, as Allport and others have suggested. However, it should be noted that the easier condition also provides more room to find an effect because performance is so good on that condition on nonswitch trials. The floor is so much lower for the easier than the harder condition on nonswitch trials that there is more room for an effect to be found for switching to the easier condition.
16. Results: interaction of local switch costs with response-site switching
16.1. Arrows test: interaction of rule switching with response-site switching
The correct response on Trial N might be in the same location as on Trial N-1 or it might be at the opposite location. We had hypothesized that when the rule switched there would be an inclination to change where to respond as well, and that when the rule remained the same, subjects would be faster when the same trial repeated (consistent with global commands to “change” or “repeat”). We thus predicted an interaction between whether the rule changed and whether the correct response-site changed. On the Arrows test, our prediction was strongly confirmed for accuracy (F(1,312) = 41.89, p < 0.0001). The effect was most marked at 6–9 years of age and smallest in adults (see Fig. 9A). Indeed, children of 6–9 years of age performed at or near chance on rule-switching response-stay trials.
Fig. 9.
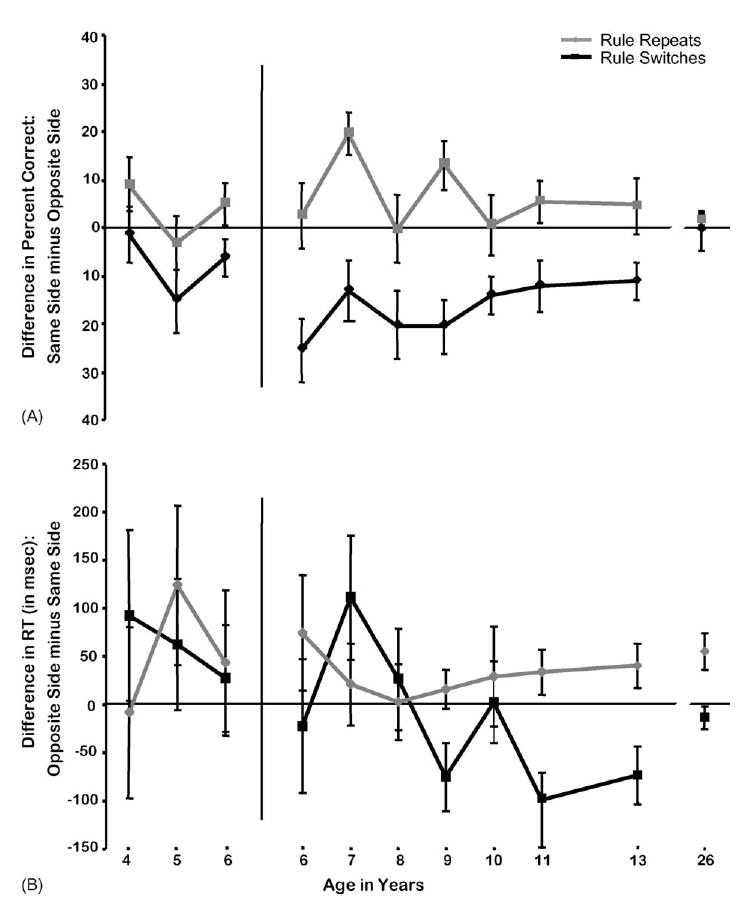
Cost of switching response locations in the Arrows task on switch trials and on nonswitch trials. (A) Difference in percent correct: opposite side minus same side and (B) difference in reaction time: opposite side minus same side.
In speed of responding the interaction of rule switch × response change on the Arrows test began to be evident at 9 years of age and was significant for adults and children of 9–13 years (RT: t[126] = 3.55, p < 0.0005). Among children younger than 9 years, however, there was a tendency for the effects of a rule switch and of a response-site change to be additive. Children younger than 9 years were slower on rule-switch trials, whether or not the correct response-site changed (main effect of a rule switch: t(163) = 6.81, p < 0.0001). Similarly, they tended to be slower on response-change trials, whether or not the rule switched. They were slowest if both rule and response changed (F(1,178) = 4.42, p < 0.0001; see Fig. 9B). The adult pattern (of faster responses when neither changed or both changed) was not seen until 9 years of age on the Arrows test.
The effect on switch trials is of most interest here because nonswitch, response-stay trials are simply repeat trials. Focusing just on switch trials, the difference in error rates on response-stay and response-shift trials was smallest for the youngest children (4–6 years of age) and young adults (see Fig. 9A). Although this difference decreased from 9 to 13 years, the difference in accuracy at 13 years was still greater than seen in young adults (F(1,42) = 4.1, p < 0.05). At all ages, this difference was for fewer errors to be made when both rule and response-site changed than when just the rule changed. This accuracy difference was most pronounced from 6 to 13 years.
The difference in response time on switch trials depending on whether the response-site changed or not decreased from 4 to 6 years when given lots of time to respond, and was insignificant among children of 6, 8, and 10 years and young adults (see Fig. 9B). Not until 9 years of age were children faster when both rule and response-site switched (t[133] = 3.58, p < 0.0005). Children of 4–8 years tended to be faster when the response-site remained the same, even on switch trials (t(163) = 1.77, p = 0.08).
16.2. Dots test: interaction of rule switching with response-site switching
When both the rule and response-site remained the same, precisely the same trial was repeated. One would expect RT to be fast then, and faster than when the response location changed. What is more interesting is that, as predicted, performance on switch trials was faster and more accurate when the correct response location also switched than when it remained the same (t(308) = 8.03, p < 0.0001 [accuracy]; 2.8, p < 0.005 [RT]; see Fig. 10). The corresponding results for only the younger subjects are: (t[89] = 4.25, p < 0.0001 (accuracy); NS (RT). The corresponding results for just the older subjects are: (t[223] = 8.25, p < 0.0001 [accuracy]; 4.05, p < 0.0001 [RT]). Indeed, from age 6 to 11 (and especially 9–11 years) the effect on accuracy was particularly large (see Fig. 10A). The adult pattern (of faster responses when neither changed or both changed) was not seen until 13 years of age on the Dots (see Fig. 10B); 4 years later than that pattern appears on the Arrows test.
Fig. 10.

Cost of switching response locations in the Dots task on switch trials and nonswitch trials. (A) Difference in percent correct: opposite side minus same side. (Across the age spectrum, and especially at 6–11 years, participants were correct on more switch trials when the response-site also switched from the previous trial.) and (B) difference in reaction time: opposite side minus same side. (The typical adult pattern of faster responding on switch trials if the response-site also switched from the previous trial, seen here and reported in numerous studies, was not evident until 13 years of age.)
On switch trials, there was little difference in children’s error rates on response-stay and response-shift trials from 4 to 7 years of age. From 8 to 10 years accuracy was far better on switch trials when the response-site also changed. The difference in accuracy was again small among young adults (see Fig. 10A). The difference in response time on switch trials depending on whether the response-site switched or not decreased from 4 to 6 years when given lots of time to respond (as it did for Arrows [compare Figs. 9B and 10B]), was large again from 6 to 8 years when given less time to respond, but not until 13 years was response time faster when both the rule and the response-site switched. Before then RTs were faster on switch trials when the response-site did not change (see Fig. 10B).
17. Discussion: interaction between rule switching and response-site switching
When the rule and response remained the same, precisely the same trial was repeated. One would expect RTs to be faster on such exact trial repetitions than on other trials. More interesting is what happens on switch trials. We predicted, consistent with the findings of others (Hommel et al., 2001; Kleinsorge, 1999; Meiran, 2000a,b; Rogers & Monsell, 1995; Schuch & Koch, 2004) and Diamond’s all-or-none hypothesis (Diamond, 2005), that performance would be better when both the rule and the response changed than when the rule changed but the response did not.
On both Arrows and Dots-Mixed, across the age spectrum, people were more accurate when both the rule and the response changed than when just the rule switched. At all ages, fewer errors were made on the Arrows test when both the rule and response-site changed than when just the rule changed, with the difference in error rate being smallest for young adults and largest for children 6–9 years of age. The difference in accuracy at 13 years was still greater than that seen in young adults. For Dots-Mixed as well, fewer errors were made when both the rule and response-site changed than when just the rule changed, with the difference in error rate being smallest for young adults and the youngest children and largest for children 8–10 years of age. Thus, we found the predicted all-or-none pattern for accuracy throughout our age spectrum.
For speed of responding, we replicated the pattern previously reported for adults: faster responses when both the rule and response switched than when only the rule switched but not the response. In children, the RT interaction of rule-switch with response-switch was not evident until the age of 9 on the Arrows task and the age of 13 on the more difficult Dots-Mixed condition. On the Arrows test, children younger than 9 years of age showed a tendency for the effects of a rule switch and response switch to be additive. Children of 4–8 years were slowest if both the rule and correct response-site changed. They were faster when the response-site remained the same, even on switch trials. Similarly, in the Dots-Mixed condition, children of 4 and 5 years given a long time to respond, and children of 6–8 years given less time to respond, were faster when the response-site remained the same whether the rule changed or not. Especially at 4 years, and at 6 years on the short-version, the RT effect of task-switching and response-switching appeared to be additive. Thus, for RT we found the predicted all-or-none pattern in older children and young adults, but for younger children we found worse RT performance on switch than nonswitch trials and on response-switch than response-stay trials and those effects tended to be additive.
18. Results: comparing across the tests that required inhibition (Pictures, Arrows, and Dots)
18.1. Comparing performance in the Mixed block of each of the tests
The Pictures test (our classic Simon task with minimized memory load) was substantially easier for children of all ages than were the Arrows or Dots-Mixed tests (spatial incompatibility tasks with higher level rules). Children showed far better accuracy, faster response times, and markedly fewer instances of anticipatory reaching in the Pictures test compared with either the Arrows or Dots tests (see Fig. 11; Pictures versus Arrows: t[293] = 13.3 [%correct], 9.42 [RT], 9.06 [AR]; Pictures versus Dots-Mixed: t[293] = 18.53 [%correct], 8.59 [RT], 12.92 [AR]; all six t-values significant at p < 0.0001). By 9 years of age, anticipatory responses had all but disappeared on the Pictures test.
Fig. 11.

Comparison of Mixed conditions of Dots, Arrows and Pictures. (A) Percent correct, (B) reaction time and (C) percentage of anticipatory responses.
Children tested in the faster presentation condition (children ≥6 years of age) found the Arrows test to be almost as difficult as the Dots-Mixed condition, judging by their comparable speed in the two conditions (see Fig. 11), though other aspects of their performance were still significantly worse on Dots-Mixed than on Arrows (children 6–13 years of age: t[203] = 4.05, p < 0.001 [accuracy]; NS [RT]; 2.72, p < 0.01 [AR]). Without question however, the difference in performance of children 6–13 years on the Pictures test compared with performance on either the Arrows test or Dots-Mixed condition was far greater than any difference in their performance on the Arrows test and the Dots-Mixed condition (Pictures minus Arrows versus Dots-Mixed minus Arrows: t[293] = 15.15 [%correct], 8.92 [RT], 11.17 [AR], all significant at p < 0.0001).
For adults and the youngest children, the results were different. Adults found the Pictures and Arrows tests to be of comparable ease and found both of those conditions to be significantly easier than the Dots-Mixed condition (see Fig. 11; Dots-Mixed versus Pictures: t(20) = 7.47 [RT]; Dots-Mixed versus Arrows: t(20) = 7.21 [RT], both p < 0.0001; no comparisons between Pictures and Arrows yielded any significant results). Thus, while performance on Arrows and Dots was roughly comparable for children of 6–13 years, performance on Arrows and Pictures was comparable for adults.
While children of 4–6 years, tested with the longer stimulus presentation times, found the Pictures test to be easier than the Arrows test or Dots-Mixed condition as did older children, children of 4–6 years (unlike older children) found the Arrows test to be much easier than the Dots-Mixed condition (see Fig. 11). All pair-wise comparisons between any two of the three tests were significant except for Arrows versus Dots-Mixed on RT, and all those were significant at p < 0.0001, except for Pictures versus Arrows on AR [t(59) = 2.6, p < 0.01] and Arrows versus Dots-Mixed on AR [t(59) = 3.8, p < 0.005]. Thus, for the youngest children no two tests were comparable in difficulty. The Dots-Mixed condition was significantly harder than the Arrows test and the latter was significantly harder than the Pictures test (except that in their RT data they showed the same pattern as older children [comparable performance on the Arrows and Dots-Mixed tests with much faster responses in the Pictures test]).
18.2. Comparing performance across conditions that differed in their demands on inhibition but required little or no memory
Comparing performance in the Pictures test and the Dots-Congruent condition enabled us to compare performance in the presence versus absence of an inhibitory demand with memory load held relatively constant. Both the Pictures test and the Dots-Congruent condition required holding two rules in mind. (In the Pictures test that memory demand could be minimized by referring to the visible icons that showed which picture was mapped to the left or right. In the Dots-Congruent condition, the memory demand could be minimized by remembering the single higher-order rule.) While the memory demand was roughly equivalent in Pictures and Dots-Congruent, the former required inhibiting the tendency to make the spatially compatible response on half the trials whereas the spatially compatible response was always the correct response in the Dots-Congruent condition (no inhibition required). Thus more inhibitory control was required in the Pictures test than in the Dots-Congruent condition. Across all ages, performance was consistently better in the Dots-Congruent condition than in the Pictures test on all dependent measures (all subjects: t[313] = 23.16 [% correct] and 24.0 [RT]; 2.31; subjects ≥6 years: t[223] = 13.9 [%correct] and 27.82 [RT]; subjects ≤6 years: t[89] = 4.16 [%correct] and 10.26 [RT]; all p < 0.0001; see Table 2). The difference in accuracy on Dots-Congruent versus Pictures decreased over the age range of 6–26 years (F(1,222) = 5.32, p < 0.02).
Another way to assess inhibitory costs, and change in their size over age, is to look at the cost of steady-state inhibition (consistently inhibiting the prepotent response in Dots-Incongruent) versus consistently making the prepotent response in Dots-Congruent. These results were presented above (see Fig. 2 and Table 3). These costs (in both speed and accuracy) were significant for children at all ages, including the oldest children (13 years old), but were not significant for adults. The accuracy difference between the Dots-Congruent and -Incongruent conditions decreased over age, while the RT on Dots-Incongruent as a percentage of RT on Dots-Congruent remained constant (see Fig. 6).
A third way to assess the relative cost of increasing inhibitory demands is to compare performance on the Arrows task (which required inhibition on the half the trials [the spatially incompatible ones] and required task-switching if encoded as two superordinate rules, but required little memory as each stimulus pointed to its correct response-site) to performance (a) where inhibition of the spatially compatible response was required on all trials (rather than switching between spatially compatible and incompatible trials—Dots-Incongruent) or (b) where inhibition of that response was never required (Dots-Congruent). Inhibiting the spatially-compatible response some of the time despite the minimal memory requirements (in the Arrows task) took a greater toll on speed and accuracy at every age than did inhibiting the spatially-compatible response on all trials (Dots-Incongruent, see Table 2), though those differences were of course smaller than that between Arrows and Dots-Congruent (where no inhibition was required). Accuracy differences between the Arrows task and Dots-Incongruent condition were greatest at intermediate ages (children of 6–11 years tested in the faster condition) and smallest among the youngest children (4–6 years, given a much longer time to respond) and among the two oldest groups (13-year-olds and adults). Accuracy differences between Arrows and Dots-Congruent were sizeable at all ages except among young adults and decreased significantly from 7 to 26 years (F(1,192) = 15.73, p = 0.0001). Differences in response speed in the Arrows test and Dots-Incongruent condition were roughly 200 ms at all ages, except among 4-year-olds, where the mean difference was only 100 ms (all subjects: t[313] = 14.76; subjects ≥6 years: t[223] = 15.76; subjects ≤6 years: t[89] = 6.27; all p < 0.0001). Mean RT differences between Arrows and Dots-Congruent were roughly 350 ms or more for the youngest children (4–6 years, given a long time to respond) and decreased linearly from 325 ms at 6 years (adult condition) to 180 ms among young adults (except for a spike at 10 years; all subjects: t[313] = 27.00; subjects ≥6 years: t[223] = 31.19; subjects ≤6 years: t[89] = 12.49; all p < 0.0001; significant decrease from 6 years to young adulthood: F(1,222) = 5.32, p < 0.0001).
Finally, comparing performance in the Pictures condition to the Dots-Incongruent condition, like the comparison of Arrows to Dots-Incongruent, provides a measure of (a) inhibition in a switching context where it is only required on some trials (with little or no memory requirement) versus (b) inhibition in a steady-state context where it is required on every trial (and memory of a higher order rule and instantiating it on each trial are required). Accuracy was comparable in these two conditions across all ages but when given only 1250 ms to respond subjects consistently responded faster in Dots-Incongruent than in Pictures (all subjects: t[313] = 7.2, p < 0.0001; only those ≥6 years: t[223] = 8.57, p < 0.0001; only those ≤6 years: t[89] = 1.9, p = 0.06; see Table 2). The RT difference (faster in Dots-Incongruent than in Pictures) tended to increase over age from 6 years through young adulthood (F(1,222) = 3.44, p = 0.065) and was over twice as large among young adults as among 6-year-olds.
19. Discussion: comparing performance on the tests that required inhibition (Pictures, Arrows, and Dots)
When minimal memory was required and no task switching, very young children were reasonably successful at overcoming the prepotent tendency to respond on the same side as the stimuli consistently on all trials (Dots-Incongruent; 86% correct at 4–5 years) and on half the trials as long as the rules did not change (Incongruent trials in the Pictures task; 80% correct at 4–5 years). On the Dots-Incongruent task the youngest children were able to perform at a relatively high level when required to combine (a) holding a superordinate rule in mind (mentally translating that into the appropriate embedded rule on each trial) plus (b) inhibiting the dominant tendency to respond on the same side as a stimulus, but importantly inhibition was required in steady-state and the rule remained constant. They had to exercise that inhibition on every trial, not switching back and forth between sometimes exercising it and sometimes not. In the Pictures task, there were no higher-order rules to mentally instantiate. Only two stimulus–response associations were relevant and memory demands were minimized by having a picture of each stimulus mounted immediately above its associated response button. Critically, the rules never changed. However, inhibition rather than being continuously required, was needed on only half the trials. The performance of 4- to 5-year-olds on the Pictures task indicates that they could obey two stimulus–response rules even though that meant switching between sometimes responding on the Congruent side (the same side as the stimulus) and sometimes on the Incongruent side.
Since it is harder to switch back and forth between inhibiting a dominant response and making it than to consistently inhibit that response, we had predicted that performance at all ages would be better in Dots-Incongruent than in Arrows (which required switching, but minimized memory demands). That prediction was confirmed. Accuracy was better and speed faster at every age in the Dots-Incongruent condition than on the Arrows test. Thus, inhibiting the spatially-compatible response some of the time even when the stimuli pointed to the correct response (in the Arrows task) took a greater toll on speed and accuracy at every age than did inhibiting the spatially-compatible response all the time (Dots-Incongruent). We had also predicted that performance differences between Dots-Incongruent and Arrows would decrease over age as cognitive flexibility improved. While the accuracy difference between these conditions was smaller in adults, otherwise the markedly better performance on Dots-Incongruent than Arrows was equally true across all ages, contrary to our prediction.
20. Results: the Abstract Shapes test: conditions that differed in their demands on memory but required little or no inhibition
The Abstract Shapes test contained two conditions (two shapes and six shapes), designed to vary working memory load (two arbitrary rules versus six). The inhibition requirement was minimal, as all shapes were presented at central fixation (no spatial incompatibility). As predicted, the six-shape condition was significantly harder than the two-shape condition (all subjects: t[313] = 13.60 [accuracy], 20.36 [RT], and 6.49 [AR], all significant at p < 0.0001; subjects ≥6 years, 1250-ms ISI: t[223] = 12.27 [accuracy], 20.49 [RT], and 6.39 [AR], all significant at p < 0.0001; subjects ≤6 years, 3000-ms ISI: t[89] = 6.16, p < 0.0001 [accuracy], 9.89, p < 0.0001 [RT], and 2.48, p < 0.02 [AR]; see Fig. 12). Both conditions showed age-related improvements in performance on all three dependent measures. For two shapes, age-related improvement was significant on all three dependent measures at p < 0.0001 for subjects ≥6 years and for all the subjects together (subjects ≥6 years: F(1,222) = 21.87 [accuracy], 78.70 [RT], and 16.06 [AR]; all subjects: F(1,312) = 26.84 [accuracy], 140.13 [RT], and 39.10 [AR]). For children 4–6 years of age there was no difference in speed in the two-shapes condition over age, but the improvements in accuracy and impulsivity were significant (accuracy: F(1,88) = 5.13, p < 0.03; AR: F(1,88) = 12.75, p < 0.001). Results are similar for the six-shapes condition; age-related improvement was significant at p < 0.0001 for all subjects together and for those ≥6 years of age, except on RT which was significant at p < 0.0005 for those ≥6 years (subjects ≥6 years: F(1,222) = 30.48 [accuracy], 12.72 [RT], and 24.79 [AR]; all subjects: F(1,312) = 29.64 [accuracy], 81.11 [RT], and 39.56 [AR]). For the youngest participants there was no significant age difference in speed on the six-shapes condition, but accuracy and impulsivity showed significant improvements over age (accuracy: F(1,88) = 11.32, p < 0.001; AR: = 4.88, p < 0.03), as was found for the two-shapes condition.
Fig. 12.
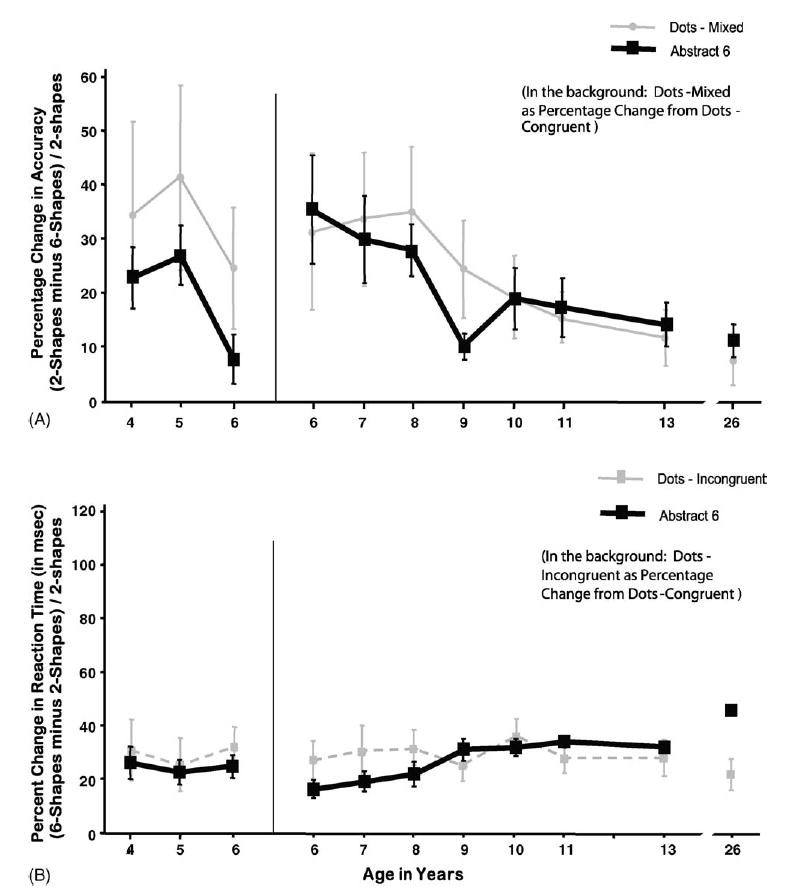
The six-Abstract-Shapes condition as percentage change from the two-Abstract-Shapes condition. (A) Percent change in accuracy-(two-shape minus six-shapes) divided by two-shapes (in the background: Dots-Mixed as percentage change from Dots-Congruent) and (B) percent change in reaction time-(two-shape minus six-shapes) divided by two-shapes (in the background: Dots-Incongruent as percentage change from Dots-Congruent).
To test whether there was more change in performance over age in the six-shapes condition than in the two-shapes condition, difference scores were calculated for each participant (performance in six-shapes minus two-shapes) for each of the dependent measures. None of these difference scores (for accuracy, speed, or anticipatory responses) varied significantly as a function of age when all subjects were included in the analyses. The degree to which the six-shapes condition was more difficult than the two-shapes condition generally did not change over age. This suggests that although participants of all ages were affected by the increased memory load (i.e., all showed positive difference scores) the size of this effect changed little over age.
The magnitude of the difference in performance on the six-versus two-shapes conditions showed no significant change over age on any of the three dependent measures when all subjects were included or only the youngest children were used. However, for the 6-year-old through young-adult subjects, the speed-accuracy tradeoff seemed to vary by age: Accuracy on the six-shapes condition more closely approximated that on the two-shape condition in older subjects [9 years through adults versus 6–8 years old: F(1,162) = 7.39, p < 0.01] while the difference in RT on the two conditions showed an opposite tendency, with a smaller RT difference between the two conditions in younger subjects [9 years through adults versus 6–8 years old: F(1,162) = 11.73, p < 0.001].
Performance in the six-shape condition can also be viewed as a percentage change from performance in the two-shape condition ([six-shapes minus two-shapes] divided by two-shapes). Overall, and for the older subjects, there was no significant change over age in the accuracy difference between the six-shape and two-shape conditions as a percentage of accuracy in the two-shape condition. For the youngest children (4–6 years, tested with 2500-ms stimulus presentation time) the error rate on six-shapes declined over age and so the percentage change in accuracy on the six-shape condition compared to the two-shape condition declined over this age range (F(1,89) = 2.65, p < 0.0001)—showing the same pattern as seen for percentage change in accuracy on Dots-Mixed compared with Dots-Congruent though the difference for the latter was larger and remained larger (see Fig. 12A). Indeed, the size of the differences, as well as the pattern, among the older subjects for six-shapes compared to two-shapes was similar to that for Dots-Mixed compared to Dots-Congruent except that the difference between six- and two-shapes was particularly small among 9-year-olds and hence the linear trend did not reach significance (see Fig. 12A).
For speed of responding, a different picture emerged. Whereas change over age in the difference between accuracy in the six- and two-shape conditions as a percentage of two-shapes performance was significant only for the younger children, the reverse was true for the RT difference. The RT difference in the two conditions as a percentage of speed in the two-shapes condition changed significantly overall and among subjects ≥6 years, but not among the younger children (all subjects: F(1,312) = 29.39, p < 0.0001; subjects ≥6 years: F(1,222) = 38.10, p < 0.0001). Further, whereas the percentage change in accuracy in six-Abstract-Shapes over age resembled that on Dots-Mixed, the percentage change in RT on six-Abstract-Shapes over age resembled that on Dots-Incongruent (see Fig. 12B). The change over age in speed on Dots-Mixed as a percentage of Dots-Congruent dwarfed the age-related change in six-Abstract-Shapes as a percentage of two-Abstract-Shapes or Dots-Incongruent as a percentage of Dots-Congruent. Finally, whereas the accuracy difference between six- and two-shapes decreased over age, the difference in response speed on the two conditions increased over age, as older subjects preserved their accuracy in the harder condition by sacrificing their speed (see Fig. 12).
21. Discussion: the Abstract Shapes test: conditions that differed in their demands on memory but required little or no inhibition
We had predicted that even very young children would perform well at holding two rules in mind when inhibition is not taxed. Consistent with that prediction, the performance of even our youngest subjects was excellent in the two-Abstract-Shapes condition.
Based on our hypothesis that the ability to hold items in mind matures early, we had predicted that although it would be harder for everyone to hold more items in mind than fewer, the relative difficulty of that would not change over age. Overall within-subject difference scores (six-Abstract-Shapes versus two) did not show any change over age in relative difficulty on any dependent measure. How to answer whether the relative difficulty changed over age is not straightforward, however, because how the difference in difficulty was handled changed over age, i.e., the speed-accuracy tradeoff changed over age. Accuracy in the six-shapes condition more closely approximated that on the two-shape condition in older subjects (9-year-olds through young adults) while the difference in speed of responding in the two conditions showed the opposite tendency, with a greater RT difference between the two conditions in the same older subjects. This suggests that older subjects preserved their accuracy in the harder six-shapes condition by sacrificing their speed. Hence the speed differential between the two conditions was largest for these subjects but the accuracy differential was smallest.
Another measure of whether the relative difficulty of these two conditions changes over age might be performance on six-Abstract-Shapes as a percentage change from performance on two-Abstract-Shapes (thus correcting for differences in baseline performance). In general, there was no significant change over age in the accuracy difference between the six-shape and two-shape conditions as a percentage of accuracy in the two-shape condition. However, for the youngest children (4–6 years) the percentage change in accuracy on the six-shape condition relative to the two-shape condition declined over age (this same pattern was seen for percentage change in accuracy on Dots-Mixed compared with Dots-Congruent though the difference between the Dots conditions was larger and remained larger).
Whereas change over age in accuracy on six-Abstract-Shapes as a percentage change from performance on two-Abstract-Shapes was significant only for the youngest children, the reverse was true for the RT difference. Here, again, the change over age in speed on Dots-Mixed as a percentage of Dots-Congruent dwarfed the age-related change in six-Abstract-Shapes as a percentage of two-Abstract-Shapes.
22. Results: comparison of performance on the Abstract Shapes test and the other tests
22.1. First-order comparisons among conditions
The easiest condition of all, across all ages, was Dots-Congruent (see Table 2). Accuracy was consistently highest and RT consistently quickest in that condition at all ages. Indeed, accuracy and speed were significantly better (in all cases at p < 0.0001) in the Dots-Congruent condition than on the three next easiest conditions, two-Abstract-Shapes, Pictures, and Dots-Incongruent, with all participants included in the analyses, only the older subjects, or only the youngest subjects (see Table 4).
Table 4.
T values for planned comparisons between experimental conditions
| Percentage of correct responses | Response speed | |
|---|---|---|
| The three of the four easiest conditionsa | ||
| Dots-Congruent vs. two-Abstract-Shapes | ||
| All subjects (d.f. = 1,313) | 13.52 | −19.78 |
| Younger subjects (4–6 years; d.f. = 1,89) | 12.54 | −26.91 |
| Older subjects (6–26 years; d.f. = 1,223) | 5.72 | −6.29 |
| Dots-Congruent vs. Pictures | ||
| All subjects (d.f. = 1,313) | 13.20 | −24.00 |
| Younger subjects (4–6 years; d.f. = 1,89) | 13.91 | −27.84 |
| Older subjects (6–26 years; d.f. = 1,223) | 4.23 | −10.31 |
| The three hardest conditionsb | ||
| Dots-Mixed vs. six-Abstract-Shapes | ||
| All subjects (d.f. = 1,313) | −6.24 | 2.46 (p < 0.01) |
| Younger subjects (4–6 years; d.f. = 1,89) | −4.22 | 1.30 NS |
| Older subjects (6–26 years; d.f. = 1,223) | −4.78 | 2.24 |
All significant at p < 0.0001, unless otherwise noted.
Dots-Incongruent was the other very easy condition. For performance on Dots-Congruent vs. Dots-Incongruent, see Table 3. Accuracy on two-Abstract-Shapes, Pictures, and Dots-Incongruent was fully comparable. Response speed was faster on two-Abstracts-Shapes than on Pictures (all three comparisons significant at p < 0.0001) and on Dots-Incongruent than Pictures (for all subjects and older subjects, p < 0.0001; for younger subjects, p = 0.06). Younger children were faster on Dots-Incongruent than two-Abstract-Shapes (t[89] = 3.54, p < 0.001), while our older subjects were faster on two-Abstract-Shapes than on Dots-Incongruent (t[223] = −2.46, p < 0.02).
Arrows was the other relatively difficult task. For Dots-Mixed vs. Arrows, see the section comparing performance in the three Mixed conditions. There were no significant differences in either speed or accuracy on six-Abstract-Shapes and Arrows.
Across all ages, accuracy on the two-Abstract-Shapes condition, the Pictures test, and the Dots-Incongruent condition was excellent and fully comparable (see Fig. 13A and Table 2). RT (as opposed to accuracy) was better on the two-Abstract-Shapes and Dots-Incongruent conditions than on the Pictures test (see Table 2; RT on two-Abstract-Shapes versus Pictures: all subjects: t[313] = 10.55; only those ≥6 years: t[223] = 8.82; only those ≤6 years: t[89] = 6.81; all p < 0.0001; RT on Dots-Incongruent versus Pictures: see above).
Fig. 13.
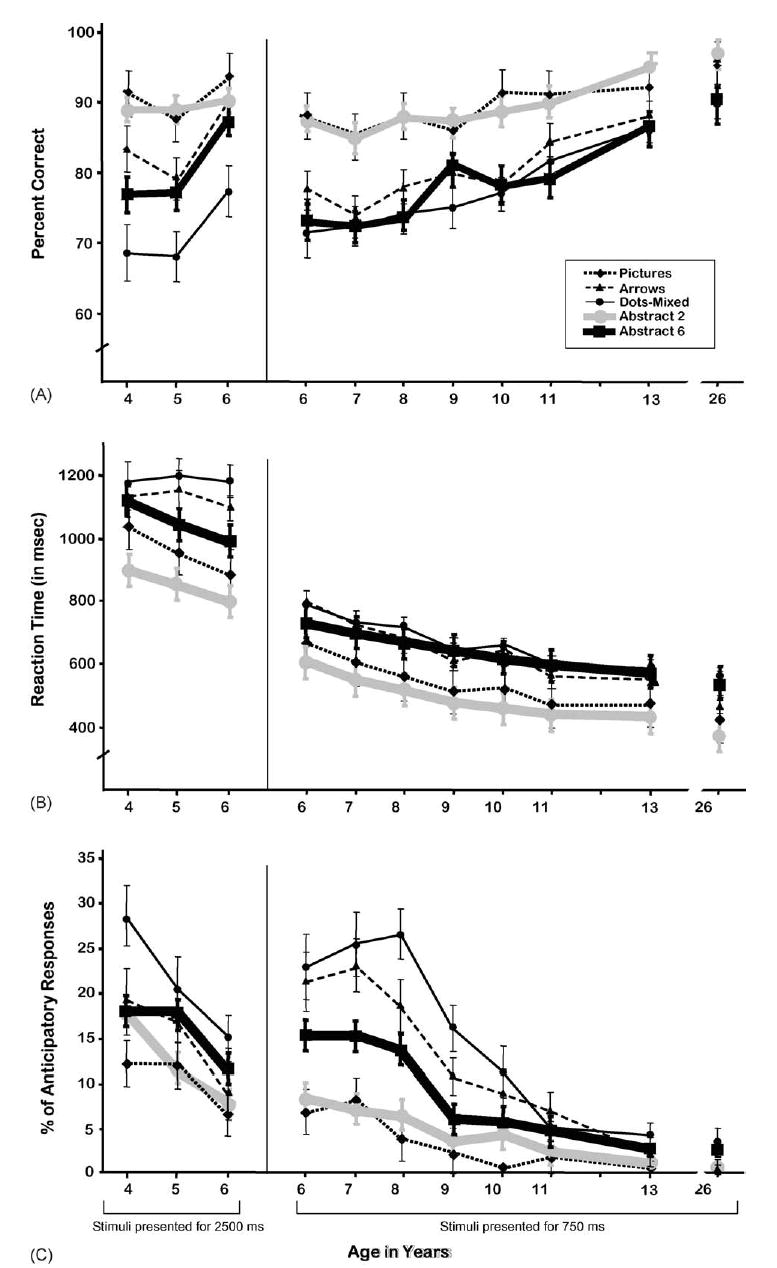
Comparisons of the two-Abstract-Shapes conditions with each other and with all the other tasks.
Despite significant differences in performance among these conditions, these four conditions (Dots-Congruent, two-Abstract-Shapes, Dots-Incongruent, and Pictures) clearly proved the easiest for participants. Across the age span, accuracy was intermediate on the Arrows test and the six-Abstract-Shapes condition between those four conditions, on the one hand, and Dots-Mixed condition, on the other (significantly worse performance on the Arrows test than on the four easier tests: all subjects: t[313] = 16.42 [accuracy] and 19.15 [RT]; only those ≥6 years: t[223] = 15.41 [accuracy] and 20.36 [RT]; only those ≤6 years: t[89] = 6.68 [accuracy] and 9.30 [RT]; all p < 0.0001; significantly better performance on Arrows than on Dots-Mixed, see above; significantly worse performance on the six-Abstract-Shapes condition than on the four easier tests: all subjects: t[313] = 18.57 [accuracy] and 23.73 [RT]; only those ≥6 years: t[223] = 16.89 [accuracy] and 23.66 [RT]; only those ≤6 years: t[89] = 8.26 [accuracy] and 10.15 [RT]; all p < 0.0001).
Although no inhibitory or task-switching demands were present in the six-Abstract-Shapes condition, holding six arbitrary rules in mind for hard-to-name, abstract stimuli was challenging at all ages. However, no test, not even six-Abstract-Shapes, was as difficult as the Dots-Mixed condition (where subjects had to hold two superordinate rules in mind, instantiate them on each trial, inhibit a prepotent response tendency on incompatible trials, and switch between same-side and opposite-side rules) all subjects: t(313) = 6.2 (accuracy) and 2.4 (p < 0.04; RT); only those ≥6 years: t(223) = 4.2 (accuracy) and NS (RT); only those ≤6 years: t(89) = 4.78 (accuracy) and 2.15 (p < 0.01;RT); all p < 0.0001 except where otherwise noted; see Fig. 13 and Tables 2 and 4).
Performance on the Arrows test was roughly comparable to that in the six-Abstract-Shapes condition in both speed and accuracy. Accuracy on both was significantly better than accuracy in the Dots-Mixed condition (see Fig. 13A). RT on the Arrows test was generally intermediate between that on the six-Abstract-Shapes and Dots-Mixed conditions, especially between 4 and 8 years of age (see Table 2), not significantly different from either. Thus, the task that taxed memory most heavily and included no inhibitory or task-switching component (six-Abstract-Shapes) proved approximately equivalent in difficulty at all ages to the task that taxed memory only minimally (since each stimulus pointed to its correct response), but required inhibition on incompatible trials and task-switching when subjects used two hierarchical rules (though one superordinate rule could be used instead).
22.2. Difference-score analyses of the relative costs of increasing memory or inhibitory demands
For the youngest children (4–5 years old), inhibitory demands even in steady-state, took a greater toll on RT than did memory demands. Their RT difference on Dots-Incongruent versus Dots-Congruent (which differed only in their inhibitory requirements) was greater than their RT difference on two-versus six-Abstract-Shapes (which differed only in their memory requirements; subjects 4–5 years old: t[59] = 2.33, p < 0.03; see Table 2). That was very surprising because the inhibitory demand in Dots-Incongruent feels rather minimal to adults while the memory demand in six-Abstract-Shapes feels quite substantial. For older children ≥8 years and adults’ memory took a greater toll on RT than did inhibition (subjects ≥8 years: Dots-Incongruent minus Dots-Congruent versus six-Abstract-Shapes minus two-Abstract-Shapes, within-subject t[163] = 5.32, p < 0.0001). The individual age group for which this comparison between difference scores was most highly significant was young adults (t[19] = 10.5, p < 0.0001).
The size of the differences in the percentage of correct responses (Dots-Congruent minus Dots-Incongruent compared with two-Abstract-Shapes minus six-Abstract-Shapes) did not differ significantly over the age range of 4–9 years. However, beginning at 10 years, increased memory demands (six versus two rules) took a greater toll on accuracy than did consistently inhibiting the tendency to respond on the same side as the stimulus (subjects ≥10 years: t[103] = 3.62, p < 0.0005).
Another way to look at the relative cost of increasing inhibitory demands is to compare performance on the Arrows task to that on Dots-Incongruent or Dots-Congruent. At all intermediate ages (6–11 years, all tested under adult conditions), having to switch between inhibiting and not (Arrows) versus settling in to exercising inhibition on all trials (Dots-Incongruent) tended to take a greater toll on accuracy and speed than did having to hold six arbitrary rules in mind rather than two (subjects 6–11 years, adult conditions: t[203] = 1.72, p = 0.08 [%correct]; 1.88, p = 0.06 [RT]). For the youngest subjects (4–6 years) given much longer to respond and for the two oldest age groups (13-year-olds and young adults), the accuracy and RT costs were comparable. At all ages, the RT costs for having to exercise inhibition in the Arrows task versus not having to do so in the Dots-Congruent condition took a much greater toll on response speed than did increasing the memory load from two to six arbitrary rules (all subjects: t[313] = 11.37; only those ≥6 years: t[223] = 12.70; only those ≤6 years: t[89] = 5.12; all p < 0.0001). A larger accuracy difference between Arrows versus Dots-Congruent than between two- versus six-Abstract-Shapes, however, was only true for the younger two-thirds of the subjects (subjects ≤9 years: t[210] = 6.65, p < 0.0001). This was not significant for the older subjects; indeed for adults there was almost a trend in the reverse direction (see Table 2).
22.3. Correlations between performance on memory-demanding and inhibition-demanding conditions
If working memory and inhibition are independent then one might expect little relation between performance on the two-Abstracts-Shapes condition (that requires little or no inhibition) and the Pictures test (that requires little or no memory), despite their relatively equivalent levels of difficulty judging by subjects’ performance. Contrary to our predictions, performance on the two was highly correlated, even after including age in the partial correlation analyses. This was especially true for speed of responding. The correlation between the two-Abstracts-Shapes condition and the Pictures test, controlling for age, was roughly twice as high for speed as it was for accuracy (all subjects: r[313] = 0.37 [accuracy] and 0.82 [RT]; only those ≥6 years: r[223] = 0.32 [accuracy] and 0.67 [RT]; only those ≤6 years: r[90] = 0.44 [accuracy] and 0.65 [RT]; all significant at p < 0.0001).
Similarly, if working memory and inhibition are independent then one might expect little relation between performance on the six-Abstracts-Shapes condition (which heavily taxed memory but required little or no inhibition) and the Arrows task (which taxed inhibition, but required little memory), even though both presented relatively equivalent levels of difficulty judging by subjects’ performance. The partial correlations were high, though not quite as high as those for two-Abstract-Shapes and Pictures, and were again higher for RT than for accuracy (all subjects: r[313] = 0.30 [accuracy] and 0.74 [RT]; only those ≥6 years: r[223] = 0.31 [accuracy] and 0.45 [RT]; all four significant at p < 0.0001; only those ≤6 years: r[89] = 0.24, p < 0.01 [accuracy] and 0.43, p < 0.001 [RT]).
23. Discussion: comparison of performance on the Abstract Shapes test and the other tests
We predicted that the most difficult condition at all ages would be the one that taxes inhibition and memory in a switching context (Dots-Mixed), and that at all ages that would be even more difficult than holding more information in mind (six rules) but without an inhibition or switching component (six-Abstract-Shapes). Consistent with our prediction, we found that Dots-Mixed was indeed the hardest condition for participants of all ages and showed the longest developmental progression. At every age, even holding six arbitrary associations in mind between responses and stimuli that did not easily lend themselves to verbal labels was easier than holding two superordinate rules in mind and switching randomly between the rule for making a prepotent response and the rule for inhibiting that to make the opposite response. Even young adults found Dots-Mixed to be the most difficult condition and their performance was worse there than on even six-Abstract-Shapes. Accuracy did not differ between Dots-Mixed and six-Abstract-Shapes, but beginning as early as 5 years of age, participants could perform at that accuracy level in six-Abstract-Shapes going at a faster pace than they could in Dots-Mixed and at the cost of fewer anticipatory response errors.
Because we hypothesized that memory and inhibition are independent functions, we had predicted that performance on the memory-alone conditions of the Abstract Shapes task would not be highly correlated with performance on conditions that primarily taxed only inhibition. We had predicted this would be true both for quite easy and very difficult tasks matched on difficulty. Our prediction of weak correlations between conditions that primarily taxed working memory and conditions that primarily taxed inhibition, matching those conditions on difficulty, was not confirmed. Our basic Simon task (Pictures) with visible memory aids taxed inhibition while placing little or no demand on memory. We predicted it would be roughly as easy as remembering the rules for two-Abstract-Shapes when no inhibition was required (our two-Abstract-Shapes condition) but that performance on Pictures and two-Abstract-Shapes would not be highly correlated. As predicted, these two conditions were indeed roughly matched in difficulty and both relatively easy. However, contrary to our prediction, their correlation was 0.82 for speed and 0.37 for accuracy.
Similarly, the six-Abstracts-Shapes condition (which required memory but little or no inhibition) and the Arrows test (which required inhibition but little or no memory) were roughly matched in difficulty and both relatively difficult. Contrary to our prediction, they were correlated 0.74 for speed and 0.30 for accuracy. Subjects who were better at exercising inhibition also tended to be better at holding information in mind and this was especially true for how fast they could execute their responses.
We also predicted that differences in inhibitory demands would matter more for young children and differences in memory demands would matter more for young adults. Findings consistent with that prediction include that the difference in accuracy between Arrows versus Dots-Congruent (which differ in inhibitory demands) was larger for younger children than the accuracy difference between two- versus six-Abstract-Shapes (which differ in their memory demands), with a trend in the reverse direction being found for adults. Other evidence that inhibition appears to have been more difficult for younger children than holding information in mind can be seen in the greater RT cost exacted by inhibitory demands even in steady-state than memory demands for the youngest children (4–5 years old). For example, the RT difference on Dots-Incongruent versus Dots-Congruent (which differ only in their inhibitory demands) was greater for the youngest children than their RT difference on two-versus six-Abstract-Shapes (which differ only in their memory requirements). Consistent with our prediction that this would reverse with age, beginning at age 10 years, increased memory demands (six versus two rules) took a greater toll on accuracy than did consistently inhibiting the tendency to respond on the same side as the stimulus (Dots-Incongruent versus Dots-Congruent).
24. Results: effect of greater presentation time
Comparison of the performance of the two groups of 6-year-olds (tested with the two presentation times) showed that children of 6 years were able to get significantly more responses correct, and made significantly fewer anticipatory responses, when they had more time to view the stimuli and compute their responses.
Often, the 6-year-olds allowed 2.5 s to view each stimulus and up to 3 s to compute their responses performed at the level of children 3–4 years older (children 9–10 years of age) who were given only 0.75 s to view each stimulus and up to 1.25 s to compute their responses. This was true for the size of the spatial incompatibility effect on accuracy in the Pictures task (Fig. 3A), the size of the spatial incompatibility effect on accuracy averaged over the Pictures, Arrows, and Dots tasks (Fig. 11A), the incidence of anticipatory responses in the Arrows test (Fig. 11C), percentage of correct responses in the Incongruent and Mixed blocks of the Dots test (Fig. 3A), and the incidence of anticipatory responses in the Mixed block of the Dots test (Fig. 3C).
Indeed, on some measures the 6-year-olds given more time to view the stimuli and compute their answers performed better than children of even 11 or 13 years tested with shorter stimulus presentation times. The accuracy of 6-year-olds tested with the longer stimulus presentation time was comparable to that of 13-year-olds given less time on the Pictures test (Fig. 11A), the Arrows test (Fig. 11A), and six-Abstract-Shapes test (Fig. 13A). In the interaction of rule switching and response switching on accuracy in the Arrows and in the Dots test, the 6-year-olds given more time showed a pattern that more closely approximated that seen in adults than did children of any other age (Figs. 9A and 10A). The accuracy of these 6-year-olds in remembering six arbitrary stimulus–response associations compared with their accuracy in remembering only two showed less of a cost than did that of even young adults (Fig. 12A).
In most cases the 6-year-olds tested at the shorter stimulus presentation times performed roughly comparable to the children of 4 and 5 years given more time. The one and only place that no advantage was found for giving 6-year-olds more time and no cost was found for giving them less time was on the two-Abstract-Shapes task, where time to view the stimuli and determine their response had no effect on performance.
25. General discussion
We investigated the development, and interactions over development, of inhibitory control, memory, and task switching. Our computerized battery included tasks designed to manipulate demands on retaining, and working with, information held in mind and/or inhibition, independently and together, in single-task and in task-switching contexts. The ability to inhibit attention to distractors makes possible selective and sustained attention. The ability to inhibit a strong behavioral inclination helps make change possible, as well as social politeness. Inhibition, thus, provides us a measure of control over our attention and actions. External stimuli and engrained behavioral tendencies exert strong influences on our behavior, but inhibition permits us the possibility to act otherwise. The ability to hold and manipulate information in mind makes it possible for us to remember our plans and others’ instructions, relate one thing to another, including relating the present to the future and the past, and to act on the basis of information not perceptually present. Cognitive flexibility is critical in a changing world. It is essential for adaptability and for the creativity that comes from being able to see things in new or different ways.
25.1. How our predictions concerning memory and inhibition fared
We predicted that inhibitory demands would account for a greater proportion of the variance in children’s performance than in adults, and the more so the younger the child. Consistent with our prediction, Dots-Incongruent (where inhibition of the spatially-compatible response was required on all trials) was more difficult than Dots-Congruent (where the spatially-compatible response was correct on all trials) and the more so the younger the children. Accuracy and impulsivity differences between those two conditions decreased over age. The RT difference on Dots-Incongruent versus Dots-Congruent (which differed only in their inhibitory demands) was greater for the youngest children than the RT difference on two- versus six-Abstract-Shapes (which differed only in their memory requirements). This finding surprises adults who have taken our task battery because for adults the inhibitory demand in Dots-Incongruent feels rather minimal while the memory demand in six-Abstract-Shapes feels quite substantial. However, this is fully consistent with greater costs being exacted by inhibitory demands even in steady-state than memory demands for the youngest children. Also consistent with this prediction, we found that the spatial incompatibility effect (the cost of inhibiting the pull to respond on the same side as the stimulus) was greater the younger the children. This suggests that the younger the children, the harder it was for them to muster inhibition at either (a) the level of attention to disregard an irrelevant aspect of the stimulus (its spatial location) and/or (b) the level of response to override the prepotent tendency to respond on the same side as the stimulus.
We looked at the spatial incompatibility effect in the context of lower- and higher-order rules, different memory loads, and in the context of task-switching. While in the Pictures test, the typical low-level rules pertaining to individual stimuli were used and memory demands were minimized by the use of icons over the response-sites, we also investigated the spatial-incompatibility effect in hybrid, conceptual tasks (Arrows and Dots) where the rules were more abstract, spatial location had to be integrated with stimulus identity, and no icons were provided to remind subjects of the stimulus–response mappings. The working memory requirements were greater for these later tasks because they required mental computation to determine the correct response. Instead of the rule being “for A press left” (the typical rule on Simon tasks, which requires attending only to the stimulus or a particular property of the stimulus), the rule for the Arrows and Dots tasks was “for A press on the side opposite A.” Knowledge of only which stimulus appeared or only where it appeared was insufficient on these tasks; those two pieces of information had to be integrated. The Arrows and Dots tasks differed from each other in that the memory demands were minimal on the Arrows task because the stimulus pointed to the correct response on each trial.
In the Pictures task spatial incompatibility effects were significant for both RT and accuracy for children of all ages, produced the greatest effect on RT, and decreased more in size as a function of age than on the Arrows or Dots tasks. Even at 13 years of age, children still showed a significantly greater Simon effect on the Pictures task than did young adults. The spatial incompatibility effect was weakest and showed the least change over age in the Dots task, even though in the Dots and Arrows tasks the spatial location of the stimuli had to be explicitly taken into account and on the Pictures task it did not. The Dots-Mixed condition was the only task in which the spatial incompatibility effect was not evident in both RT and accuracy. In Dots-Mixed, the spatial incompatibility effect was evident only in RT, its effect on RT was weaker for the youngest children (4–6 years old) and adults than in the Arrows or Pictures tests, and the size of its effect on RT did not change significantly over age. The Arrows condition produced a significant spatial incompatibility effect on both RT and accuracy, and a decrease in the size of the effect on accuracy (though not on RT) over age. These results – a stronger spatial incompatibility effect the easier the task – are consistent with results in the literature showing that this effect decreases as a function of task difficulty (Hommel, 1993, 1994; Vu & Proctor, 2004). However, those results have previously been interpreted to mean that anything that increases response time will decrease the Simon effect (giving the automatic activation of the irrelevant stimulus location information time to decay). In contrast, we found that although younger children took much longer to respond than older children, they showed a larger spatial incompatibility effect.
The larger Simon effect found for the younger children might indicate that their ability to exercise inhibition of the pull to respond on the same side as the stimulus was weaker than that of older children. It may have also been affected by the greater likelihood of younger children to use verbal mediation. Though the Pictures task could be solved by simple perceptual matching, some younger children named the stimulus out loud on some trials. Similarly, on the Dots task, younger children often called out the rule (“same” or “different”) on trials in the Mixed condition. In adults, the Simon effect is stronger, and does not diminish with consecutive incompatible trials, when the stimulus or response has a verbal property (Proctor & Vu, 2002).
The lack of an accuracy cost on spatially incompatible (Incongruent) trials in the Mixed block of the Dots task is in sharp contrast to the results when comparing separate blocks of Congruent and Incongruent trials on the task, which showed a significant spatial incompatibility effect for children of all ages in both speed and accuracy, though not for adults. The lack of a spatial incompatibility effect for accuracy within the Dots-Mixed condition, in conjunction with the increase in RT and reduction in accuracy for all trials in that condition relative to the other two Dots conditions, may indicate that participants exercised inhibition on both Congruent and Incongruent trials when those trials were intermixed in a difficult task-switching context. It may also be due to an order effect (see below).
The results of Crone et al. (in press), with a task similar to our Dots task and with subjects ages 7–8, 10–12, and 20–25 years, are similar to ours. Like us, they found a stronger Simon effect comparing across the single-task blocks than in the Mixed block, but unlike us they found that the Simon effect disappeared altogether in the Mixed block. Like us, they found the single-task block Simon effect to be significant for both accuracy and RT, but unlike us they found no change in the size of the accuracy or RT Simon effects over age.
Our study of spatial compatibility effects with conceptual rules and switching between those rules, as well as stimulus-level S–R associations, deserves additional follow-up. Our Dots and Arrows tests required integrating stimulus-appearance information with spatial location information. Since spatial location was relevant to the correct response they were not pure Simon tasks, but hybrid spatial incompatibility tasks. There is evidence that the neural bases for working memory of object-appearance and spatial-location information are somewhat different (Haxby, Petit, Ungerleider, & Courtney, 2000; Levy & Goldman-Rakic, 2000; Mecklinger & Mueller, 1996) and spatial location is exactly the stimulus property that feeds the spatial-compatibility bias. In standard Simon tasks, subjects would perform better if they could (theoretically) screen out the location of the stimulus, but on our Dots and Arrows tasks information about the location of the stimulus is critical for determining the correct response. Will results be similar to those on our Dots test, if the conceptual rules are, for example, “If the stimulus is an animal, press right; if the stimulus is a vehicle, press left”? Here, the same number of mental steps ([1] Is the stimulus an animal or a vehicle? [2] Where do I press for that?) would be required as in our Dots test ([1] Which rule [same side or opposite side] pertains to this stimulus? [2] Which side is the stimulus on?). That would be a conceptual task with higher-order rules (like our Dots task), but unlike our Dots task it would be a pure Simon task, not a hybrid.
Similarly, Wascher and colleagues (Wascher, Schatz, Kuder, & Verleger, 2001; Wascher & Wolber, 2004; Wiegand & Wascher, 2005) provide evidence for the involvement of both visuomotor and cognitive mechanisms in Simon task performance. Modifying the task slightly (e.g., presenting vertical rather than horizontal stimuli) changed the distributions of RT scores, and was taken as evidence for involvement of a cognitive component. An interesting developmental question is, “Does the effect function across Simon task variants change over age and if so in what ways and why?” Our manipulations (increasing or decreasing the working memory load) might also change the distribution of RT scores but, unfortunately, the vincentization procedure used by Wascher and colleagues involves splitting the RT distributions into quantiles (i.e., quartiles or even deciles) and would result in relatively few datapoints per bin in the current study. Future research with these tasks with more data per subject could allow more thorough investigation of the RT distributions across development.
In young adults, in whom inhibitory control is more mature, we had predicted that memory demands would exact a greater cost than inhibitory demands. Beginning at 10 years, increased memory demands (six versus two rules) took a greater toll on accuracy than did consistently inhibiting the tendency to respond on the same side as the stimulus (Dots-Incongruent versus Dots-Congruent)—the opposite of the pattern observed in the youngest children. Also in line with the greater role of memory in what is demanding for adults, taxing cognitive flexibility and inhibition in a switching context was not that hard for adults if memory demands were minimal. Unlike the case for children of all ages in our study, the Arrows test was easy for adults. However, the Dots-Mixed condition, which presented the same demands on inhibition and cognitive flexibility as did the Arrows test but in addition taxed working memory more, was difficult even for adults. The difference in accuracy on Dots-Mixed and Arrows, which differed only in the greater working memory demands in the Dots condition, was greater for young adults than for children at any age from 7 to 13 years. The greater memory demands in the Dots condition made a big difference for adults and the youngest children, but not for the majority of children (aged 7–13 years).
Since we are discussing performance on Dots-Incongruent and Dots-Congruent here, it is appropriate to note that one of the striking differences between the results for children and adults was that effects elicited only in Mixed blocks (e.g., in Dots-Mixed) with adults were found in children even in single-task blocks (e.g., Dots-Incongruent versus Dots-Congruent Blocks). For example, even though switching between rules that require inhibiting a prepotent response or making it is what was most difficult at all ages, even inhibition in steady state (Dots-Incongruent) was more difficult for children than going with their prepotent response on every trial (Dots-Congruent). At every age, without exception, children were slower and less accurate in the Dots-Incongruent block than the Dots-Congruent Block. Thus, inhibition, even in steady state, was sufficiently difficult for children to elicit a cost in their performance. This was not true for adults. Adults performed comparably in both speed and accuracy in the Dots-Congruent and Dots-Incongruent conditions. They required having to switch between the two conditions for a significant effect on their performance to be evident.
These results for adults are consistent with those reported in other studies of the spatial incompatibility effect in adults. As we found here, adults tend not to show the spatial incompatibility effect if Congruent and Incongruent trials are administered in separate, single-task blocks (Stürmer et al., 2002; Valle-Inclán et al., 2002; Verbruggen et al., 2005; Wühr, 2004, 2005). Adults evidently re-set their default response if several trials in a row are Incongruent and so are no slower on those Incongruent trials than on Congruent ones. Indeed, adults can show a reverse spatial incompatibility effect when switching back to responding on the same side as a stimulus after several trials of responding on the side opposite the stimulus (e.g., Logan & Zbrodoff, 1979). Children from 4 to 13 years of age, on the other hand, evidently did not re-set their default response. They showed the spatial incompatibility effect with Congruent and Incongruent trials administered in separate, single-task blocks. Inhibition in steady state took a toll on the performance of children even as old as 13 years, but not on that of adults.
Note that participants (including adults) in our study showed a spatial compatibility effect in Dots-Mixed although all subjects were tested on Dots-Incongruent immediately before that. Just as adults typically show little cost in making spatially-incompatible responses if they are tested on a block where they need to do that on every trial or most trials, Tagliabue, Zorzi, Umiltà, and Bassignani (2000) found no Simon effect in Mixed blocks when adults were given a run of Incongruent trials beforehand. Given those results it is possible that we might have found a much stronger spatial incompatibility effect in Dots-Mixed if it had been preceded by a block of Congruent trials. In order to test participants at all ages under the same conditions we had not varied task order, so further study would be needed to address that interesting possibility. It is also possible that other effects observed here might appear different if the tasks were administered in a different order. For example, Dots-Incongruent was always tested after Dots-Congruent. We do not think that caused a difference in the memory demands between the two conditions because the rule for Dots-Incongruent was taught and practiced immediately before that block just as the rule for Dots-Congruent was taught and practiced immediately before that block, but given that we did not vary task order we cannot prove that that is the case.
Based on our hypothesis that inhibitory control is extremely problematic for very young children, we predicted they would perform poorly on all trials requiring inhibition (Incongruent trials and switch trials) and that those effects would be additive. That is, we predicted they would perform worst on switching to the Incongruent (more difficult) condition; opposite to the pattern typically reported in adults. We predicted that after that early period, we would see greater switch costs at all ages for switching to the easier (Congruent) condition than to the harder (Incongruent) condition (consistent with the asymmetric switch costs previously reported in adults [Allport & Wylie, 2000; Allport et al., 1994; De Jong, 1995; Kleinsorge & Heuer, 1999; Los, 1996 and Stoffels, 1996; Wylie & Allport, 2000]). Further, for intermediate-age children, who are beginning to exercise better inhibitory control, we reasoned that doing so should require greater effort than in older participants and so predicted that undoing that inhibition (switching back to making the dominant response) should exact a greater cost in those children than in adults. Thus, we predicted that beginning after 6 or 7 years of age, asymmetric switch costs would be larger in younger than older participants. These predictions were confirmed for RT. On both Arrows and Dots-Mixed, children of 4–6 years showed a greater RT cost for switching to the Incongruent rule than the Congruent one. In both of those conditions, adults and older children showed a greater RT switch cost for switching to the Congruent than the Incongruent rule (consistent with previous reports of asymmetric switch costs). In both Arrows and Dots-Mixed, the differential RT cost of switching to the Congruent rather than the Incongruent rule was largest at 7–10 years of age. For accuracy, on the other hand, across the age spectrum on both Arrows and Dots-Mixed, people were more accurate when both the rule and the response changed than when just the rule changed. Thus the asymmetric switch costs reported in the literature primarily for RT, were found here for accuracy at all ages (even among the youngest children).
Crone et al. (in press) found results for RT that resemble ours for accuracy and results for accuracy that resemble ours for RT. Across their age spectrum (7–23 years), they found faster responses when both the rule and the response changed than when just the rule switched. Across our age spectrum (4–45 years), we found a greater percentage of correct responses when both the rule and the response changed than when just the rule switched. We found this effect on accuracy to be largest at 8–10 years; they found this effect on RT to be largest at 7–8 years. Mirroring in reverse these greater effects in young children versus older children and adults, Mayr (2001) found the RT effect (faster rule switching when the response also changed) to be greater in older versus younger adults. Crone et al. found that accuracy costs did not show the pattern they expected; more errors occurred on switch trials when the response-site also changed than when it remained the same. We similarly found slower responding on rule-switch, response-switch trials until age 13 on our Dots-Mixed task which resembles Crone et al.’s (this encompasses two of Crone et al.’s three age groups), but unlike Crone et al.’s results for accuracy, we found faster responding when both rule and response switched for adults.
We had predicted that even the youngest children would find it easy to hold two rules in mind and that although it would be harder for everyone to hold more rules in mind than fewer, the relative difficulty of this would not change over age. Indeed, as predicted, holding two arbitrary rules in mind was easy even for our youngest participants. At all ages performance was excellent on the two-Abstracts-Shapes and Dots-Congruent conditions (the latter requiring holding a superordinate rule in mind and mentally translating that into the appropriate embedded rule on each trial). This is consistent with other evidence that children can hold two conditional rules in mind by 4(½)–5 years of age (Campione & Brown, 1974; Doan & Cooper, 1971; Gollin, 1964, 1965, 1966; Gollin & Liss, 1962; Osier & Kofsky, 1965; Shepard, 1957). Although everyone found the increased memory load (six versus two Abstract Shapes) more difficult, the size of the effect changed little over age in either speed or accuracy when all subjects were included in the analyses. More fine-grained analyses, however, showed that how the difference in difficulty was handled differed over age. The speed-accuracy tradeoff changed over age. Accuracy on six-shapes more closely approximated that on two-shapes the older the subjects. RT in the two conditions, however, diverged more the older the subjects.
Across conditions, older participants slowed down to preserve their accuracy on more difficult trials. Thus they showed sizeable RT differences and small accuracy differences. An elegant analysis of this tendency of adults to alter their response times to preserve a constant level of accuracy in the face of variations in task difficulty is provided by Usher and McClelland (2001) and Usher, Olami, and McClelland (2002). In our study, younger children often showed less of a change in speed and hence showed very large differences in accuracy across trials of differing difficulty. For example, older participants were better able to modulate their speed and slowed down in the difficult Dots-Mixed condition relative to the easy Dots-Congruent condition to minimize a reduction in accuracy. Younger participants (even those given a very long time to respond) kept their response speed more constant across conditions, perhaps because they were too impulsive to take more time when they needed it, at the cost of accuracy in the difficult conditions. Hence, for example, the accuracy difference between the Dots-Mixed and Dots-Congruent conditions decreased with age but RT differences between those two conditions increased over age (see Fig. 6). Similarly, the mixing cost of Congruent and Incongruent trials being mixed together declined over age for accuracy but increased over age for RT (see Fig. 7). Likewise over age, differences in performance on the six- and two-Abstract-Shapes conditions declined in accuracy but increased in RT.
The very youngest children (4–5 years of age) were given a long time to respond (3000 ms) so it is unlikely that they lacked sufficient time to modulate their response speed. It is more likely that they had difficulty inhibiting impulsive responding, i.e., difficulty withholding their response long enough to take the time they really needed. For instance, the RTs for children of 4–5 years on nonswitch Incongruent trials in the Dots-Mixed condition differed little from their RTs in the easier single-task Dots-Incongruent block. This was true even though their RTs in both conditions were on average less than half of the time allotted, so they had time to compute their responses but did not make use of that extra time. Their inhibitory problems can also be seen in their greater likelihood to respond impulsively before a stimulus appeared and to fail to promptly stop pressing a response button after responding.
These findings concerning not taking the time they needed are fully consistent with results reported by Gerstadt, Diamond, and Hong (1994) and Diamond, Kirkham, and Amso (2002) on a different task, the Day–Night Stroop-like task, where children had to say “night” to a daytime image and “day” to a nighttime image. Gerstadt et al. (1994) found that: (a) those children of 3(½)–4(½) years who took more time to compute their answers were able to answer correctly on more trials than children who answered more quickly and (b) within child, on those trials where a child of 3(½)–4(½) years took longer to respond, the child was more likely to be correct. Diamond et al. (2002) manipulated time to view the stimulus and compute the response by chanting a ditty to the child either after the stimulus was presented but before the child could respond or between trials before the stimulus was presented. Diamond et al. found that 4- and 4(½)-year-old children were correct on significantly more trials in the manipulation that gave them more stimulus-viewing and more response-computation time (ditty chanted while stimulus was visible) but performed no better than in the basic condition when the extra time could be used to remind themselves of the rules but not to instantiate the correct rule for the current trial (ditty chanted between trials, before stimulus was visible).
On the other hand, we have evidence here that if a task is sufficiently easy that 4–5-years-olds can compute the answer in roughly a second, they will modulate their speed to preserve their accuracy. On the Pictures test, for example, children of 4–5 years slowed way down on Incongruent trials relative to Congruent ones, thereby preserving their accuracy so that the difference in their accuracy on Incongruent versus Congruent trials was smaller than that seen by older children of 6–8 years given less time to compute their responses (see Fig. 3). Similarly, on the Arrows test, children of 4–5 years used the ample time allowed them to maintain an accuracy level of over 80%, a level of accuracy not seen when given less time to respond until children were 10–11 years old. Children of 4–6 years also showed smaller local switch costs on the Arrows test than did the older children; they achieved this by using their allotted time to slow down on the switch trials; their RT switch costs were over twice those of participants at any other age.
Certainly there is considerable evidence that 6-year-olds benefited from having a longer time to respond (3000 ms versus 1250 ms). The results clearly show that by 6 years, if allowed more time to respond, children will take advantage of that to reduce their errors.
The relative lack of response speed modulation in children of 6–8 years tested in the adult condition probably had a different cause than that for children of 4–5. In the case of the 6–8-year-olds, the response window (1250 ms ISI; 750 ms stimulus presentation) was likely too brief to allow them the time they needed to slow down sufficiently in the more difficult conditions to preserve their accuracy. Thus, even on the easy Pictures task, they could not slow down sufficiently on incompatible trials to preserve their accuracy, and so although their RTs were longer on incompatible trials, their accuracy suffered on those trials more than was found at any other age.
Given our hypothesis that working memory and inhibition are independent, we had predicted that performance on tasks that tax primarily memory or primarily inhibition would not be highly correlated. Instead, when the tasks were matched for difficulty, speed on working memory and inhibition tasks was highly correlated. Individuals who were fast at exercising inhibition also tended to be fast on working memory measures, even after accounting for age effects. Accuracy across working memory and inhibition measures was also correlated, though not as strongly.
Finally, we had predicted that the most difficult condition at all ages would be the one that taxed inhibition and memory in a switching context (Dots-Mixed) and that that would even be more difficult than having to hold much more information in mind but with no inhibition or switching component (six-Abstract-Shapes). Indeed, as predicted, we found that at every age, including for young adults, holding two superordinate rules in mind and switching randomly between the rule for making a prepotent response and the rule for inhibiting that to make the opposite response (Dots-Mixed) was the most difficult condition, harder even than holding six arbitrary rules in mind for stimuli that did not easily lend themselves to verbal labels (six-Abstract-Shapes).
25.2. How our predictions concerning cognitive flexibility and task switching fared
Cognitive flexibility (switching, overcoming inertial tendencies) was far harder than consistent inhibition in steady state or than holding and manipulating a couple of items in mind, and showed a much longer developmental progression. The cost, and longer developmental progression, of cognitive flexibility can be seen most clearly on the Arrows test, where little or no memory was required as the arrow pointed to the correct response on every trial. Since we hypothesized that switching is so difficult, we had predicted that having to switch between tasks even when memory demands were minimized (as in the Arrows test) would show a long developmental progression. This was confirmed. Even by age 10, the percentage of correct responses did not exceed 80% on the Arrows test, and even by the age of 13, children were not yet performing at adult levels on the Arrows task.
At all ages, the RT costs for having to exercise inhibition in a switching context on the Arrows task versus not having to exercise inhibition or switch (Dots-Congruent condition) took a much greater toll on response speed than did increasing the memory load from two to six arbitrary rules However, consistent with inhibition in a switching context being disproportionately difficult for young children and memory being disproportionately difficult for adults, even young adults, the larger accuracy difference between Arrows versus Dots-Congruent than between two- versus six-Abstract-Shapes was found only for the younger two-thirds of the subjects (children ≤9 years).
Consistent with cognitive flexibility improving with age, performance differences on Dots-Incongruent and Dots-Mixed decreased over age. If cognitive flexibility is improving, however, one would also expect the difference in performance on Dots-Incongruent and Arrows to decrease over age. The markedly faster speed and better accuracy in the Dots-Incongruent condition compared with the Arrows test, however, remained strong throughout the age range for children, though the accuracy difference disappeared among young adults. This may suggest that much of the age-related reduction in the cost of exercising cognitive flexibility comes after 13 years of age.
Consistent with the “all or none” principle (Diamond, in preparation), it should be easier to inhibit a dominant response all the time than only some of the time. We thus predicted that performance at all ages would be better in Dots-Incongruent (where inhibition was consistently required on all trials) than in Mixed blocks of Dots or Arrows (where inhibition is only required on the 50% of trials that are Incongruent), and that this difference would be greater the younger the children. Indeed, we found that inhibiting the spatially-compatible response some of the time in a switching context despite minimal memory requirements (the Arrows task) took a greater toll on speed and accuracy at every age than did inhibiting the spatially-compatible response consistently on all trials (Dots-Incongruent). Not surprisingly, the differences were even larger between Dots-Mixed and Dots-Incongruent. Our prediction that the difference in performance between Dots-Mixed and Dots-Incongruent would decrease over age as cognitive flexibility improved fared less well as these differences remained large at all ages, though the difference in accuracy was larger the younger the children. Similarly, the accuracy difference between Dots-Incongruent and Arrows was smaller in adults than in children, but otherwise the markedly better performance on Dots-Incongruent than on Arrows was equally true across all ages.
Also consistent with the “all or none” principle is that performance should be better on not-switching anything (repeat-rule, repeat-response trials) and on switching everything (switch trials where the response-site also switches) than on trials where either the rule or response-site changes but not the other. We had predicted that these effects, heretofore documented only in adults and older children (Kleinsorge, 1999; Meiran, 2000a,b; Rogers & Monsell, 1995; Schuch & Koch, 2004), would also be found in young children. We predicted that throughout our age span, participants would do better at switching tasks if the response-site also changed and would be slower and less accurate on switch trials when the response-site remained the same as on the previous trial. In both Dots-Mixed and Arrows, older children and adults were indeed better at switching tasks if the response-site also changed than if the response-site remained the same as on the preceding trial. However, contrary to our prediction, the youngest children (children of 4–8 years) performed better on switch trials where the response-site remained the same. They were faster on switch than nonswitch trials and on response-switch rather than response-stay trials and those effects tended to be additive. These results raise the possibility that perhaps the hypothesized “all or none” default of cognitive systems is an efficient characteristic of the mature cognitive system. It appears, in this particular context anyway, that piecemeal, additive effects are more characteristic of young children’s performance.
Research in adults has shown that performance on nonswitch trials (where the rule remains the same as on the previous trial) is worse when these trials are presented in the context of periodically having to switch than in the context of a block of all nonswitch trials (e.g., Fagot, 1994; Mayr, 2000a). We found that indeed performance was worse-slower, less accurate, and characterized by more anticipatory errors-on nonswitch trials within the Mixed block of the Dots task than within either single-task block of the task. Such global switch costs were among the strongest effects found in this study. It is not that participants forgot the rules when they had to hold both the Congruent and Incongruent rules in mind for the Mixed block. Indeed, children often called out the correct higher-order rule on trials in the Mixed condition (e.g., “same,” “opposite,” “opposite,” “same”) even as they were making errors. The problem seemed to be in quickly translating that rule into the correct response. The presence of global switch costs at all ages in our study is consistent with task-switching studies in children, young adults, and older adults; all studies consistently find global switch costs throughout the age spectrum.
We had predicted that global switch costs would decrease over age. That prediction was only partially confirmed. The global switch cost in accuracy declined from 9 to 13 years of age, as predicted, but the global switch cost in RT increased from 6 years through early adulthood (see Fig. 7). Adults adjusted their speed to preserve their accuracy; younger children did that much less hence the difference in the speed-accuracy trade-off with age. This mirrors exactly what was found by Cohen et al. (2001) using a very different task-switching paradigm. They used Meiran’s (1996) task-switching paradigm presented as a computer game. A smiley face appeared at one of four quadrants of a square, preceded by a cue indicating the relevant dimension (horizontal [“is the cue in the left or right half?”] or vertical [“is the cue in the top or bottom half?”]). This was administered to 150 children (ages 5–11 years) and 16 young adults. They found that global switch costs in accuracy decreased from 5 to 11 years, and even 11-year-olds were not as accurate in mixed blocks as young adults, but global switch costs in speed of responding increased over age (just as we found here).
Contrary to our findings, however, though with only a few overlapping ages, Reimers and Maylor (2005) found that global RT switch costs decreased linearly from 10 to 18 years. Like us, Crone et al. (in press) found significant global switch costs in both speed and accuracy. However, unlike us, Cohen et al. (2001), or Reimers and Maylor (2005), Crone and colleagues found no change in the size of global switch costs with age in either speed or accuracy from 7 to 8 years to 23 years. Most studies in older adults report greater global RT switch costs in elders than in young adults (Kray et al., 2004; Kray & Lindenberger, 2000; Mayr, 2000; van Asselen & Ridderinkhof, 2000), though Kray et al. (2002) report that no difference in global RT switch costs is found between younger and older adults when switches between tasks are unpredictable. The difference between predictable and unpredictable switches would also explain the differences across studies in whether global RT switch costs differ between young children and adults. The only study to find smaller global RT switch costs with increasing age from young children to adults was the one study that also predictably switched between tasks (Reimers & Maylor, 2005), where a predictable double-alternation switching pattern was used in the Mixed block.
Cepeda et al. (2001) calculated global switch costs differently from the studies above. They compared performance on all trials in Mixed blocks (not just the nonswitch trials) to performance in single-task blocks. Just as we found that the difference in performance on Dots-Mixed versus Dots-Congruent or Dots-Incongruent decreased over age from 6 years to young adulthood, so too Cepeda and colleagues found that the difference in performance in their Mixed blocks versus their single-task blocks decreased from their youngest age (7 years) to young adulthood. Cohen et al. (2001) similarly report a linear decline in the difference in performance in Mixed blocks versus single-task blocks for both speed and accuracy, with their oldest children (age 11 years) still showing a larger difference in both dependent measures than young adults.
This illustrates an important point. Inhibiting a dominant response requires effort, but it is not nearly as difficult if that inhibition needs to be consistently maintained (as in Dots-Incongruent). What is far more demanding is switching back and forth between sometimes inhibiting a dominant response and sometimes making it. What is truly difficult is overcoming one’s inertial tendency to continue in the same mindset, switching between one mental set and another. Even now many investigators still administer the Stroop task in single-task blocks (blocks of always reading the word and blocks of always naming the ink color). While it requires effort to focus on the ink color (and one can see that toll in slowed responding) one can get in the mode of always focusing on the ink color and the task is quite manageable. It is far harder not to be able to rely on always ignoring the word; to have to switch back and forth between sometimes reading the word and sometimes naming the ink color.
Because of floor effects (people are already slower and more error-prone in the Incongruent-only block), we predicted that the effect of context (the Mixed block versus single-task block) would be greater on Congruent than Incongruent trials. We further predicted that this should be more evident the younger the child. That is, we predicted that the younger the child, the closer performance on “easy” (Congruent, nonswitch) trials would fall to the level of “harder” trials in the context of sometimes having to switch back and forth. Consistent with this prediction, we found that the cost of mixing nonswitch trials in with switch trials and mixing Congruent trials in with Incongruent ones, versus having single-task blocks, was greater for the Congruent (easier) nonswitch trials than the Incongruent nonswitch trials. When these trials were administered in separate single-task blocks, fewer errors occurred on Congruent trials (except for adults where accuracy did not differ in the Congruent and Incongruent single-task blocks) but when they were intermixed within the same block comparable numbers of errors occurred on Congruent and Incongruent trials. Participants were able to respond much faster on nonswitch Congruent trials when all trials in the block were Congruent than when some were Incongruent, though only Congruent trials following a Congruent trial were included in these analyses. The same was true for Incongruent trials but to a lesser extent. The effect of context (Mixed block versus single-task block) appears to have been larger for the faster, more automatic response (responding on the same side as the stimulus) than for the slower, more demanding response (inhibiting the dominant response and responding opposite to it). However, contrary to the portion of our prediction concerning development, the size of the greater effect of context on Congruent versus Incongruent trials did not change significantly over age.
The difficulty of the harder condition is underestimated in single-task blocks (always having to respond opposite to the side of the stimulus tends to reduce its difficulty because you get in the mode of doing that) and the ease of the easier condition is underestimated in Mixed blocks (because people tend to slow down across the board on such blocks). Comparing non-switch Incongruent trials in the Dots-Mixed block to nonswitch Congruent trials in the Dots-Congruent block may come closest to approximating the full difficulty of inhibiting the tendency to make the spatially compatible response.
A striking difference in our findings for children and adults was that while RT was unquestionably a more sensitive measure than percentage of correct responses for adults, the latter was often the more sensitive measure for children, especially younger children. For example, for our youngest children (4–6 years of age), age-related improvements in each of the three conditions of the Dots task and in each of the two conditions of our Abstract Shapes task were far more evident in accuracy than in speed. Age-related improvements in the ability to inhibit spatially compatible responses were far more evident in reduced accuracy differences between Congruent and Incongruent trials over age than in reduced RT differences in each of the tasks that tested this (Pictures, Arrows, and Dots; see Fig. 11). Similarly, age-related improvements in the ability to hold multiple items in mind were far more evident in the reduced change in accuracy over age for holding six rather than two arbitrary rules in mind than it was in reduced change in RT (see Fig. 12).
26. Final comments and conclusions
Because we wanted to include very young children, we did not test our subjects for nearly as many trials as is typically done in studies of adults. We did not discard the trial following an error (only error trials), contrary to what is often done in RT analyses with adult subjects, because with young children error rates are sufficiently high that to discard the trials immediately after an error as well as error trials would have resulted in the loss of too many datapoints. We are impressed, however, with the consistency of our results with those of other published studies despite procedural differences.
Certainly there are a number of unanswered questions that could fruitfully be followed up in later studies. We stopped testing children at 13 years of age, but on a number of our measures children of 13 were not yet performing at adult levels. Older children should be tested to better understand the developmental progression between 13 and 26 years. We will test our Simon task (the Pictures task) without the posted memory aids to see how much our having removed the usual memory demand present in most Simon tasks affected results with our paradigm in children. Arrows may be a more compelling symbol for adults than children, so we will re-administer that condition using eyes looking straight down or diagonally to the other side. It is likely that adults reduced the separate rules for vertical and diagonal Arrows to one rule (“Press where the arrow is pointing”) but that children did not spontaneously do that. We will see what effect explicitly instructing children to code this as one rule has on their performance. Some of our adults had a little difficulty distinguishing the gray and striped Dots. We will therefore use stimuli of different shapes (hearts and flowers) in future testing.
Among the most important additional work to be done is to complement the work of O’Craven, Davidson, Bergida, Savoy and Diamond (in preparation) on the neural systems recruited for performance of the various conditions tested here in adults with neuroimaging studies of the neural systems recruited by children in performance of these conditions and how, and why, that changes over age. Certainly there are important functional and structural changes in the neural network recruited for cognitive control and executive functions throughout the age range investigated here (Diamond, 2002). Functional changes in the neural basis for cognitive control appear to be characterized by increasingly focal activation during early childhood and then decreasingly intense activation of the focal regions during adolescence (e.g., Brown et al., 2005; Casey, Galvan, & Hare, 2005; Durston et al., 2005).
Besides characterizing effects (such as local switch costs, global switch costs, and asymmetric switch costs), previously studied only in older children or adults, in young children and throughout a wide age span, some of the most important findings to come out of this study include the following:
Inhibiting the tendency to make a spatially compatible response exacted a greater toll on young children’s performance than did memory demands and than it did on older participants’ performance. The spatial incompatibility effect (the cost of inhibiting the pull to respond on the same side as the stimulus) was greater the younger the participant. Even at 13 years of age, children still showed a greater Simon effect than did young adults.
Inhibitory control was sufficiently problematic for very young children that they took especially long on all trials requiring inhibition (Incongruent trials and switch trials) and those effects were additive. Thus, they responded slower when switching to the Incongruent rule than the Congruent one; opposite to the pattern seen in adults. Intermediate-age children, who were beginning to exercise better inhibitory control, exerted more effort to do that than older children and adults and so showed a heightened cost to undoing that inhibition (a more exaggerated version of the asymmetric switch costs seen in adults and older children here and reported in the literature for adults).
As inhibitory control improved with age, memory demands started to exact a greater cost than inhibitory ones. Beginning at 10 years of age, increased memory demands (holding of six versus two arbitrary, hard-to-verbalize rules) took a greater toll on accuracy than did consistently inhibiting the tendency to respond on the same side as the stimulus (Dots-Incongruent versus Dots-Congruent)—the opposite of the pattern observed in the youngest children. Also in line with the greater role of memory in what is demanding for adults, taxing cognitive flexibility and inhibition in a switching context was not that hard for adults if memory demands were minimal. Unlike the case for children of all ages in our study, the Arrows test was easy for adults.
Given that we hypothesized that working memory and inhibition are independent, we had predicted that performance on tasks that tax primarily memory or primarily inhibition would not be highly correlated. We were wrong. When matched for difficulty, these tasks were very highly correlated for RT (0.7–0.8) and respectably correlated for accuracy (0.3–0.4).
Cognitive flexibility (switching between rules), even with memory demands minimized, showed a long developmental progression. On the Arrows test, where little or no memory was required since the stimulus pointed to the correct response, even by 13 years, children were not yet performing at adult levels. Global switch costs were among the strongest effects found in this study. It is not that participants forgot the rules when they had to hold both the Congruent and Incongruent rules in mind for the Mixed block. The problem seemed to be in quickly translating the rules into the correct response.
The most difficult condition at all ages was the one that taxed inhibition and memory in a switching context (Dots-Mixed). At every age, holding two superordinate rules in mind and switching randomly between the rule for making a prepotent response and the rule for inhibiting that to make the opposite response (Dots-Mixed) was harder even than holding six arbitrary rules in mind for stimuli that did not easily lend themselves to verbal labels (six-Abstract-Shapes).
The speed-accuracy tradeoff changed over age. Across conditions, older participants slowed down on more difficult trials to preserve their accuracy. Thus they showed large response time differences and small accuracy differences. Younger children often showed less of a change in speed and but very large differences in accuracy. Young children were often too impulsive to take the time they needed; their response speed remained more constant across conditions, but at the cost of accuracy on the more difficult conditions. Thus, for example, global switch costs in accuracy declined over age, but global switch costs in RT increased over age.
Effects elicited only in Mixed blocks with adults were found in young children even in single-task blocks. While young children could exercise inhibition in steady state, it was sufficiently difficult for them that it exacted a cost not seen in adults. Adults (but not young children) seemed to re-set their default response when inhibition of the same tendency was required throughout a block. Adults required having to switch between the two conditions for a significant effect on their performance to be evident.
Acknowledgments
The authors gratefully acknowledge the support of this work by NIH. Grant #R01-HD35453 from NICHD funded the empirical work and grant #R01-DA19685 from NIDA funded preparation of the manuscript. Sarah Munro and Cecil Chau helped greatly with the figure preparation. The authors would also like to acknowledge their gratitude to Robert Proctor and two anonymous reviewers for comments on an earlier draft, and to all the parents and children who made this research possible. Reprint requests should be addressed to: Adele Diamond, UBC, Dept. of Psychiatry, 2255 Wesbrook Mall, Vancouver, BC, Canada V6T 2A1, or via email: adele.diamond@ubc.ca.
References
- Allport, A., Styles, E. A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umilta & M. Moscovitch (Eds.), Attention and performance XV (pp. 421–452). Cambridge, MA: MIT Press.
- Allport, A., & Wylie, G. (2000). Task switching, stimulus–response bindings, and negative priming. In S. Monsell & J. Driver (Eds.), Control of cognitive processes: Attention and performance XVII (pp. 35–70). Cambridge, MA: MIT Press.
- Anderson MC, Spellman BA. On the status of inhibitory mechanisms in cognition: Memory retrieval as a model case. Psychological Review. 1995;102:68–100. doi: 10.1037/0033-295x.102.1.68. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aron AR, Sahakian BJ, Robbins TW. Distractibility during selection-for-action: Differential deficits in Huntington’s disease and following frontal lobe damage. Neuropsychologia. 2003;41:1137–1147. doi: 10.1016/s0028-3932(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Overtoom CC, Verbaten MN. The ability to activate and inhibit speeded responses: Separate developmental trends. Journal of Experimental Child Psychology. 2000;75:263–290. doi: 10.1006/jecp.1999.2538. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Science. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brass M, Ruge H, Meiran N, Rubin O, Koch I, Zysset S, et al. When the same response has different meanings: Recoding the response meaning in the lateral prefrontal cortex. Neuroimage. 2003;20:1026–1031. doi: 10.1016/S1053-8119(03)00357-4. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Brown, A. L. (1975). The development of memory: Knowing, knowing about knowing, and knowing how to know. In H. W. Reese (Ed.), Advances in child development and behavior: 10, (10, (pp. 103–152). New York: Academic Press. [DOI] [PubMed]
- Brown RG, Marsden CD. An investigation of the phenomenon of “set” in Parkinson’s disease. Movement Disorders. 1988;3:152–161. doi: 10.1002/mds.870030207. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The multi-source interference task: Validation study with fMRI in individual subjects. Molecular Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Campione JC, Brown AL. The effects of contextual changes and degree of component mastery on transfer of training. Advances in Child Development and Behavior. 1974;9:69–114. doi: 10.1016/s0065-2407(08)60315-8. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinion in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology. 2001;37:715–730. [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97:332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen, S., Bixenman, M., Meiran, N., & Diamond, A. (2001). Task switching in children. In Proceedings of the paper presented at the South Carolina Bicentennial Symposium on Attention
- Craft JL, Simon JR. Processing symbolic information from a visual display: Interference from an irrelevant directional cue. Journal of Experimental Psychology. 1970;83:415–420. doi: 10.1037/h0028843. [DOI] [PubMed] [Google Scholar]
- Crone, E. A., Bunge, S. A., Van der Molen, M. W., & Ridderinkhof, K. R. (in press). Switching between tasks and responses: A developmental study. Developmental Science [DOI] [PubMed]
- Crone EA, Ridderinkhof RK, Worm M, Somsen RJM, van der Molen MW. Switching between spatial stimulus–response mappings: A developmental study of cognitive flexibility. Developmental Science. 2004;7:443–455. doi: 10.1111/j.1467-7687.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- Crone, E. A., Wendelken, C., Donohue, S. E., & Bunge, S. A. (2005). Neural evidence for dissociable components of task-switching. Cerebral Cortex [July 6 Epub ahead of print]. [DOI] [PubMed]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus–response compatibility on cortical motor activation. NeuroImage. 2001;131:1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- De Jong R. Strategical determinants of compatibility effects with task uncertainty. Acta Psychologica. 1995;88:187–207. [Google Scholar]
- DeLuca C, Wood SJ, Anderson V, Buchanan JA, Proffitt T, Mahony K, et al. Normative data from CANTAB: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology. 2003;25:242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Dempster, F. N. (1985). Short-term memory development in childhood and adolescence. In C. J. Brainerd & M. Pressley (Eds.), Basic processes in memory development: Progress in cognitive development research New York: Springer–Verlag.
- Diamond, A. (1990). The development and neural bases of higher cognitive functions NY: New York Academy of Sciences. [DOI] [PubMed]
- Diamond, A. (1991). Neuropsychological insights into the meaning of object concept development. In S. Carey & R. Gelman (Eds.), The epigenesis of mind: Essays on biology and knowledge (pp. 67–110). Hillsdale, NJ: Lawrence Erlbaum Associates.
- Diamond A. Evidence of robust recognition memory early in life even when assessed by reaching behavior. Journal of Experimental Child Psychology. 1995;59:419–456. doi: 10.1006/jecp.1995.1020. [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2002). Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In D. T. Stuss & R. T. Knight (Eds.), Principles of frontal lobe function (pp. 466–503). London, UK: Oxford University Press.
- Diamond, A. (2005). Some generalizations concerning cognition and cognitive development Presented to the Psychology Dept., University of Victoria, October 27, 2005.
- Diamond, A. A theory of “all or none.” In preparation.
- Diamond A, Carlson SM, Beck DM. Preschool children’s performance in task switching on the dimensional change card sort task: Separating the dimensions aids the ability to switch. Developmental Neuropsychology. 2005;28:689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Kirkham NZ, Amso D. Conditions under which young children CAN hold two rules in mind and inhibit a prepotent response. Developmental Psychology. 2002;38:352–362. [PubMed] [Google Scholar]
- Diamond A, O’Craven KM, Savoy RL. Dorsolateral prefrontal cortex contributions to working memory and inhibition as revealed by fMRI. Society for Neuroscience Abstracts. 1998;24:1251. [Google Scholar]
- Diedrichsen J, Mayr U, Dhaliwal H, Keele S, Ivry RB. Task-switching deficits in patients with prefrontal lesions or Parkinson’s disease. Cognitive Neuroscience Society Annual Meeting Abstracts. 2000;1:99. [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad B, Cepeda NJ, Weissman DH, Milham MP, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Doan HM, Cooper DL. Conditional discrimination in children: Two relevant factors. Child Development. 1971;42:209–220. [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon YD. Prefrontal cortex activation in task switching: An event-related fMRI study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW. Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: Evidence for a specific attentional dysfunction. Neuropsychologia. 1989;27:1329–1343. doi: 10.1016/0028-3932(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Dreher J, Berman FK. Fractionating the neural substrate of cognitive control processes. Proceedings of the National Academy of Sciences. 2002;99:14595–14600. doi: 10.1073/pnas.222193299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cerebral Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Molecular Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Fagot, C. (1994). Chronometric investigations of task switching. Ph.D. Thesis, University of California, San Diego.
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. NeuroImage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fischer B, Biscaldi M, Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Seger CM. S–R compatibility: Spatial characteristics of stimulus and response codes. Journal of Experimental Psychology. 1953;81:174–176. doi: 10.1037/h0062827. [DOI] [PubMed] [Google Scholar]
- Flowers KA, Robertson C. The effect of Parkinson’s disease on the ability to maintain a mental set. Journal of Neurology Neurosurgery, and Psychiatry. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. Population activity in the control of movement. International Review of Neurobiology. 1994;37:103–119. doi: 10.1016/s0074-7742(08)60241-x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Lurito JT, Petrides M, Schwartz AB, Massey JT. Mental rotation of the neuronal population vector. Science. 1989;243:234–236. doi: 10.1126/science.2911737. [DOI] [PubMed] [Google Scholar]
- Gerardi-Coulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24–36 months of age. Developmental Science. 2000;3:397–404. [Google Scholar]
- Gernsbacher MA, Faust ME. The mechanism of suppression: A component of general comprehension skill. Journal of Experimental Psychology. 1991;17:245–262. doi: 10.1037//0278-7393.17.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstadt C, Hong Y, Diamond A. The relationship between cognition and action: Performance of 3 1/2–7 year old children on a Stroop-like day–night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic, P. S. (1987). Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In F. Plum (Ed.), Handbook of physiology: V, (V, (pp. 373–417). Bethesda, MD: American Physiological Society.
- Gollin ES. Reversal learning and conditional discrimination in children. Journal of Comparative & Physiological Psychology. 1964;58:441–445. doi: 10.1037/h0045362. [DOI] [PubMed] [Google Scholar]
- Gollin ES. Factors affecting conditional discrimination in children. Journal of Comparative and Physiological Pyschology. 1965;60:422–427. doi: 10.1037/h0022583. [DOI] [PubMed] [Google Scholar]
- Gollin ES. Solution of conditional discrimination problems by young children. Journal of Comparative and Physiological Psychology. 1966;62:454–456. [Google Scholar]
- Gollin ES, Liss P. Conditional discrimination in children. Journal of Comparative and Physiological Psychology. 1962;55:850–855. doi: 10.1037/h0045951. [DOI] [PubMed] [Google Scholar]
- Harnishfeger KK, Pope RS. Intending to forget: The development of cognitive inhibition in directed forgetting. Journal of Experimental Child Psychology. 1996;62:292–315. doi: 10.1006/jecp.1996.0032. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. Journal of Experimental Psychology. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11:380–391. doi: 10.1006/nimg.2000.0592. [DOI] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience. 1998;10:178–198. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Hedge A, Marsh NWA. The effect of irrelevant spatial correspondence on two-choice response-time. Acta Psychologica. 1975;39:427–439. doi: 10.1016/0001-6918(75)90041-4. [DOI] [PubMed] [Google Scholar]
- Heidbreder EF. Problem solving in children and adults. Journal of Genetic Psychology. 1928;35:522–545. [Google Scholar]
- Hommel B. The relationship between stimulus processing and response selection in the Simon task: Evidence for a temporal overlap. Psychological Research/Psychologische Forschung. 1993;55:280–290. [Google Scholar]
- Hommel B. Effects of irrelevant spatial S compatibility depend on stimulus complexity. Psychological Research/Psychologische Forschung. 1994;56:179–184. doi: 10.1007/BF00419705. [DOI] [PubMed] [Google Scholar]
- Hommel B. Stimulus–response compatibility and the Simon effect: Toward an empirical clarification. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:764–775. doi: 10.1037//0096-1523.17.1.246. [DOI] [PubMed] [Google Scholar]
- Hommel B, Musseler J, Aschersleben G, Prinz W. The Theory of Event Coding (TEC): a framework for perception and action planning. Behavioral & Brain Sciences. 2001;24(5):849–878. doi: 10.1017/s0140525x01000103. [DOI] [PubMed] [Google Scholar]
- Hommel B, Proctor RW, Vu KP. A feature-integration account of sequential effects in the Simon task. Psychological Research. 2004;68:1–17. doi: 10.1007/s00426-003-0132-y. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Mazziotta JC. Bimodal (auditory and visual) left frontoparietal circuitry for sensorimotor integration and sensorimotor learning. Brain. 1998;121:2135–2143. doi: 10.1093/brain/121.11.2135. Pt 11. [DOI] [PubMed] [Google Scholar]
- Jeffrey WE. Variables in early discrimination learning. III. Simultaneous vs. successive stimulus presentation. Child Development. 1961;32:305–310. doi: 10.1111/j.1467-8624.1961.tb05028.x. [DOI] [PubMed] [Google Scholar]
- Jersild AT. Mental set and shift. Archives of Psychology. 1927;89:5–82. [Google Scholar]
- Kail, R. (1991a). Development of processing speed in childhood and adolescence. In H. W. Reese (Ed.), Advances in child development and behavior: 23, (23, (pp. 151–185). New York: Academic Press. [DOI] [PubMed]
- Kail R. Developmental change in speed of processing during childhood and adolescence. Psychological Bulletin. 1991b;109:490–501. doi: 10.1037/0033-2909.109.3.490. [DOI] [PubMed] [Google Scholar]
- Kail R. Processing time declines exponentially during childhood and adolescence. Developmental Psychology. 1991c;27:259–266. [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychologica. 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Keele, S., & Rafal, R. (2000). Deficits of task set in patients with left prefrontal cortex lesions. In S. Monsell & J. Driver (Eds.), Control of cognitive processes: Attention and performance: XVIII, (XVIII, (pp. 627–652). Cambridge, MA: MIT Press.
- Kimberg DY, Aguirre GK, D’Esposito M. Modulation of task-related neural activity in task-switching: An fMRI study. Cognitive Brain Research. 2000;10:189–196. doi: 10.1016/s0926-6410(00)00016-1. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: The role of working memory in complex, organized behavior. Journal of Experimental Psychology. 1993;122:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Kirkham NK, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6(5):449–476. [Google Scholar]
- Kleinsorge T. Response repetition benefits and costs. Acta Psychologica. 1999;103:295–310. doi: 10.1016/s0001-6918(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Kleinsorge T, Heuer H. Hierarchical switching in a multidimensional task space. Psychological Research. 1999;62:300–312. [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychologica. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kray J, Eber J, Lindenberger U. Age differences in executive functioning across the lifespan: The role of verbalization in task preparation. Acta Psychologica. 2004;115:143–165. doi: 10.1016/j.actpsy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kray J, Li KZ, Lindenberger U. Age-related changes in task-switching components: The role of task uncertainty. Brain and Cognition. 2002;49:363–381. doi: 10.1006/brcg.2001.1505. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Experimental Brain Research. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Logan GD, Zbrodoff NJ. When it helps to be misled: Facilitative effects of increasing the frequency of conflicting stimuli in a Stroop-like task. Memory & Cognition. 1979;7:166–174. [Google Scholar]
- Los SA. On the origin of mixing costs: Exploring information processing in pure and Mixed blocks of trials. Acta Psychologica. 1996;94:145–188. [Google Scholar]
- Los SA. Identifying stimuli of different perceptual categories in pure and Mixed blocks of trials: Evidence for stimulus-driven switch costs. Acta Psychologica. 1999;103:173–205. doi: 10.1016/s0001-6918(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. NeuroImage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Lu CH, Proctor RW. The influence of irrelevant location information on performance: A review of the Simon and spatial Stroop effects. Psychonomic Bulletin and Review. 1995;2:174–207. doi: 10.3758/BF03210959. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luciana M, Nelson CA. Assessment of neuropsychological function in children using the Cambridge Neuropsychological Testing Automated Battery (CANTAB): Performance in 4 to 12 year-olds. Developmental Neuropsychology. 2002;22:595–623. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Lyons-Warren A, Lillie R, Hershey T. Short and long-term spatial delayed response performance across the lifespan. Developmental Neuropsychology. 2004;26:661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- Maclin EL, Gratton G, Fabiani M. Visual spatial localization conflict: An fMRI study. Neuroreport. 2001;12:3633–3636. doi: 10.1097/00001756-200111160-00051. [DOI] [PubMed] [Google Scholar]
- Mayr U. Spatial attention and implicit sequence learning: Evidence for independent learning of spatial and nonspatial sequences. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:350–364. doi: 10.1037//0278-7393.22.2.350. [DOI] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The role of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R. Complex semantic processing in old age: Does it stay or does it go? Psychology and Aging. 2000a;15:29–43. doi: 10.1037//0882-7974.15.1.29. [DOI] [PubMed] [Google Scholar]
- Mayr U, Kliegl R. Task-set switching and long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000b;26:1124–1140. doi: 10.1037//0278-7393.26.5.1124. [DOI] [PubMed] [Google Scholar]
- Mayr U, Liebscher T. Is there an age deficit in the selection of mental sets? European Journal of Cognitive Psychology. 2001;13:47–69. [Google Scholar]
- Mecklinger A, Mueller N. Dissociations in the processing of “what” and “where” information in working memory: An event-related potential analysis. Journal of Cognitive Neuroscience. 1996;8:453–473. doi: 10.1162/jocn.1996.8.5.453. [DOI] [PubMed] [Google Scholar]
- Mecklinger AD, von Cramon DY, Springer A, Matthes-von Cramon G. Executive control functions in task switching: Evidence from brain injured patients. Journal of Clinical and Experimental Neuropsychology. 1999;21:606–619. doi: 10.1076/jcen.21.5.606.873. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:1423–1442. [Google Scholar]
- Meiran N. Modeling cognitive control in task-switching. Psychological Research. 2000a;63:234–249. doi: 10.1007/s004269900004. [DOI] [PubMed] [Google Scholar]
- Meiran, N. (2000b). Reconfiguration of stimulus task-sets and response task-sets during task-switching. In S. Monsell & J. Driver (Eds.), Control of cognitive processes: Attention and performance: XVIII, (XVIII, (pp. 377–400). Cambridge, MA: MIT Press.
- Meiran N. Task rule congruency and Simon-like effects in switching between spatial tasks. Quarterly Journal of Experimental Psychology A. 2005;58:1023–1041. doi: 10.1080/02724980443000421. [DOI] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with pattern of relatively intact and relatively impaired task-set switching abilities. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 1996;56(2):P88–P102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Meiran N, Chorev Z, Sapir A. Component processes in task switching. Cognitive Psychology. 2000;41:211–253. doi: 10.1006/cogp.2000.0736. [DOI] [PubMed] [Google Scholar]
- Meiran N, Gotler A, Perlman A. Old age is associated with a pattern of relatively intact and relatively impaired task-set switching abilities. Journals of Gerontology: Series B: Psychological Sciences & Social Sciences B. 2001;56:88–102. doi: 10.1093/geronb/56.2.p88. [DOI] [PubMed] [Google Scholar]
- Meiran N, Levine J, Meiran N, Henik A. Task-set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- Meyer, D. E., Evans, J. E., Lauber, E. J., & Gmeindl, L. (1998). The role of dorsolateral prefrontal cortex for executive processes in task switching. Cognitive Neuroscience Society Annual Meeting Abstracts
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Freidman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell, S., & Driver, J. (Eds.). (2000). Control of cognitive processes: Attention and performance: XVIII, (XVIII,. Cambridge, MA: MIT Press.
- Morton JB, Munakata Y. Are you listening? Exploring a developmental knowledge action dissociation in a speech interpretation task. Developmental Science. 2002;5:435–440. [Google Scholar]
- Munakata Y. Challenges to the violation of expectation paradigm: Throwing the conceptual baby out with the perceptual processing bathwater? Infancy. 2000;1:471–477. doi: 10.1207/S15327078IN0104_7. [DOI] [PubMed] [Google Scholar]
- Munoz D, Broughton J, Goldring J, Armstrong I. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;217:1–10. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- O’Craven, K. M., Davidson, M. C., Bergida, R., Savoy, R. L., Diamond, A. (in preparation). Prefrontal cortex contributions to working memory and inhibition as revealed by fMRI.
- O’Craven, K. M., Savoy, R. L., & Diamond, A. (1998). Working memory and inhibition in dorsolateral prefrontal cortex. In Proceedings of the Human Brain Mapping Annual Meeting
- Omori M, Yamada H, Murata T, Sadato N, Tanaka M, Ishii Y, et al. Neuronal substrates participating in attentional set-shifting of rules for visual guided motor selection: A functional magnetic resonance imaging investigation. Neuroscience Research. 1999;33:317–323. doi: 10.1016/s0168-0102(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Osier SF, Kofsky E. Stimulus uncertainty as a variable in the development of conceptual ability. Journal of Experimental Child Psychology. 1965;2:264–279. [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set shifting in patients with frontal lobe damage or Parkinson’s disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kleine BU, Schnitzler A. Magnetic stimulation of the dorsal premotor cortex modulates the Simon effect. NeuroReport. 1999;10:3671–3674. doi: 10.1097/00001756-199911260-00038. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Vu KPL. Mixing location-irrelevant and location-relevant trials: Influence of stimulus mode on spatial compatibility effects. Memory & Cognition. 2002;30:281–293. doi: 10.3758/bf03195289. [DOI] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: Effects of age on general and specific switch costs. Developmental Psychology. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof, K. R. (2002). Activation and suppression in conflict tasks: Empirical classification through distributional analyses. In W. Prinz & B. Hommel (Eds.), Attention and performance XIX: Common mechanisms in perception and action (pp. 494–519). Oxford: Oxford University Press.
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121:815–842. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe NM, Lineweaver TT, Coon VE. Aging of attention: Does the ability to divide decline? Memory and Cognition. 1995;23:56–71. doi: 10.3758/bf03210557. [DOI] [PubMed] [Google Scholar]
- Schuch S, Koch I. The role of response selection for inhibition of task sets in task shifting. Journal of Experimental Psychology: Human Perception & Performance. 2003;29:92–105. doi: 10.1037//0096-1523.29.1.92. [DOI] [PubMed] [Google Scholar]
- Schuch S, Koch I. The costs of changing the representation of action: Response repetition and response-response compatibility in dual tasks. Journal of Experimental Psychology: Human Perception and Performance. 2004;30:566–582. doi: 10.1037/0096-1523.30.3.566. [DOI] [PubMed] [Google Scholar]
- Shaffer LH. Choice reaction with variable S–R mapping. Journal of Experimental Psychology. 1965;70:284–288. doi: 10.1037/h0022207. [DOI] [PubMed] [Google Scholar]
- Shallice, T., & Burgess, P. W. (1991). Higher-order cognitive impairments and frontal lobe lesions in man. In H. S. Levin, H. M. Eisenberg, & A. L. Benton (Eds.), Frontal lobe function and dysfunction (pp. 125–138). Oxford: Oxford University Press.
- Shepard WO. Learning set in preschool children. Journal of Comparative & Physiological Psychology. 1957;50:15–17. doi: 10.1037/h0043206. [DOI] [PubMed] [Google Scholar]
- Simon FR, Small AM. Processing auditory irrelevant information: Interference from an irrelevant cue. Journal of Applied Psychology. 1969;53:433–435. doi: 10.1037/h0028034. [DOI] [PubMed] [Google Scholar]
- Simon, J. R. (1990). The effects of an irrelevant directional cue on human information processing. In R. W. Proctor & T. G. Reeve (Eds.), Stimulus–response compatibility: An integrated perspective (pp. 31–86). Amsterdam: North-Holland.
- Simon JR, Berbaum K. Effect of conflicting cues on information processing: the ‘Stroop effect’ vs. the ‘Simon effect’. Acta Psychologica. 1990;73(2):159–170. doi: 10.1016/0001-6918(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A, Biederman I. Mental set and mental shift revisited. American Journal of Psychology. 1976;89:669–679. [Google Scholar]
- Stoffels EJ. Uncertainty and processing routes in the selection of a response: an S–R compatibility study. Acta Psychologica. 1996;94(2):227–252. doi: 10.1016/0001-6918(95)00063-1. [DOI] [PubMed] [Google Scholar]
- Stürmer B, Leuthold H, Soetens E, Schröter H, Sommer W. Control over location-based response activation in the Simon task: Behavioral and electrophysiological evidence. Journal of Experimental Psychology: Human Perception & Performance. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- Sudevan P, Taylor DA. The cueing and priming of cognitive operations. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:89–103. doi: 10.1037//0096-1523.13.1.89. [DOI] [PubMed] [Google Scholar]
- Sylvester CC, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, et al. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tagliabue M, Zorzi M, Umiltà C, Bassignani F. The role of long-term-memory and short-term-memory links in the Simon effect. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:648–670. doi: 10.1037//0096-1523.26.2.648. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Frazen PI, Welsh TF, Berkowitz AL, Noll DC, et al. A developmental functional MRI study of spatial working memory. NeuroImage. 1999;10:327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Usher M, McClelland JL. The time course of perceptual choice: The leaky, competing accumulator model. Psychological Review. 2001;108:550–592. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
- Usher M, Olami Z, McClelland JL. Hick’s law in a stochastic race model with speed-accuracy tradeoff. Journal of Mathematical Psychology. 2002;46:704–715. [Google Scholar]
- Valle-Inclán F. The locus of interference in the Simon effect: An ERP study. Biological Psychology. 1996;43:147–162. doi: 10.1016/0301-0511(95)05181-3. [DOI] [PubMed] [Google Scholar]
- Valle-Inclán, F., Hackley, S. A., & de Labra, C. (2002). Does stimulus-driven response activation underlie the Simon effect? In W. Prinz & B. Hommel (Eds.), Attention and performance XIX: Common mechanisms in perception and action (pp. 474–493). Oxford: Oxford University Press.
- van Asselen M, Ridderinkhof KR. Shift costs of predictable and unexpected set shifting in young and older adults. Psychologica Belgica. 2000;40:259–273. [Google Scholar]
- Verbruggen FR, Liefooghe B, Szmalec A, Vandierendonck A. Inhibiting responses when switching: Does it matter? Experimental Psychology. 2005;52:125–130. doi: 10.1027/1618-3169.52.2.125. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, De Meersman L. Aging and the Stroop effect: A meta-analysis. Psychology and Aging. 1998;13:120–126. doi: 10.1037//0882-7974.13.1.120. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Vu KPL, Proctor RW. Mixing compatible and incompatible mappings: Elimination, reduction, and enhancement of spatial compatibility effects. Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 2004;57:539–556. doi: 10.1080/02724980343000387. [DOI] [PubMed] [Google Scholar]
- Wager TD, Reading S, Jonides J. Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wascher E, Schatz U, Kuder T, Verleger R. Validity and boundary conditions of automatic response activation in the Simon task. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:731–751. doi: 10.1037//0096-1523.27.3.731. [DOI] [PubMed] [Google Scholar]
- Wascher E, Wolber M. Attentional and intentional cueing in a Simon task: An EEC-based approach. Psychological Research/Psychologische Forschung. 2004;68:18–30. doi: 10.1007/s00426-002-0128-z. [DOI] [PubMed] [Google Scholar]
- Waszak F, Hommel B, Allport A. Task-switching and long-term priming: Role of episodic stimulus-task bindings in task-shift costs. Cognitive Psychology. 2003;46:361–413. doi: 10.1016/s0010-0285(02)00520-0. [DOI] [PubMed] [Google Scholar]
- Wiegand K, Wascher E. Dynamic aspects of stimulus–response correspondence: Evidence for two mechanisms involved in the Simon Effect. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:453–464. doi: 10.1037/0096-1523.31.3.453. [DOI] [PubMed] [Google Scholar]
- Wühr P. Sequential modulation of logical-recoding operations in the Simon task. Experimental Psychology. 2004;58A:705–731. doi: 10.1027/1618-3169.51.2.98. [DOI] [PubMed] [Google Scholar]
- Wühr P. Evidence for gating of direct response activation in the Simon task. Psychonomic Bulletin & Review. 2005;12:282–288. doi: 10.3758/bf03196373. [DOI] [PubMed] [Google Scholar]
- Wylie G, Allport A. Task switching and the measurement of “switch costs”. Psychology Research. 2000;63:212–233. doi: 10.1007/s004269900003. [DOI] [PubMed] [Google Scholar]
- Yeung N, Monsell S. The effects of recent practice on task switching. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:919–936. doi: 10.1037/0096-1523.29.5.919. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Craik FIM, Booth L. Executive function across the life span. Acta Psychologica. 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. [DOI] [PubMed] [Google Scholar]


