Abstract
Although there is good evidence that immunity to the blood stages of malaria parasites can be mediated by different effector components of the adaptive immune system, target antigens for a principal component, effector CD4+ T cells, have never been defined. We generated CD4+ T cell lines to fractions of native antigens from the blood stages of the rodent parasite, Plasmodium yoelii, and identified fraction-specific T cells that had a Th1 phenotype (producing IL-2, IFN-γ, and tumor necrosis factor-α, but not IL-4, after antigenic stimulation). These T cells could inhibit parasite growth in recipient severe combined immunodeficient mice. N-terminal sequencing of the fraction showed identity with hypoxanthine guanine xanthine phosphoribosyl transferase (HGXPRT). Recombinant HGXPRT from the human malaria parasite, Plasmodium falciparum, activated the T cells in vitro, and immunization of normal mice with recombinant HGXPRT reduced parasite growth rates in all mice after challenge.
Although it is known that immunity to malaria can be mediated by different immune mechanisms (1–4), to date only target antigens for antibodies have been defined, and these targets are either variant or demonstrate allelic polymorphism (5). CD4+ T cells can adoptively transfer resistance to malaria (3, 6, 7) in rodent models, but target antigens have not been defined. Of interest, CD4+ T cells displaying a Th1 cytokine profile (IL-2- and IFN-γ-secreting) and specific for the major merozoite surface protein 1 (MSP119) of Plasmodium yoelii (a leading vaccine candidate homologue) are unable to transfer resistance, and immunization of mice with defined T cell epitopes from MSP119 did not render the mice resistant (8). Identification of a target antigen(s) would provide an additional vaccine strategy for vaccine design. The goal of this study was to define such target antigens in the rodent model, P. yoelii.
Materials and Methods
Mice.
Four- to 6-week-old normal BALB/c, athymic BALB/c nude, and BALB/c severe combined immunodeficient (SCID) mice were used when 6–8 weeks old.
Parasites and Parasitemia.
P. yoelii 17XNL was maintained by passaging between infected and uninfected mice via the i.p. route. A thin blood smear was air-dried and stained using Diff-Quick (Lab Aids, Narrabeen, Australia).
Preparation of Parasite Antigens.
Preparation of whole parasite antigen (pRBC).
Parasitized red blood cells (pRBC) in PBS were lysed by incubation in erythrocyte lysis buffer (0.17 M Tris-hydroxymethyl aminomethane/0.16 M ammonium chloride; pH 7.2) at 37°C for 10 min. The parasites were then freeze–thawed at least three times, sonicated at 4°C to break up the parasites, aliquoted, and stored at −70°C to be used for in vitro cell cultures.
Preparation of soluble parasite antigen (sAg).
RBCs were lysed by incubation of the blood in 0.01% saponin/PBS at 37°C for 20 min in the presence of protease inhibitors (Sigma). The blood was then given another wash in saponin/PBS buffer before the pRBCs were sonicated in cold PBS at 4°C.
After sonication, the lysate was centrifuged at 100,000 × g for 30 min at 4°C. The supernatant was then collected and dialyzed against three changes of PBS at 4°C. The protein concentration was determined by bicinchoninic acid assay (Pierce) and the sAg was stored at −70°C until use.
Generation of T Cell Lines in Vitro.
Mice were immunized s.c. at the hind footpads with 50 μl of antigen emulsified in complete Freund's adjuvant (CFA) (Sigma). Inguinal and popliteal lymph nodes were collected 7–10 days later, and lines were then produced as described (7).
Lymphocyte Proliferation Assay.
T cells taken after rest phase were mixed with irradiated syngeneic splenocytes (1:3) and cultured at 2 × 106 cells per ml for 4 days in 96-flat-well microtiter plates in a humidified incubator containing 5% CO2 at 37°C. Cells were in triplicate or quadruplicate wells in medium only (negative control), tuberculin purified protein derivative (PPD) (CSL, Parkville, Australia), or Con A (Sigma) as positive controls or with test antigen, for the assessment of proliferation and/or cytokine secreted into culture supernatants at 24, 48, or 72 h.
Proliferation was measured by pulsing with 0.25 μCi (1 Ci = 37 GBq) per well [3H]thymidine (Amersham Pharmacia Biotech), harvesting onto glass fiber mats, and scintillation counting.
Adoptive Transfer and Infection-Cure Regimens.
T cells harvested at resting phase were purified over Ficoll/Hypaque (Pharmacia), washed, and resuspended in PBS, and 200 μl was transfused i.v. into each mouse. Twenty-four hours after transfusion, the T cells were expanded in vivo by an i.v. infection of 105 pRBC per mouse. Two days later the mice were treated i.p. with pyrimethamine (0.2 mg/0.2 ml PBS per mouse per day) for 3 days. This cycle of infection-cure regimen was repeated before mice were given an i.v. challenge infection (105 pRBC per mouse). There were 3-week intervals between reinfections.
Cytokine ELISA.
Supernatants from cell cultures were collected. IL-4 and IFN-γ− were measured by ELISA (9), using mAb clones BVD4-1D11 (5 μg/ml) and R4-6A2 (2 μg/ml) (PharMingen), respectively.
Intracellular Cytokine Staining.
Viable rested T cells were stimulated at 37°C for 6 h, with 40 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma) and 2 μM calcium-ionophore (Sigma) in the presence of monensin (GolgiStop, PharMingen). Cells were washed twice and stained with a 1:50 FITC-conjugated rat anti-mouse CD4 mAbs (Caltag, Burlingame, CA). Cells were washed, resuspended, and fixed in 4% ice-cold paraformaldehyde for 30 min. After two washes the cells were further fixed and permeabilized by incubation in cytofix/cytoperm (PharMingen) for 20–30 min at 4°C. Cells were washed twice in permeabilization buffer and resuspended at 2 × 107 cells per ml of cytofix/cytoperm, and 106 cells were dispensed into fluorescence-activated cell sorting (FACS) tubes. Cells were stained for 30 min at 4°C in the dark for IL-2, IFN-γ, tumor necrosis factor (TNF)-α, and IL-4 by using a 1:50 phycoerythrin-conjugated rat anti-mouse IL-2, IFN-γ, TNF-α, and IL-4 mAbs (PharMingen), respectively, washed, and analyzed by FACS.
Fluorescence was analyzed using a FACSCalibur flow cytometer (Becton Dickinson) equipped with cellquest software.
Analysis of Cell Surface Phenotypes.
Cells were stained for surface expression of the following molecules: CD3, CD4, CD8, CD19, NK1.1, TCR α/β, and TCR γ/δ, as described (10).
Immunization and Challenge Infections.
Groups of three to five mice were immunized s.c. on the hind footpad with antigen emulsified in complete Freund's adjuvant (CFA) (Sigma). At 4 and 6 weeks, booster immunizations were given s.c. on the abdomen and i.p., respectively, by using same amount of antigen emulsified in incomplete Freund's adjuvant (IFA) (Sigma). PBS mixed with adjuvant was used as control antigen. Ten days after the last booster the mice were challenged i.v. with P. yoelii 17XNL parasitized RBC.
Serum Analysis by ELISA for Parasite-Specific Antibodies.
This was performed as described (10).
Preparative Isoelectric Focusing (IEF) for Fractionation of Soluble Parasite Proteins.
Preparative wide-range (pH 3.5–10.0) and narrow-range (pH 5.3–6.0) IEF was performed on a Multiphor II electrophoresis unit (Amersham Pharmacia Biotech) as described (11).
SDS/PAGE.
A standard method described by Laemmli (12) was used with few modifications. Protean II gel slab (Bio-Rad) was cast using acrylamide:bis-acrylamide (29:1).
After loading, the gel was electrophoresed at constant voltage (60 V) for ≈1 h and 30 min. The proteins were then visualized by staining with Coomassie brilliant blue R-250 (Sigma) or silver stain by the method of Rabilloud et al. (13).
Passive Elution of Proteins from SDS/PAGE Gels.
Coomassie-stained gels were washed in water for 30 min. Protein bands were excised from the gel with a sterile scalpel and the proteins were eluted overnight at 37°C with extraction buffer (100 mM sodium acetate/0.1% SDS/10 mM DTT). The supernatants were collected and analyzed by SDS/PAGE to confirm purity and subsequently transferred onto a poly(vinylidene difluoride) (PVDF) membrane.
PVDF-Membrane Electroblotting and N-Terminal Sequencing.
Proteins were separated on SDS/PAGE and blotted onto a PVDF membrane by using Multiphor II electrophoresis unit. The band of interest excised was sequenced by the Edman degradation method (14).
Statistical Analysis.
The significance of differences was determined using Student's t test.
Results
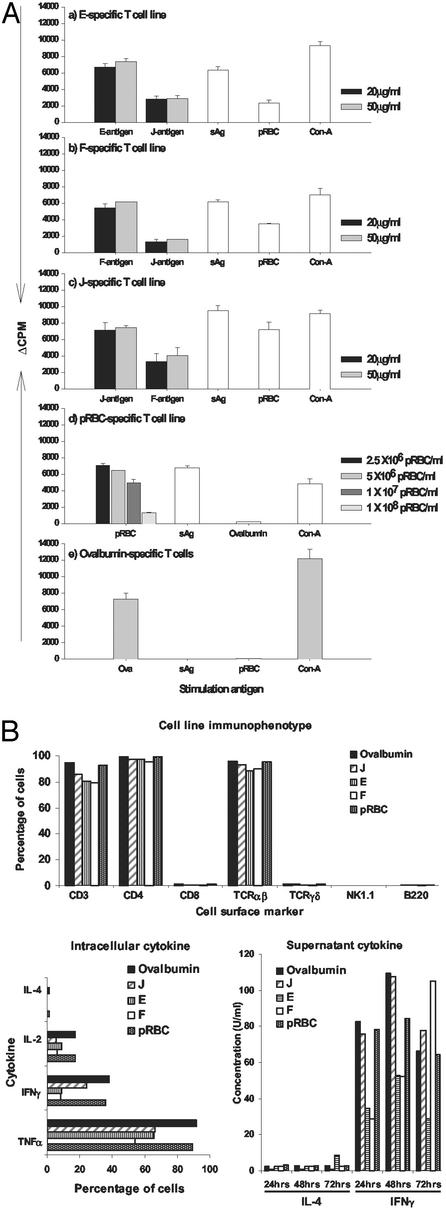
We made a soluble preparation of P. yoelii pRBC that was fractionated by IEF into 12 pools (A–L) that were then emulsified in CFA and used to immunize mice. Lymph nodes were taken 8 days later and polyclonal T cell lines were produced. Lines were successfully established for the E, F, and J fractions. The lines demonstrated antigenic specificity (Fig. 1A) and expressed CD3, CD4, and αβ T cell receptors and produced cytokines (IL-2, IFN-γ, and tumor necrosis factor-α, but not IL-4; Fig. 1B). The lines were given to athymic nude BALB/c mice that were challenged with parasites. One line (F), which demonstrated the most potent antiparasite activity, was chosen for further investigation. However, recipients of F-specific T cells died (at low parasitemia) after challenge, as did the recipients of the other lines. T cell-mediated immune pathology can be a common feature of rodent malaria infections (7, 15).
Figure 1.
(A) Specificity of T cell lines by proliferative response. Final concentrations of 100 μg/ml OVA, 500 μg/ml soluble parasite antigen (sAg), 2.5 × 106 pRBC per ml, and 5 μg/ml Con A were used. The data are mean cpm ± SD of triplicate wells. The background cpm for F-, J-, pRBC-, E-, and OVA-specific T cell lines were 2,630 ± 51, 560 ± 83, 2,573 ± 325, 3,330 ± 403, and 274 ± 46, respectively. (B) Immunophenotypic and cytokine characterization of parasite-specific and OVA T cell lines. For each cell line, the percentage of cells or cytokine concentration is a representative result of cells or supernatant harvested from two 24-well culture plates in two or three independent experiments.
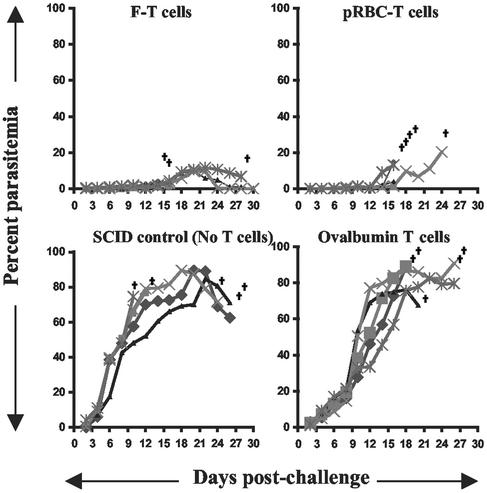
The testing procedure was thus modified to allow the cells to mature for longer periods of time in the host before final challenge. There is evidence that the natural immune response to malaria parasites does vary over time, and this is associated with reduced pathology (16–19). T cells were given to BALB/c SCID mice (deficient in both T and B cells) that were then exposed to two infectious episodes, each curtailed by antimalaria chemotherapy 2 days after challenge with 3-week interval between infections. SCID mice were chosen so that any protection could be attributed to the transferred T cells. Control mice received T cells generated to whole parasites (pRBC) or, as negative controls, either no T cells or T cells specific for an irrelevant antigen, ovalbumin (OVA), and demonstrating the same phenotypic characteristics (CD4+, Th1). After the two infectious episodes, all mice then received a final challenge not curtailed by chemotherapy. Control mice succumbed to challenge. However, two of five mice that had received the F-specific T cells survived, and all recipients demonstrated significantly lower parasite densities throughout the infection (Fig. 2). Sera from surviving mice contained no parasite-specific antibodies (data not shown).
Figure 2.
Parasitemia levels of BALB/c SCID mice after adoptive transfer of T cells and challenge infection. The mice (five mice per group) were transfused with 5 × 106 antigen-specific T cells. One day later the mice were subjected to two cycles of an infection-cure regimen before an i.v. challenge infection with 105 pRBC. †, death.
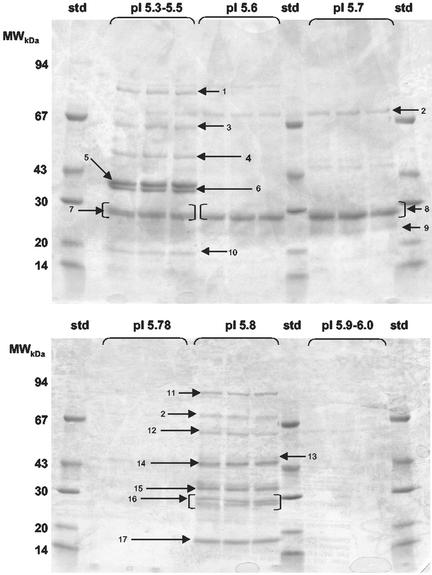
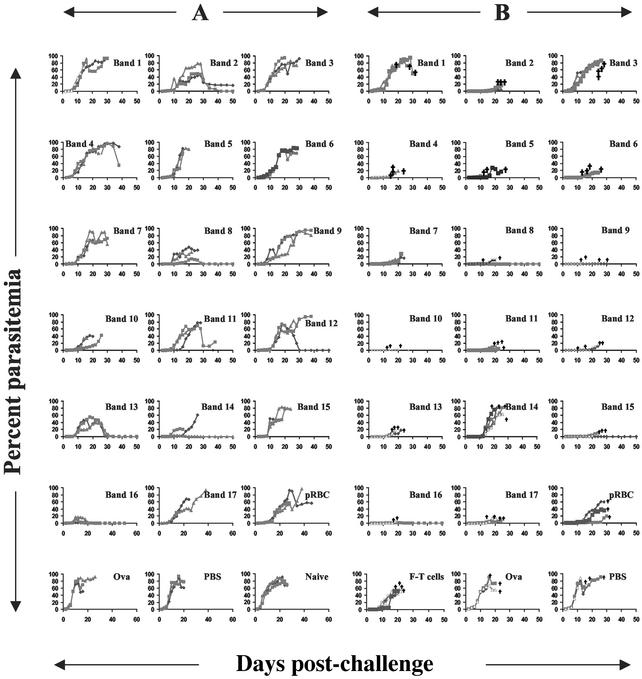
To identify the antigenic specificity or specificities of the protective T cells, fraction F and adjacent fractions of similar pI were fractionated by narrow-range IEF (pH 5.3–6.0) and proteins were separated by size on SDS/PAGE. Seventeen bands were selected for further study (Fig. 3). Two methods were followed. In the first approach (A), new T cell lines were made by immunizing naive mice with individual bands and then expanding the cells in vitro with fraction F. These new T cell lines were then tested in vitro and in vivo (as above). In the second approach (B), T cells specific for the fraction F were given to SCID mice that were then immunized with individual bands. Mice were then challenged with a live infection. The results of both approaches are given in Fig. 4, showing that T cells specific for the doublet band 16 were able to slow parasite growth in all animals and to completely protect 100% and 50% of mice in approaches A and B, respectively. Band 16-specific T cells demonstrated a Th1 profile (data not shown). Of all of the T cells, those specific for band 16 showed the most pronounced effect. In Fig. 4A the mean peak parasitemia of mice that received band 16-specific T cells was 10.3%. For all other bands the mean peak parasitemia varied from 29% to 93.7% (average 69%). In experiment B the mean peak parasitemia for recipients of F-specific T cells that were expanded in vivo by band 16 was 2.2%. For this experiment the mean peak parasitemia for the other bands ranged from 0.6% to 86.6% (average 22%). In approach B, however, 2 or 4 mice survived compared to the total of 3 of 64 mice that survived in all of the other bands combined.
Figure 3.
Resolution of proteins in the F pool of fractions on SDS/PAGE, after narrow-range IEF. Preparative SDS/PAGE gradient gel (6–18%) was stained with Coomassie blue to indicate single-band proteins nominated for excision and assessment of protective efficacy. The arrows indicate the position of the 17 bands. The relative molecular masses of standard marker proteins (std) are indicated on the left.
Figure 4.
Parasitemia levels in BALB/c SCID mice showing evaluation of protective efficacy of individual protein bands by using two independent assays, A and B. In A, the mice (three mice per group) were adoptively transfused with single-band-specific T cells (5 × 106 per mouse) expanded in vitro with the F pool of protein fraction antigens. The cells were expanded in vivo 24 h after transfer by two cycles of an infection-drug-cure regimen, as described in Materials and Methods, before the mice were challenged i.v. with parasitized erythrocytes (105 pRBC per mouse). In B, SCID mice (three or four mice per group) were transfused with F-antigen specific T cells (106 per mouse), immunized twice with single-band proteins in complete Freund's adjuvant (CFA)/incomplete Freund's adjuvant (IFA) (total of 5 μg per mouse), and subjected to two cycles of an infection-cure regimen. All mice were then challenged i.v. with parasitized erythrocytes (105 pRBC per mouse). †, death.
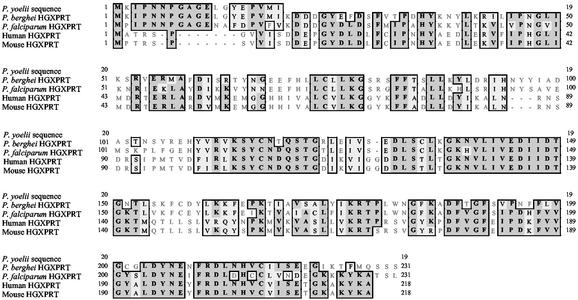
The doublet bands (Fig. 3, band 16) were then sequenced using Edman degradation, yielding MKIPNNPGAGELGYEPVMI and MKIPN, known to be the N-terminal sequence of the purine salvage enzyme, HGXPRT, from the closely related parasite Plasmodium berghei (Fig. 5). The sequence for HGXPRT from P. yoelii is not present in the database. Recombinant P. berghei or P. yoelii HGXPRT was not available, but Plasmodium falciparum HGXPRT (pfHGXPRT) was available (20) and could stimulate protective band-specific T cells (Fig. 6A).
Figure 5.
Alignment of HGXPRT sequences from Plasmodia, humans, and mice. Dark gray shading indicates identities; light shading indicates conservative substitutions.
Figure 6.
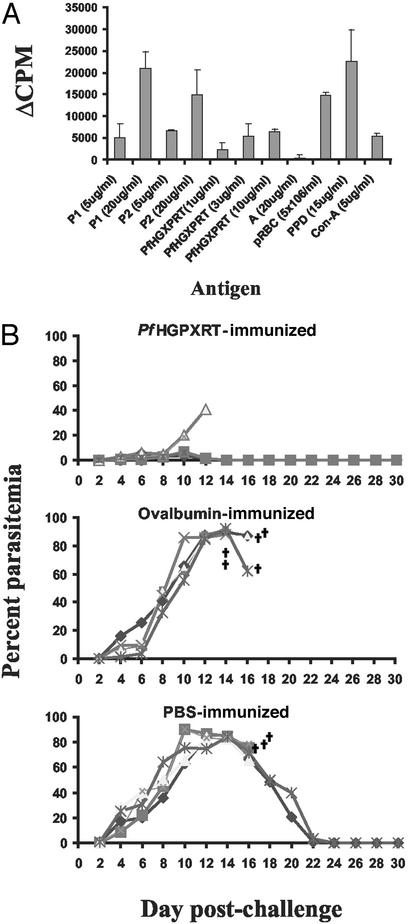
(A) Determination of specificity of lymphocytes primed in vivo with polypeptide P1 (upper band of protective doublet band 16) by proliferative assay. Normal BALB/c mice were immunized with P1. Proliferative responses of P1-primed lymph node cells to culture stimulation with P1, P2 (lower band of protective doublet) recombinant pfHGXPRT, an unrelated band (A), and P. yoelii pRBC were examined ex vivo. Purified protein derivative (PPD) and Con A were used as positive controls. Based on N-terminal sequencing, both bands (P1 and P2) appear to be HGXPRT. The data are mean cpm ± SD of triplicate wells. The background cpm was 2,551 ± 396. (B) Parasitemia levels in BALB/c mice immunized with recombinant pfHGXPRT after challenge infection with P. yoelii 17XNL. Mice immunized with OVA and PBS are included as controls. The mice (five mice per group) were administered three successive vaccinations with antigen in Freund's adjuvants following a standard schedule described in Materials and Methods. All mice were challenged i.v. with 105 pRBC parasites 10 days after final booster vaccination. †, death.
Normal BALB/c mice were immunized with recombinant pfHGXPRT and challenged with a live P. yoelii infection (Fig. 6B). Control mice were immunized with OVA or with PBS. On day 8 after challenge, the mean parasitemia of immunized mice was 3.6%, compared with 43.2% in OVA-immunized mice (P < 0.0001) and 47.8% in PBS-immunized mice (P < 0.001). Two of five HGXPRT-immunized mice were able to completely resolve their infection. There was no difference in the parasitemia of OVA-immunized and PBS-immunized mice (P = 0.545).
Discussion
This study identifies HGXPRT as a target antigen of protective CD4+ T cells. The efficacy of the T cells is independent of antibody, as shown by the ability of T cells to transfer resistance into SCID mice. Precisely how parasite-specific CD4+ T cells control parasite growth is unknown, but many studies have shown that such T cells (of unknown specificity) can control parasite growth (3, 6, 7), and many have suggested that inflammatory mediators downstream of IFN-γ and tumor necrosis factor-α, such as nitric oxide (6, 21, 22) and oxygen radicals (23, 24), are critical. We have shown that humans can be immunized by exposure to ultra-low doses of P. falciparum pRBC (25), correlating with induction of a parasite-specific Th1 T cell response and up-regulation of nitric oxide synthase, but lack of any measurable antibody response. The activation of the cells is specific but the effector function may be nonspecific. A side effect of the activation of parasite-specific T cells may be immunopathology (15), which might explain why not all vaccinated or T cell-transfused recipients survive, even though the parasite densities are significantly reduced in all mice. A vaccine aimed at inducing cell-mediated immunity may benefit greatly by combination with an antidisease vaccine (26).
We previously investigated whether T cells specific for the merozoite surface protein fragment, MSP119, could protect mice (8). The results were uniformly negative, despite the fact that MSP119 is capable of inducing a high degree of protection. Curiously, the nonprotective MSP119-specific T cells exhibited the same phenotypic characteristics (CD4+, Th1) as the protective HGXPRT-specific T cells in this study. More recently, we have identified epitopes on the MSP133 fragment that could induce T cells capable of delaying the growth of parasites after adoptive transfer. However, recipients eventually died at high parasitemia (27). Because T cells recognize antigen only after it is processed, it is unexplained why T cells of one specificity can protect, whereas those of a different specificity can not. HGXPRT is found in electron-dense regions within merozoites and in vesicles within the cytoplasm of the infected red cell (28). It is possible that antigen accumulates and that abundance is a critical factor.
Because HGXPRT is highly conserved, it is curious that natural immunity does not develop more quickly (29). It is possible such T cells are nevertheless contributing to immunity. Minor differences in HGXPRT may alter the T cell response to the protein. It is also worth noting that parasite infection is known to lead to apoptosis of human mononuclear cells (30) and of parasite-specific CD4+ T cells in vivo in rodent systems (31, 32). A further possibility is that HGXPRT liberated from pRBC cytoplasm into the plasma at the time of pRBC rupture may tolerize the immune system (33). Thus, the human T cell response may be suppressed by infection and not develop. The purpose of this study was not to define the immune response that developed after infection, because clearly that is often unable to control subsequent malaria infection, but rather to define the immune response that could be induced by subunit vaccine candidates and ask whether such a response could subsequently control infection.
Malaria parasites and their hosts express HGXPRT. Fig. 5 shows the sequence alignment for the HGXPRT sequences of different Plasmodia, humans, and mice. It would be predicted that, because of immunological tolerance to self antigens, the T cell epitopes present on parasite HGXPRT would be found in the regions that lacked similarity. Defined epitopes, as opposed to the entire parasite HGXPRT, might constitute a preferred vaccine molecule because of fears that immunization with whole parasite HGXPRT might “break” immunological tolerance.
T cell epitopes from HGXPRT may be useful alone as an immunogen or may be an ideal component that could be linked to or mixed with other vaccine molecules, all of which are currently designed to stimulate a protective antibody responses (5) or an immune response to protect against disease symptoms per se (26, 34). By adding an additional type of immune response, it is likely that the chances of developing a successful vaccine will greatly improve.
Abbreviations
- HGXPRT
hypoxanthine guanine xanthine phosphoribosyl transferase
- IEF
isoelectric focusing
- OVA
ovalbumin
- pRBC
parasitized RBC
- SCID
severe combined immunodeficient
References
- 1.Freeman R R, Parish C R. Exp Parasitol. 1981;52:18–24. doi: 10.1016/0014-4894(81)90056-4. [DOI] [PubMed] [Google Scholar]
- 2.Grun J L, Weidanz W P. Infect Immun. 1983;41:1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brake D A, Long C A, Weidanz W P. J Immunol. 1988;140:1989–1993. [PubMed] [Google Scholar]
- 4.Seixas E M, Langhorne J. J Immunol. 1999;162:2837–2841. [PubMed] [Google Scholar]
- 5.Good M F. Nat Rev Immunol. 2001;1:117–125. doi: 10.1038/35100540. [DOI] [PubMed] [Google Scholar]
- 6.Taylor-Robinson A W, Phillips R S, Severn A, Moncada S, Liew F Y. Science. 1993;25:1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- 7.Amante F H, Good M F. Parasite Immunol. 1997;19:111–126. doi: 10.1046/j.1365-3024.1997.d01-187.x. [DOI] [PubMed] [Google Scholar]
- 8.Tian J H, Good M F, Hirunpetcharat C, Kumar S, Ling I T, Jackson D, Cooper J, Lukszo J, Coligan J, Ahlers J, Saul A, et al. Parasite Immunol. 1998;20:263–278. doi: 10.1046/j.1365-3024.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- 9.Sander B, Hoiden I, Andersson U, Moller E, Abrams J S. J Immunol Methods. 1993;166:201–214. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Hodder A N, Yan H, Crewther P E, Anders R F, Good M F. J Immunol. 2000;165:389–396. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- 11.Dainese P, Hoyer-Hansen G, Bassi R. Photochem Photobiol. 1990;51:693–703. doi: 10.1111/php.1990.51.6.693. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Rabilloud T, Carpentier G, Tarrous P. Electrophoresis. 1988;9:228–291. doi: 10.1002/elps.1150090608. [DOI] [PubMed] [Google Scholar]
- 14.Edman P, Begg G. Eur J Biochem. 1967;1:80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- 15.Hirunpetcharat C, Finkelman F, Clark I A, Good M F. Parasite Immunol. 1999;21:319–329. doi: 10.1046/j.1365-3024.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown A E, Webster H K, Teja-Isavadharm P, Keeratithakul D. Clin Exp Immunol. 1990;82:97–101. doi: 10.1111/j.1365-2249.1990.tb05410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Int Immunol. 1989;1:416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 18.Baird J K. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 19.Omer F M, Riley E M. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keough D T, Ng A L, Winzor D J, Emmerson B T, de Jersey J. Mol Biochem Parasitol. 1999;5:29–41. doi: 10.1016/s0166-6851(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 21.Rockett K A, Awburn M M, Cowden W B, Clark I A. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson M M, Tam M F, Wolf S F, Sher A. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 23.Clark I A, Hunt N H. Infect Immun. 1983;39:1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wozencraft A O, Dockrell H M, Taverne J, Targett G A, Playfair J H. Infect Immun. 1984;43:664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pombo D J, Lawrence G, Hirunpetcharat C, Rzepczyk C, Bryden M, Cloonan N, Anderson K, Mahakunkijcharoen Y, Martin L B, Wilson D, et al. Lancet. 2002;360:610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 26.Schofield L, Hewitt M C, Evans K, Siomos M A, Seeberger P H. Nature. 2002;418:785–789. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 27.Wipasa J, Hirunpetcharat C, Mahakunkijcharoen Y, Xu H, Elliott S, Good M F. J Immunol. 2002;169:944–951. doi: 10.4049/jimmunol.169.2.944. [DOI] [PubMed] [Google Scholar]
- 28.Shahabuddin M, Gunther K, Lingelbach K, Aikawa M, Schreiber M, Ridley R G, Scaife J G. Exp Parasitol. 1992;74:11–19. doi: 10.1016/0014-4894(92)90134-v. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood B M, Bradley A K, Greenwood A M, Byass P, Jammeh K, Marsh K, Tulloch S, Oldfield F S, Hayes R. Trans R Soc Trop Med Hyg. 1987;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 30.Toure-Balde A, Sarthou J L, Aribot G, Michel P, Trape J F, Rogier C, Roussilhon C. Infect Immun. 1996;64:744–750. doi: 10.1128/iai.64.3.744-750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirunpetcharat C, Good M F. Proc Natl Acad Sci USA. 1998;95:1715–1720. doi: 10.1073/pnas.95.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Wipasa J, Yan H, Zeng M, Makobongo M O, Finkelman F D, Kelso A, Good M F. J Exp Med. 2002;195:881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchison N A. Immunology. 1968;15:509–530. [PMC free article] [PubMed] [Google Scholar]
- 34.Clark I A, Schofield L. Parasitol Today. 2000;16:451–454. doi: 10.1016/s0169-4758(00)01757-9. [DOI] [PubMed] [Google Scholar]