Abstract
Objective
To determine the effects of hyperdynamic endotoxemia on the motility of the small intestine.
Summary Background Data
Motility disorders of the gastrointestinal tract are a common complication of sepsis. It has been suggested that gram-negative endotoxin plays a role in the pathogenesis of the accompanying diarrhea frequently observed.
Methods
Pigs were infused with Escherichia coli lipopolysaccharide for a 24-hour period. During this fasting period jejunal motility was measured using ambulatory manometry. One and 4 days after cessation of endotoxin, pigs were enterally fed, and again motility was recorded.
Results
Hyperdynamic endotoxemia was achieved in this model. Manometric pressure recordings revealed that endotoxin infusion accelerated the migrating motor complex (MMC) migration along the jejunum. Also, a simultaneous increase in MMC cycling frequency was observed in the endotoxin-treated group. Elevated MMC migration velocity and cycling frequency were maintained the following day after endotoxin during feeding and returned to basal values 4 days after endotoxin.
Conclusions
A small dose of continuously infused endotoxin significantly provokes jejunal motility disturbances that may contribute to diarrhea.
Disturbed gastrointestinal (GI) motility is a frequently observed complication in septic patients. In septic patients, the complex interaction among anesthesia, surgical manipulation, inflammatory agents, nutritional status, and hormone levels determine the picture of altered motility. 1 Retarded passage in the large intestine leads to constipation and in the advanced stages to ileus, whereas the main symptom in accelerated passage of the large intestine is diarrhea. 1 Challenge with bacterial endotoxins is a common way to imitate septic shock or sepsis. 2 Intravenous Escherichia coli endotoxin administration was found to affect small intestinal motility in rats, 3 piglets, 4 and dogs. 5 Several lines of evidence suggest that endotoxin-induced mediators such as neuropeptides, cytokines, and nitric oxide are involved in the disturbances of GI motility observed in endotoxemia. 6–10 Besides these mediators, the rate of intestinal blood supply plays an important factor in the gut’s functioning and motility. 11
In the small bowel, the fasting GI motility pattern, the migrating motor complex (MMC), has been attributed a housekeeper function, and its maintenance has been considered essential in the prevention of gut-derived infections. 12 The effect of hyperdynamic endotoxemia with preserved visceral blood flow on small intestinal motor activity has not been well characterized. Therefore, the aim of this study was to investigate the effects of prolonged hyperdynamic endotoxemia on the MMC pattern of the small intestine. In contrast to previous endotoxemia models in which single sublethal doses of endotoxin were administrated, in this clinical model of sepsis continuous small doses of endotoxin were infused, accompanied by fluid resuscitation. This experimental set-up better resembles the hyperdynamic septic state 2 that is encountered in septic patients receiving modern intensive care. Moreover, as enteral nutrition is thought to play a role in the maintenance of gut function, 13 postendotoxemic effects on motility were assessed during nutritional intervention.
METHODS
Animals
Twelve female cross-bred pigs (Yorkshire × Dutch Landrace: 20–22 kg body weight) were fed 1 kg regular pig feed (Landbouwbelang, Roermond, The Netherlands; 16% crude protein) daily in the morning, supporting a growth rate of approximately 300 g/day. Before surgery, pigs were randomly assigned to the endotoxin treatment group (n = 6) or the control group (n = 6). The Animal Ethics Committee of the Maastricht University approved the study.
Surgical Procedure
The night before surgery, food was withheld from the pigs. One hour after premedication with 10 mg/kg intramuscular azaperone (Stresnil, Janssen Pharmaceutica, Beersse, Belgium), anesthesia was induced by a mixture of N2O/O2 (1:2) and halothane (0.8%). After intubation, the pigs were intravenously given 6.25 mg/kg lincomycin-2 HCl (Lincomycin, A.U.V., Cuyk, The Netherlands) as bactericidal prophylaxis and 12.5 mg/kg spectinomycin HCl lyophil (Spectinomycin, A.U.V.) as bacteriostatic prophylaxis. To avoid coagulation and as postoperative analgesia, 50 mg/kg flunixine (Finadyne, Schering-Plough, Brussels, Belgium) was given. During surgery, the anesthesia was maintained by the N2O/O2–halothane mixture and intravenous Lactetrol (per milliliter: 5.76 mg NaCl, 0.37 mg KCl, 0.37 mg CaCl2, 0.2 mg MgCl2, 5 mg sodium lactate, methylparahydroxybenzoic acid, Janssen Pharmaceutica). The surgical procedure has been described in detail. 14 Catheters were cannulated in (A1) the abdominal aorta with the end positioned just above the bifurcation, in (P) the portal vein for blood sampling, in (V2) the inferior caval vein with the end positioned just above the right renal vein for endotoxin infusion, and in (S) the splenic vein, which was used for infusion of para-aminohippuric acid (PAH). A gastric tube (inner diameter 1.6 mm, outer diameter 4.8 mm, Tygon, Westvaco, Cleveland, OH) was inserted for enteral feeding. The catheters were tunneled through the abdominal wall and skin. In addition, a Bishop-Koop stoma was constructed. Briefly, 20 cm distal to the ligament of Treitz (the transition from duodenum to jejunum), the jejunum was transected and the anatomic continuity of the remaining bowel was re-established by a single-layer running suture (PDS 4-0 synthetic polydioxanon; Ethicon, Norderstedt, Germany), creating an end-to-side anastomosis (Fig. 1). The transected distal part of the jejunum was sutured to the skin, creating a single-barrel stoma. On experiment days, the manometry catheter was inserted via the stoma into the lumen of the small bowel.
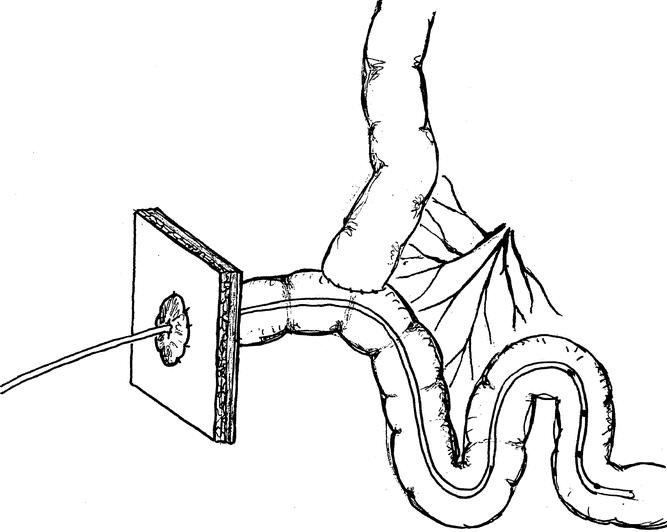
Figure 1. Surgical jejunostomy for catheter insertion according to the end-to-side single-layer anastomotic closure.
During recovery of the pigs, the stoma was closed by a filled balloon catheter (size Ch.16, 3–5 mL; Rüsch-Gold, Kernen, Germany) to avoid leakage of gastrointestinal juices from the jejunum. Pigs wore a canvas harness to protect the catheters and the stoma and to allow easy handling. On the first 2 postoperative days, pigs received twice-daily 6.25 mg/kg lincomycin and 12.5 mg/kg spectinomycin intravenously. Pigs were repeatedly placed in a movable cage to acclimate to the situation in which the experiment would be carried out.
Experimental Protocol
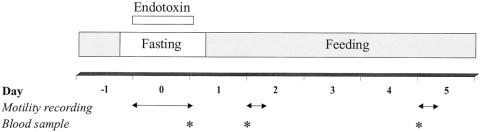
From postoperative day 10 onward, a high-protein liquid diet (Nutrison Steriflo Protein-Plus, Nutricia, Zoetermeer, The Netherlands) was enterally infused into the gastric tube via a swivel system connected to a pump. To acclimate the pigs to the diet, enteral nutrition was infused at an hourly rate of 4 mL/kg (0.3 g/kg of protein per hour) for the first 3 days. On postoperative day 10, after an 8-hour fast, the experiments were started. The design of the protocol is shown in Figure 2. At 0 hours, an intravenous infusion of endotoxin (LPS from Escherichia coli, serotype 055:B5; Sigma Chemical Co., St. Louis, MO) dissolved in saline was started at an hourly rate of 3 μg/kg. Endotoxin was infused for 24 hours. Control pigs received saline at an equal infusion rate. All pigs were given extra saline infusion to replenish intravascular volume losses and to maintain visceral flow: 30 mL/kg per hour for the first 8 hours and 20 mL/kg per hour for the following 16 hour. At 0 hours, the manometry catheter was inserted through the jejunal stoma into the small intestine and 24-hour motility recordings of the endotoxemic period were collected (day 0). Eight hours after cessation of the endotoxin infusion, enteral nutrition was restarted at the previous infusion rate (4 mL/kg per hour). One and 4 days after cessation of the endotoxin infusion (days 2 and 5, respectively), 8-hour motility readings from the postprandial postendotoxemic pigs were obtained. At days 1, 2, and 5, body temperature, mean arterial blood pressure, and heart rate were measured. Also at these days, blood samples were collected to measure visceral blood flow.
Figure 2. Experimental protocol. For blood flow measurements at days 1, 2, and 5, blood was sampled in triplicate at arterial, portal, and venous sites 60 minutes after the start of the PAH infusion.
Infusion Protocol
At days 1, 2, and 5, infusion protocols were performed to measure visceral blood flow. One hour before the start of the infusions, a primed constant infusion of PAH (25 mmol/L, A 1422, Sigma Chemical, St. Louis, MO) was given. The PAH infusion was started at a rate of about 40 mL/h for 1 hour through the S catheter after an initial bolus of 5 mL. 14 The steady-state condition for PAH was assessed in a pilot experiment in which blood samples were collected at 10-minute intervals. Steady state was achieved 60 minutes after the start of the infusion. 14 Blood samples from the arterial, portal, and venous sites were obtained in triplicate 60 minutes after the start of the PAH infusion.
Sample Processing and Biochemical Analysis
Directly after sampling, blood was distributed in prechilled, heparinized tubes (Sarstedt, Nümbrecht, Germany) on ice. For analysis of PAH, 300 μL whole blood was added to 600 μL of 0.7 mmol/L TCA solution and thoroughly mixed. Supernatant was collected after centrifuging and stored at −80°C until use. The PAH concentration in the supernatant was detected spectrophotometrically on an automated analysis system (Cobas Mira-S, Hoffmann-La Roche, Basel, Switzerland) as described before 14 by standard enzymatic methods, using commercially available kits.
Calculations
The portal-drained viscera are defined as the total of all portal-drained organs, which, except for the spleen, stomach, and pancreas, largely represent the intestines. By sampling arterially and portally, the blood flow rate (mL/kg per minute) across portal-drained viscera can be calculated using the following formula based on the principle of indicator dilution methods:
Blood flow = I/([PAH]P − [PAH]A)
in which I represents the rate of the PAH infused (nmol/kg per minute) in the splenic vein. [PAH]P and [PAH]A represent the concentration (μmol/L) of PAH in the portal and arterial blood, respectively. The dilution of [PAH]V infused via the splenic vein entering the portal vein depends on the blood flow rate through the portal-drained viscera.
Intestinal Motility Measurements
During experiments, motility was studied by ambulatory manometry using a four-channel catheter with four solid-state pressure transducers (CTO-6, Dunvegan, Isle of Skye, Scotland) spaced 5 cm apart. The transducers were calibrated before the investigations were started. Immediately following the start of the infusions, the manometry catheter was inserted into the bowel lumen of the jejunum via the Bishop-Koop jejunostomy, with the pressure transducers located 10, 15, 20, and 25 cm distal to the end-to-side anastomosis (see Fig. 1). Pressure signals were sampled at a frequency of 8 Hz and stored in a data logger (UPS-2020, MMS, Enschede, The Netherlands). A personal computer served as an on-line monitoring and retrieval system. Recordings were performed for 24 hours (fasting) or 8 hours (postprandial).
Analysis of Motility Recordings
Motility recordings were analyzed semiautomatically using commercially available software (MMS). The number of pressure waves with an amplitude of at least 1.4 kPa and their mean amplitude and motility index (MI = loge [sum of contraction amplitudes * number of contractions + 1]) were calculated after elimination of artifacts such as abdominal pressure peaks, respiration, and transducer drift. Motility events were analyzed on the fourth jejunal channel, as the most distant channel from the anastomosis was considered most reliable. The frequency of contractions, the mean amplitude, and the mean motility index (MI) were defined for 1-hour periods. Motility activity (MA) was calculated as: MA = MI * number of MMCs * h−1. The MMC cycle was defined as the time from the end of phase 3 of the complex to the end of next phase 3, as identified by the criteria of Code and Marlett. 15 The distinct phases of the MMC were determined visually; phase 3 of the MMC was identified as distally propagating regular contractions; phase 2 was a period of irregular contractions and was followed by phase 1, a period of absent contractions. The migration velocity of the MMCs was determined semiautomatically by assessing the time of passage of the end of phase 3 along at least two channels. The mean migration velocity of the MMC was calculated in millimeters per minute.
Statistical Analysis
The data were analyzed by repeated measures analysis of variance for differences between treatments and differences in time, using as within factor time with three levels and as between-factors treatment (with or without endotoxin). When significant time differences appeared, univariate F tests were used for contrasts. Levels of significance were set at P < .05. The independent-samples t test with Bonferroni correction was performed for group comparisons at day 0 (motility parameters) or day 1 (hemodynamic parameters), day 2, and day 5.
RESULTS
Effect of Enteral Feeding
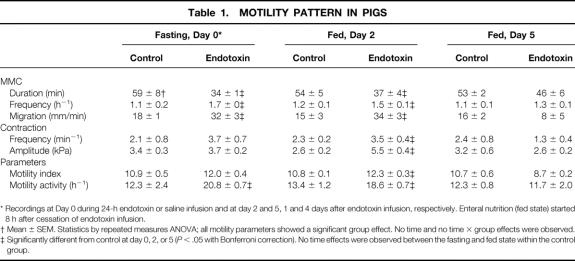
In both fasted (Fig. 3) and fed (Fig. 4) pigs, a regular MMC pattern was observed in the jejunum during the recording period. MMCs recorded during fasting and feeding recurred at average intervals of about comparable duration (Table 1). However, in the fasting state the period of quiescence (phase 1) occupied about 35% (21 ± 3 minutes) of the total MMC; this period almost disappeared during feeding (4 ± 3 minutes). Phase 1 was replaced by phase 2-like activity of irregular contractile activity during feeding. Neither the frequency nor the amplitude of the jejunal contractions observed in phase 2 in the fed state differed significantly from those observed during fasting. The average propagation velocity of the MMC cycles remained unaltered by feeding.
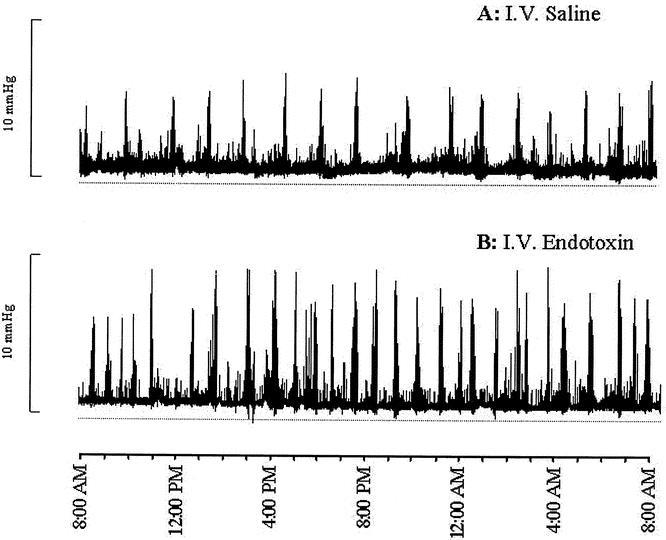
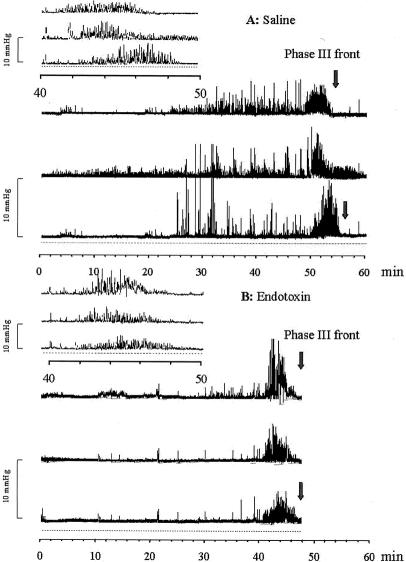
Figure 3. Manometric pressure recording at day 0 (one channel shown). Fasting jejunal motility pattern in saline-treated control pigs (A) or endotoxin-treated pigs (B). Intravenous Escherichia coli lipopolysaccharide infusion resulted in acceleration of MMC cycling frequency and migration during fasting.
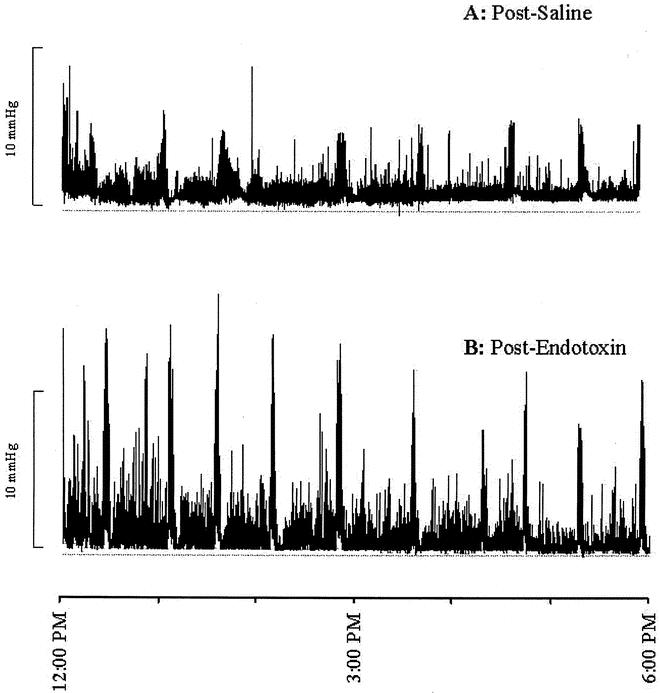
Figure 4. Manometric pressure recording at day 2 (one channel shown). Jejunal motility pattern in pigs during enteral feeding 1 day after cessation of saline (A) or endotoxin (B). After endotoxin administration, changes in the jejunal motility pattern were maintained during feeding, with an increased contraction frequency and amplitude in addition.
Table 1.MOTILITY PATTERN IN PIGS
* Recordings at Day 0 during 24-h endotoxin or saline infusion and at day 2 and 5, 1 and 4 days after endotoxin infusion, respectively. Enteral nutrition (fed state) started 8 h after cessation of endotoxin infusion.
† Mean ± SEM. Statistics by repeated measures ANOVA; all motility parameters showed a significant group effect. No time and no time × group effects were observed.
‡ Significantly different from control at day 0, 2, or 5 (P < .05 with Bonferroni correction). No time effects were observed between the fasting and fed state within the control group.
Effect of Endotoxin Infusion
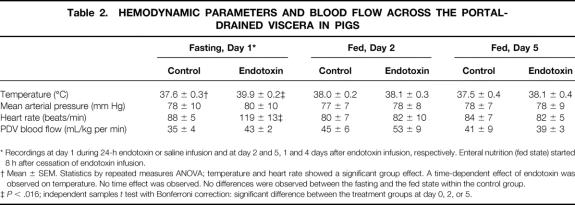
At day 1, the body temperature of the pigs receiving the 24-hour endotoxin infusion (Table 2) was higher than in control pigs. The mean arterial pressure was not affected by endotoxin infusion, while the heart rate of the endotoxin-infused pigs was elevated at day 1. At days 2 and 5, 1 and 4 days after cessation of the endotoxin infusion, the body temperature and heart rate had normalized. The blood flow to the portal-drained viscera remained unchanged by endotoxin infusion. Except for body temperature, no statistically significant interactions between time and endotoxin treatment were observed for measured hemodynamic variables. All six endotoxin-treated pigs showed no disruption of the regular MMC pattern (see Fig. 3). However, MMC duration was reduced in the endotoxin-treated pigs compared with controls (see Table 1) due to shortening and/or disappearance of the irregular contraction phase 2 (Fig. 5). Phase 1 and phase 3 duration did not significantly change as a result of endotoxin infusion. All endotoxin-treated pigs were able to tolerate the intragastric feeding that succeeded the fasting period since vomiting and intragastric retention were not observed. However, the recordings of three fasted and two fed endotoxin-treated pigs showed retrograde contractions that were of temporary origin (<1.5 hours). The increase in MMC frequency as observed in the fasting state was still present during feeding at 1 day after endotoxin infusion (see Fig. 4, Table 1). Moreover, the migration velocity of the MMC cycles was significantly increased at day 0 during fasting endotoxemia, and MMC phase 3 occurred almost simultaneously at all four recording channels (see Fig. 5). This increase in MMC migration velocity was maintained at day 2, 1 day after endotoxin administration during feeding. While the mean contraction frequency and amplitude in the endotoxin-treated pigs did not differ significantly from controls on the day of endotoxin administration, they both were significantly elevated in the fed state 1 day after endotoxin administration (see Table 1). The MI the jejunal contractions therefore remained unchanged during endotoxin infusion and increased the following day compared with controls. Due to the increased MMC cycle frequency, the motility activity of the endotoxin-treated pigs was higher compared with the saline-treated pigs at day 0. Motility activity remained relatively high for at least 1 day after endotoxin administration. The changes in motility pattern observed in the endotoxin-treated pigs were accompanied by watery diarrhea in these pigs. The motility pattern at 4 days after endotoxin administration (day 5) was not significantly different from control values.
Table 2.HEMODYNAMIC PARAMETERS AND BLOOD FLOW ACROSS THE PORTAL-DRAINED VISCERA IN PIGS
* Recordings at day 1 during 24-h endotoxin or saline infusion and at day 2 and 5, 1 and 4 days after endotoxin infusion, respectively. Enteral nutrition (fed state) started 8 h after cessation of endotoxin infusion.
† Mean ± SEM. Statistics by repeated measures ANOVA; temperature and heart rate showed a significant group effect. A time-dependent effect of endotoxin was observed on temperature. No time effect was observed. No differences were observed between the fasting and the fed state within the control group.
‡P < .016; independent samples t test with Bonferroni correction: significant difference between the treatment groups at day 0, 2, or 5.
Figure 5. Manometric pressure recording of one MMC cycle (three jejunal channels shown) in the jejunum of fasting saline-treated control pigs (A) or endotoxin-treated pigs (B). The upper-left corner shows phase 3 in more detail.
DISCUSSION
In this model of endotoxemia, the continuous infusion of endotoxin evoked an acceleration of MMC migration and MMC cycling frequency in the jejunum. Moreover, 1 day after endotoxin challenge, a pronounced increase in the MI was manifest due to increased contraction frequency and amplitude. Endotoxin infusion also resulted in increased motility activity of the jejunum that lasted until at least 1 day after endotoxin infusion during enteral feeding. Normal motility was restored after 4 days of feeding.
Many of the endotoxemia models studying GI motility disturbances use large single doses of endotoxin (in the milligram to microgram range), reproducing hemodynamic alterations that resemble hypodynamic circulatory shock rather than hyperdynamic sepsis. In the current hyperdynamic endotoxemia model, pigs were challenged with a continuous low dose of endotoxin (in the nanogram to milligram range) accompanied by fluid support to imitate compensated sepsis that better represents modern intensive care. 2 The endotoxin dose used in this reproducible sepsis model is comparable with the endotoxin doses used in other experimental hyperdynamic sepsis models. 16,17 One of the fundamental features of resuscitation during slow endotoxin infusion is the mesenteric blood flow, 16,17 which is maintained, as in the case of our pigs. Moreover, the pigs receiving 24-hour endotoxin exhibited a hyperdynamic cardiovascular and metabolic profile including elevated body temperature and heart rate and preserved mean arterial pressure. 16,17 We conclude that this model reproduces several of the systemic hemodynamic characteristics of the sepsis syndrome in humans.
To imitate clinical sepsis in this model, endotoxin infusion was followed by enteral feeding, as enteral nutrition often is indicated in septic patients with a protracted disease course. All enterally fed pigs were able to tolerate the enteral nutrition, as no vomiting and gastric retention was observed. Generally, when fed one large meal, the characteristic interdigestive repetitious MMC pattern is disrupted and replaced by postprandial irregular contractions. However, studies in pigs that were fed freely showed that the basic MMC pattern persisted regardless of feeding. 18 Accordingly, continuous enteral feeding preserved the MMC pattern in our pigs, although the quiescence period (phase 1) was absent due to an increased phase 2 to phase 1 ratio. The observation that MMC cycles were maintained during enteral feeding might be because, as in humans, MMCs are preserved when only small amounts of nutrition are continuously infused. 19 The MMC cycling frequency in the proximal jejunum was similar during fasting and enteral feeding and showed a cycle duration of 54 to 60 minutes, comparable to previously reported values for fasted pigs of about the same age. 20
Hyperdynamic endotoxemia exerted pronounced and transient effects on jejunal motility. The induced changes included increased frequency of MMC cycling due to shortening or even disappearance of phase 2, both during endotoxin and 1 day thereafter during feeding 1 day after endotoxin infusion. A comparable increase in MMC cycling was reported in the jejunum of piglets that received a bolus injection of endotoxin. 4 Although the MMC cycle in these pigs initially completely disappeared, the MMC cycling pattern of our pigs did not disappear. Possibly the maintenance of visceral flow, due to fluid support, was an important contributor to the preservation of peristalsis and MMC cycling in the jejunum. The increase in MMC cycle frequency during and 1 day after endotoxin infusion was paralleled by acceleration of the MMC phase 3 migration along the jejunum. This increased jejunal phase 3 migration may contribute to rapid transit of small bowel content. Rapid GI transit was also observed in dogs 3,21 challenged with a single dose of E. coli endotoxin at 1 day after endotoxin administration, with simultaneously increased MIs in the colon. In addition to changes in the MMC pattern, 1 day after endotoxin administration the jejunal contractions were more intense and were characterized by increased amplitude and frequency and a subsequently higher MI. The increased jejunal MI in this porcine model of endotoxemia contrasts with the decreased MI shown in a canine model of endotoxemia. 22 The differences observed in jejunal MI may be due to differences in the model and animal species used.
Although symptoms of large bowel dysmotility in sepsis caused by gram-negative endotoxins are relatively well recognized, small intestinal disturbances are less well understood. E. coli endotoxin is an important stimulus involved in the pathogenesis of secretory diarrhea, 23–25 which is characterized by accelerated GI transit, notably through the colon. The marked increase in propulsive activity in the jejunum due to an increased incidence of propagated contractions may have caused accelerated small intestinal transit to such a degree that it resulted in diarrhea. In patients with secretory diarrhea, similar spike-burst complexes with increased incidence are present in the small bowel. 26 Moreover, characteristics of increased motility activity and accelerated MMC migration in the jejunum of our continuously endotoxin-infused pigs resemble the colonic motility alterations in E. coli endotoxin-treated dogs. 21 However, it remains to be established whether jejunal motility changes are comparable or occur simultaneously with the colonic motility changes that are observed in the pathogenesis of endotoxin-induced diarrhea.
From this study we can conclude that a hemodynamic profile characteristic of the hyperdynamic state was present, displaying all the features representative of the human compensated sepsis profile. However, this experimental model of endotoxemia induced by continuous endotoxin infusion may not necessarily reproduce the pathophysiologic conditions and the compromised immune status of many of the patients admitted to the ICU, since these patients often receive mechanical ventilation and additional therapy such as antibiotics or opiates that may severely disturb the motility pattern. 27 This hyperdynamic endotoxemia model reveals that the jejunal motility disorders in the response to endotoxin infusion include increased MMC migration velocity and increased MMC cycling frequency, with absence or shortened phase 2. These changes were still manifest 1 day after endotoxin administration during feeding, indicating a protracted effect of endotoxin challenge, whereas the MMC pattern was restored 4 days after endotoxin administration. Acceleration of the jejunal MMC migration velocity may contribute to the increased transit of jejunal contents, which would be supported by the observation that our pigs developed diarrhea. The jejunal manometric recordings of this prolonged pig model of endotoxemia showed that MMC cycles are preserved during mild endotoxemia and in the postendotoxemic and fed condition. Similar endotoxin-induced changes in jejunal motility along the small bowel may contribute to the complications manifest in critically ill patients with endotoxin-induced diarrhea.
Acknowledgments
The authors thank Mrs. G.A.M. ten Have for her assistance during the experiments.
Footnotes
Supported by grant 902-23-098 from the Netherlands Organization for Scientific Research.
Correspondence: Maaike J. Bruins, PhD, Division of Biopharmaceutics, Leiden/Amsterdam Center for Drug Research, Leiden University, Gorleus Laboratories, P.O. Box 9503, 2300 RA, Leiden, The Netherlands
E-mail: m.bruins@chem.leidenuniv.nl
Accepted for publication January 9, 2002.
References
- 1.Thompson JS. The intestinal response to critical illness. Am J Gastroenterol 1995; 90: 190–200. [PubMed] [Google Scholar]
- 2.Fink MP, Heard SO. Laboratory models of sepsis and septic shock. J Surg Res 1990; 49: 186–196. [DOI] [PubMed] [Google Scholar]
- 3.Cullen JJ, Mercer D, Hinkhouse M, et al. Effects of endotoxin on regulation of intestinal smooth muscle nitric oxide synthase and intestinal transit. Surgery 1999; 125: 339–344. [PubMed] [Google Scholar]
- 4.Li JX, Oliver JR, Philips JB 3d. Endotoxin induces biphasic alterations in small intestinal myoelectric activity in fasted newborn piglets. Pediatr Res 1996; 40: 822–826. [DOI] [PubMed] [Google Scholar]
- 5.Pons L, Droy-Lefaix MT, Braquet P, et al Role of free radicals and platelet-activating factor in the genesis of intestinal motor disturbances induced by Escherichia coli endotoxins in rats. Gastroenterology 1991; 100: 946–953. [DOI] [PubMed] [Google Scholar]
- 6.Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol 1999; 277: G478–486. [DOI] [PubMed] [Google Scholar]
- 7.Nissan A, Zhang JM, Lin Z, et al. The contribution of inflammatory mediators and nitric oxide to lipopolysaccharide-induced intussusception in mice. J Surg Res 1997; 69: 205–207. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom PM, al-Saffar A, Ljung T, et al. Endotoxin actions on myoelectric activity, transit, and neuropeptides in the gut. Role of nitric oxide. Dig Dis Sci 1997; 42: 1640–1651. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Cuesta MA, Baracchina MD, Beltran B, et al. Nitric oxide modulates the acute increase of gastrointestinal transit induced by endotoxin in rats: a possible role for tachykinins. J Pharm Pharmacol 1997; 49: 988–990. [DOI] [PubMed] [Google Scholar]
- 10.Wirthlin DJ, Cullen JJ, Spates ST, et al. Gastrointestinal transit during endotoxemia: the role of nitric oxide. J Surg Res 1996; 60: 307–311. [DOI] [PubMed] [Google Scholar]
- 11.Knapik Z. Motility disturbances of the large intestine. Z Gesamte Inn Med 1981; 36: S194–196. [PubMed] [Google Scholar]
- 12.Nieuwenhuijs VB, Verheem A, van Duijvenbode-Beumer H, et al. The role of interdigestive small bowel motility in the regulation of gut microflora, bacterial overgrowth, and bacterial translocation in rats. Ann Surg 1998; 228: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mainous MR, Block EF, Deitch EA. Nutritional support of the gut: how and why. New Horiz 1994; 2: 193–201. [PubMed] [Google Scholar]
- 14.Ten Have GA, Bost MC, Suyk-Wierts JC, et al. Simultaneous measurement of metabolic flux in portally-drained viscera, liver, spleen, kidney and hindquarter in the conscious pig. Lab Anim 1996; 30: 347–358. [DOI] [PubMed] [Google Scholar]
- 15.Code CF, Marlett JA. The interdigestive myo-electric complex of the stomach and small bowel of dogs. J Physiol (Lond) 1975; 246: 289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreimeier U, Frey L, Dentz J, et al. Hypertonic saline dextran resuscitation during the initial phase of acute endotoxemia: effect on regional blood flow. Crit Care Med 1991; 19: 801–809. [DOI] [PubMed] [Google Scholar]
- 17.Murphey ED, Traber DL. Cardiopulmonary and splanchnic blood flow during 48 hours of a continuous infusion of endotoxin in conscious pigs: a model of hyperdynamic shock. Shock 2000; 13: 224–229. [DOI] [PubMed] [Google Scholar]
- 18.Ruckebusch Y, Bueno L. The effect of feeding on the motility of the stomach and small intestine in the pig. Br J Nutr 1976; 35: 397–405. [DOI] [PubMed] [Google Scholar]
- 19.Riachi G, Ducrotte P, Guedon C, et al. Duodenojejunal motility after oral and enteral nutrition in humans: a comparative study. J Parenter Enteral Nutr 1996; 20: 150–155. [DOI] [PubMed] [Google Scholar]
- 20.Rayner V, Wenham G. Small intestinal motility and transit by electromyography and radiology in the fasted and fed pig. J Physiol 1986; 379: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen JJ, Caropreso DK, Ephgrave KS. Effect of endotoxin on canine motility and transit. J Surg Res 1995; 58: 90–95. [DOI] [PubMed] [Google Scholar]
- 22.Cullen JJ, Caropreso DK, Ephgrave KS, et al. The effect of endotoxin on canine jejunal motility and transit. J Surg Res 1997; 67: 54–57. [DOI] [PubMed] [Google Scholar]
- 23.Meyer W. Clinical aspects, pathogenesis and therapy of shock due to sepsis caused by gram-negative organisms. Schweiz Med Wochenschr 1966; 96: 137–143. [PubMed] [Google Scholar]
- 24.Powell DW. Approach to the patient with diarrhea. In Yamada T, ed. Textbook of gastroenterology. Philadelphia: JB Lippincott, 1995: 813–840.
- 25.Moon HW. Pathogenesis of enteric diseases caused by Escherichia coli. Adv Vet Sci Comp Med 1974; 18: 179–211. [PubMed] [Google Scholar]
- 26.Vantrappen G, Janssens J, Coremans G, Jian R. Gastrointestinal motility disorders. Dig Dis Sci 1986; 31: 5S–25S. [DOI] [PubMed] [Google Scholar]
- 27.Bosscha K, Nieuwenhuijs VB, Vos A, et al. Gastrointestinal motility and gastric tube feeding in mechanically ventilated patients. Crit Care Med 1998; 26: 1510–1517. [DOI] [PubMed] [Google Scholar]