Abstract
Objective
To evaluate the effect of interleukin-1α (IL-1α) on the mesenteric circulation, intestinal mucosal integrity, and bacterial translocation in a burn/endotoxemia chronic porcine model.
Summary Background Data
Major burn and sepsis are associated with a high mortality, ischemia/reperfusion injury to the intestine, and an increased rate of bacterial translocation. Pathologic alterations of IL-1 synthesis, degradation, and binding to receptors have been reported. Manipulation of IL-1-mediated effects might be of therapeutic utility.
Methods
Twenty-one female pigs were instrumented with an ultrasonic flow probe on the superior mesenteric artery and a catheter into the superior mesenteric vein. After 5 days, all animals were anesthetized, and 14 received 40% total body surface area third-degree burn. IL-1α was administered intravenously at 1,000 ng/kg to seven pigs immediately after burn. Eighteen hours after burn, 100 μg/kg Escherichia coli lipopolysaccharide (LPS) was administered intravenously. Systemic and splanchnic hemodynamics were measured and blood samples were drawn for blood gas analysis. Intestinal permeability was assessed every 6 hours by measuring the lactulose/mannitol (L/M) excretion ratio. At the end of the study (42 hours), tissue samples were harvested for bacteriologic cultures.
Results
Mesenteric blood flow was significantly decreased after burn and endotoxin. Administration of IL-1α significantly improved mesenteric blood flow postburn and post-LPS. Mesenteric oxygen supply and consumption showed a significant reduction after burn. In contrast, animals treated with IL-1α showed an increase in postburn mesenteric oxygen supply and consumption. LPS-induced mesenteric hypoxia was also ameliorated by IL-1α treatment. Intestinal permeability, as assessed by the L/M ratio, showed a 7- and 10-fold elevation after thermal injury and LPS, respectively. In contrast, IL-1α-treated animals showed an increase of only three- and fourfold in the L/M ratio, respectively. Bacterial translocation was significantly increased in the burn/endotoxin group. IL-1α significantly reduced the rates of bacterial translocation.
Conclusions
IL-1α treatment attenuates mesenteric ischemia and reperfusion injury induced by thermal injury and endotoxemia by improving mesenteric blood flow and oxygenation. Subsequently, IL-1α reduces intestinal permeability and bacterial translocation after burn and sepsis.
The prognosis of extensively burned patients is dependent on the presence of sepsis. The importance of ischemic damage and systemic inflammatory response syndrome (SIRS), initiated by mediators or cytokines in the pathogenesis of postburn multiple organ dysfunction syndrome (MODS), has been demonstrated in serial clinical and experimental studies. 1 Sepsis syndrome also results from bacterial translocation, in which gut bacteria and/or endotoxins (LPS) are thought to enter the portal bloodstream and/or lymph system. 2,3 Circulating LPS was suggested to be the trigger for increased proinflammatory cytokine production, SIRS, and septic complications in injured patients. 4 The pathophysiologic mechanism of sepsis is the increased release of inflammatory mediators and resulting imbalances between these substances and their antagonists. In cases of severe sepsis, the sequelae of the imbalance between inflammatory mediators and their antagonists can lead to endothelial injury, disseminated intravascular coagulation, and finally MODS. 5 Strategies used to prevent sepsis include hospital-wide infection control measures, modifying the immune system function, and minimizing the occurrence of bacterial translocation. 6
Several mediators have been reported to be involved in the process of burn- and endotoxemia-induced ischemia–reperfusion injury to the intestine and bacterial translocation. 7 There are some data implicating interleukin-1 (IL-1) as one of these mediators. IL-1 production by blood monocytes has been documented to be markedly decreased in severely burned patients. 8 These changes were more evident in patients complicated with organ injury, multiple organ failure, and systemic infection. The administration of interleukin-1α (IL-1α) was shown to improve survival in an animal model of burn wound sepsis. 9 This survival was associated with a decrease in positive blood cultures. IL-1 given pretreatment was also shown to decrease ischemia–reperfusion injury. 10 Hence, we examined the ability of IL-1α to counteract intestinal ischemia–reperfusion injury and bacterial translocation in a burn/sepsis chronic porcine model.
METHODS
The experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Medical Branch (ACUC # 90–09–103).
Surgical Preparation
Studies were performed in 21 female mini-pigs (weight 20–25 kg). After an overnight fast, the pigs were sedated with intramuscular ketamine (10 mg/kg) and mechanically ventilated with 2% to 2.5% halothane after endotracheal intubation. A bilateral subcostal incision was performed. A transit time ultrasonic flow probe (6–8 mm, Transonic Systems Inc., Ithaca, NY) was placed on the superior mesenteric artery. A 6.5F catheter was positioned in the superior mesenteric vein. A Witzel jejunostomy was also performed using a 12F Foley catheter.
After surgery, the animals were kept in recovery slings for 24 hours, then placed in runs for 5 days with free access to food and water. On the day of the experiment, the animals were reanesthetized. Through a neck incision a catheter was placed via the right common carotid artery into the abdominal aorta, and a Swan-Ganz thermal dilution catheter (Model 93 A-131–5F, American Edwards Laboratories, Anasco, PR) was positioned in the pulmonary artery through the right jugular vein. A 12F Foley catheter was inserted in the urinary bladder.
Experimental Design
The animals were kept in special slings for monitoring. Throughout the study, all animals received enteral feeding (Osmolite) at 25 mL/h and nothing orally. Baseline data were collected after complete recovery from anesthesia.
The pigs were randomized into three groups. The burn/LPS group (n = 7) had a 40% total body surface area third-degree flame burn under general anesthesia as described above. The pigs were resuscitated according to the Parkland formula and received lactated Ringer’s solution (4 mL/kg/percentage of total body surface area burned) starting immediately after the burn; half was given in the first 8 hours after burn and the remainder in the next 16 hours. Eighteen hours after burn, 100 μg/kg E. coli LPS (0111:B4; Difco, Detroit, MI) was administered intravenously. During the second day of the experiment, burned animals received lactated Ringer’s solution at 3.5 mL/m2 burned area and 2 mL/kg/h for daily maintenance.
The sham group (n = 7) had a sham burn under anesthesia. Eighteen hours later, the animals received the diluent (0.9% NaCl) used for the endotoxin. Lactated Ringer’s solution was administered at 2 mL/kg/h for daily maintenance.
The treatment group (n = 7) underwent the same procedure as the burn/LPS group, except for the administration of IL-1α (recombinant human IL-1α, lot IL-1 1/92, with specific activity of 8.8 × 108 units/mg, provided by Hoffmann-La Roche Inc., Nutley, NJ) intravenously at 1,000 ng/kg, immediately after burn.
Mean arterial (MAP) and central venous (CVP) pressures were measured using transducers (P231D, Statham Gould, Oxnard, CA) connected to an Electronic Medicine Honeywell Recorder (Honeywell Inc., Pleasantville, NY) for electronic calculation of mean pressures. Cardiac output (CO) was determined by the thermal dilution technique using a Swan-Ganz catheter and a cardiac output computer (Model 9520, American Edwards Laboratories, Irvine, CA).
Superior mesenteric artery (SMA) blood flow was measured with a transit time ultrasonic flow probe connected to a T101 ultrasonic meter (Transonic Systems Inc.).
Systemic and splanchnic hemodynamics were measured and blood samples were drawn for determination of arterial, mixed venous, and portal blood gases at baseline and 14 consecutive time points, starting 1 hour after burn.
Systemic vascular resistance index (SVRI) and mesenteric vascular resistance (MVR) were calculated with the following formulas:
Cardiac index (L/min/m2) = cardiac output (L/min)/body surface area
SVRI (dyne · sec · cm–5 · m2) = ([mean arterial pressure –central venous pressure] × 80)/cardiac index
MVR (dyne · sec · cm–5) = ([mean arterial pressure – central venous pressure] × 80)/mesenteric arterial blood flow
Systemic oxygen delivery (DO2), systemic oxygen consumption (VO2), mesenteric oxygen delivery (mDO2), and mesenteric oxygen consumption (mVO2) were calculated as follows:
DO2 (mL/min/m2) = cardiac index × arterial oxygen content × 10
VO2 (mL/min/m2) = cardiac index × (arterial oxygen content – mixed venous oxygen content) × 10
mDO2 (mL/min) = mesenteric arterial blood flow × arterial oxygen content/100
mVO2 (mL/min) = mesenteric arterial blood flow × (arterial oxygen content – mesenteric oxygen content)/100
Arterial oxygen content (mL/dL) equals (hemoglobin × 1.34) SaO2 + (PaO2 × 0.0031); mixed venous oxygen content (mL/dL) equals (hemoglobin × 1.34) SvO2 + (PvO2 × 0.0031); mesenteric oxygen content (mL/dL) equals (hemoglobin × 1.34) SmO2 + (PmO2 × 0.0031).
Permeability Assessment
After the animals had recovered from the surgical instrumentation, a solution of 10 g lactulose and 5 g mannitol, diluted in 60 mL distilled water (1,160 mOsm/kg), was given via the jejunostomy tube and urine was collected for a 6-hour period to obtain baseline measurements. Lactulose/mannitol (L/M) assessment was repeated every 6 hours. At the completion of collection, the urine was divided into aliquots and frozen at 20°C until assayed. Urinary lactulose and mannitol concentrations were simultaneously determined by the technique described by Fleming et al., 11 using high-pressure liquid chromatography coupled with pulsed amperometric detection (HPLC-PAD). Urine was 2- to 20-fold diluted with deionized water, depending on the collection volume. One milliliter of diluted urine was mixed with internal saccharide standards, desalted, vortex mixed, centrifuged, and filtered. Fifty microliters of the filtrate was injected onto a 250 × 40-mm anion exchange column (Dionex Carbopak, PAI, Houston, TX) and eluted with 0.15 mol/L NaOH, 1 mL/min at 20°C. Detection was by PAD with a working gold electrode and silver/silver chloride reference electrode, with a detection potential of +0.05, oxidation potential of +0.06, and reduction potential of −0.95 V. Quantification was by peak height analysis and peak height ratios, with internal standardization. This method offers excellent separation of the carbohydrates and precise detection at low concentrations (lactulose 0.3 mg/L). The amount of each sugar excreted in the urine during 6 hours was then converted to a percentage of the amount of the given sugar, reflecting the excretion fraction of each sugar. By dividing the lactulose and mannitol excretion fractions, a permeability index (the L/M ratio) was calculated.
Testing for Bacterial Translocation
At the end of the 42 hours, the animals were anesthetized with 10 mg/kg intravenous ketamine and killed with 5 mL intravenous saturated KCl. Using aseptic technique, through a midline laparotomy incision, peritoneal fluid and tissue samples from the proximal and distal mesenteric lymph nodes, spleen, liver, kidney, lung, jejunum, ileum, cecum, and colon were taken for bacteriologic cultures. Collected tissue samples were weighed and 0.5 g of each was homogenized in a tissue grinder with 4.5 mL nonbacteriostatic saline to create a 1:10 dilution of the original sample; 0.1 mL and 0.01 mL (of the 1:10 dilution) were inoculated onto a MacConkey agar plate and a Columbia Nutrient Agar (CNA) plate for isolation of gram-negative and gram-positive organisms, respectively. Therefore, one colony would represent 1 × 102 and 1 × 103 colony-forming units per gram of tissue, respectively, for each inoculum size. Limits of detection were 100 organisms per gram of tissue. Inoculated plates were incubated at 37°C for 24 and 48 hours and read with a Darkfield Quebec Colony Counter (Model 3330, American Optical Co., Buffalo, NY). Cultures were considered positive when more than 100 colonies per gram of tissue were found. All bacterial isolates were identified by biotype using a Microscan 4 bacterial analyzer (Baxter, Sacramento, CA).
Statistical Analysis
Data are presented as mean ± SEM. Within-group analysis was performed by the analysis of variance for repeated measurements with the Dunnett post-hoc test. Between-groups analysis was performed by analysis of variance for factorial analysis with the Bonferroni post-hoc test. Bacteriologic tissue culture results were analyzed by the Fisher exact test. P < 0.05 was considered statistically significant.
RESULTS
Systemic Hemodynamics
Baseline hemodynamic measurements were similar in all groups. All animals survived the study period (Figs. 1, 2).
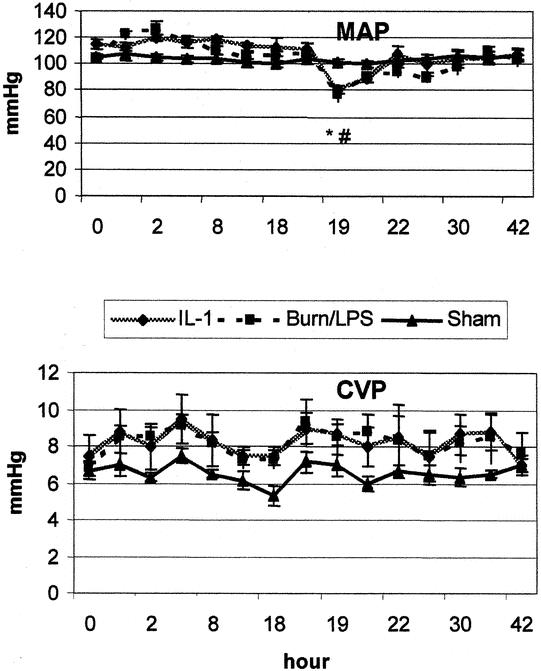
Figure 1. Mean arterial pressure (MAP) and central venous pressure (CVP) after burn (0 hour) and endotoxin (18 hours). IL-1α treatment had a marginal effect. P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
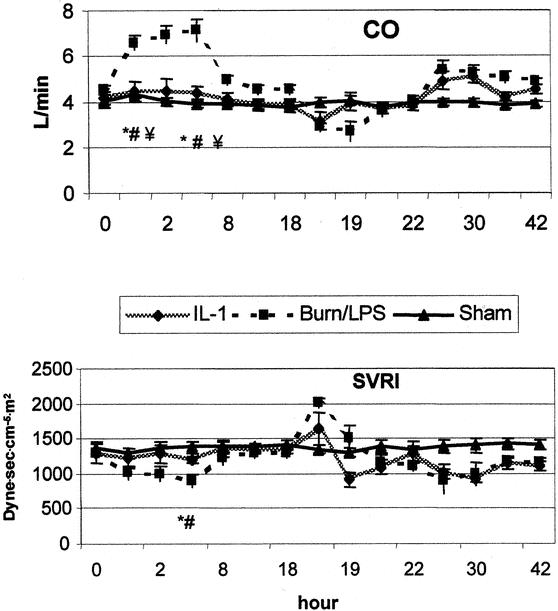
Figure 2. IL-1α ameliorated the changes in cardiac output (CO) and systemic vascular resistance index (SVRI) postburn (0 hour) and early after endotoxin (18 hours). P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
After burn, MAP and CVP showed a slight elevation, but no significant differences were observed between groups. CO was elevated to 158% of baseline during the first 4 hours, returning to baseline 8 hours after burn. This increase was associated with a concomitant fall in SVRI to 65% of baseline level. The second insult (administration of LPS) led to a typical biphasic response. During the early post-LPS phase, a decrease was noticed in MAP to 80% of baseline, and in CO to 67% of baseline. Four hours after LPS administration, all measurements were stabilized to baseline levels. A hyperdynamic period began to be manifest 8 hours after endotoxin infusion. Whereas CO showed a 20% increase, SVRI dropped to 64% of baseline at this time point. Throughout the study period, a slight elevation of CVP was shown in all burned animals. IL-1α treatment had a moderate impact on the hemodynamic alternations seen after burn and LPS.
Mesenteric Hemodynamics
Although thermal injury resulted in a hyperdynamic status with an increased CO, SMA blood flow decreased significantly to approximately 59% of baseline during the first 2 hours after burn (Fig. 3). In contrast to the previously observed postburn reduction in systemic vascular resistance, MVR showed a significant increase (195% of baseline) during the early postburn phase (Fig. 4). During the late postburn phase, starting 4 hours after insult, a mesenteric reperfusion phase became manifested, as SMA blood flow increased by 45% over baseline. Compared to burned animals not receiving IL-1α treatment, IL-1α-treated animals showed no reduction in SMA blood flow following thermal insult; on the contrary, SMA blood flow in this group showed an increase of 40% of baseline (see Fig. 3). Animals in the IL-1α group maintained, in contrast to nontreated burned animals, a stable MVR near baseline during this early mesenteric vasoconstrictive phase (see Fig. 4). At 18 hours postburn, mesenteric hemodynamic measurements were comparable to baseline levels in all groups.
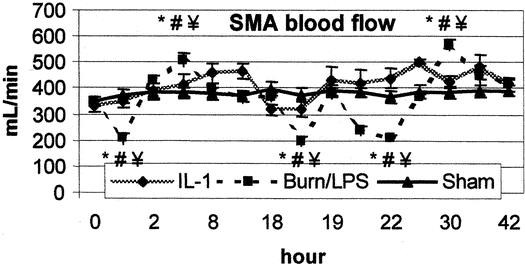
Figure 3. Superior mesenteric artery (SMA) blood flow was significantly reduced and showed a pattern of ischemia and reperfusion after burn (0 hour) and endotoxin (18 hours). IL-1α administration significantly improved SMA blood flow. P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
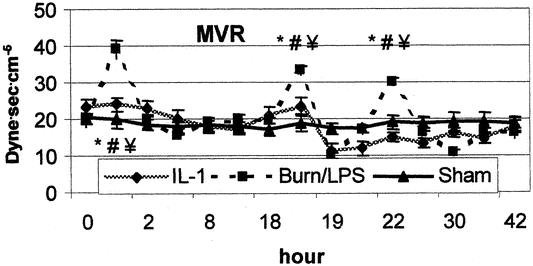
Figure 4. Alterations in mesenteric vascular resistance (MVR) after burn (0 hour) and endotoxin (18 hours). IL-1α had a positive impact on MVR. P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
Administration of LPS to burned animals resulted in a biphasic response of mesenteric ischemia and reperfusion. The second insult yielded a significant mesenteric vasoconstriction with an increase of MVR to 158% of baseline during the first 8 hours post-LPS. Similarly, SMA blood flow decreased significantly to 60% of baseline during the same time of maximum increase of MVR. Burned animals treated with IL-1α showed no signs of mesenteric vasoconstriction after the second insult (LPS). Compared to nontreated animals, IL-1α treatment resulted in a significant improvement of SMA blood flow after the second impact (LPS). Eight hours post-LPS, SMA blood flow reached a value of 152% of baseline in the IL-1α group, whereas MVR was significantly reduced to 56% of baseline (see Figs. 3, 4).
Systemic Oxygen Delivery and Consumption
During the first 4 hours after burn, the burn/LPS group showed a significant increase in both DO2 (150% of baseline) and VO2 (204% of baseline). The IL-1α group showed moderate changes in DO2 and VO2 after burn, with an increase to 113% and 126% of baseline, respectively (Fig. 5). One hour after LPS administration, a significant drop in DO2 was noticed in the burn group (57% of baseline). VO2 was reduced to 68% of baseline during this early post-LPS phase. During the post-LPS hyperdynamic phase, DO2 and VO2 showed no significant alterations. Animals treated with IL-1α showed some changes post-LPS, but of no significance.
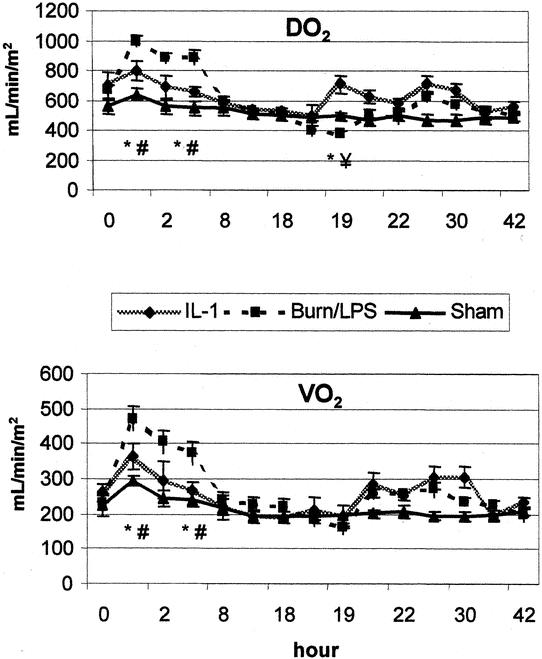
Figure 5. Administration of IL-1α slightly affected systemic oxygen delivery (DO2) and oxygen consumption (VO2) after burn (0 hour) and endotoxin (18 hours). P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
Mesenteric Oxygen Delivery and Consumption
In the early phase after burn, mDO2 and mVO2 showed a significant fall to 60% and 54% of baseline levels, respectively. On the contrary, animals treated with IL-1α showed an increase in postburn mDO2 and mVO2 to 124% and 128% of baseline, respectively (Fig. 6).
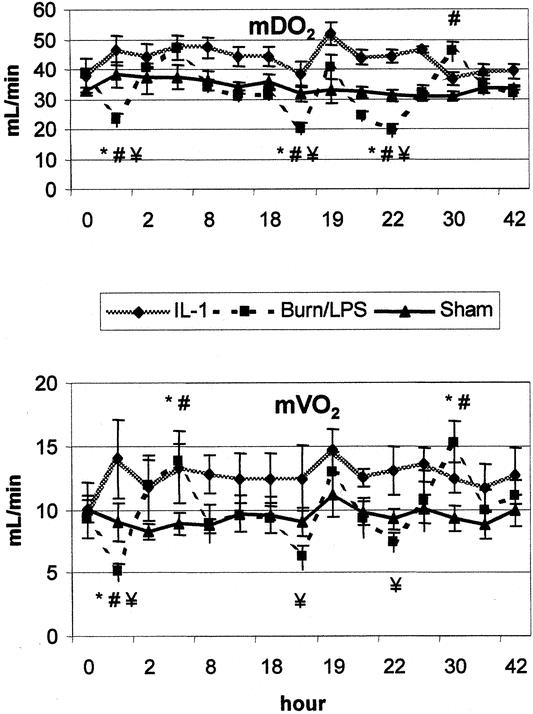
Figure 6. Burn (0 hour) and endotoxin (18 hours) significantly reduced mesenteric oxygen supply (mDO2) and oxygen consumption (mVO2). These detrimental effects were attenuated by IL-1α treatment. P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
Administration of LPS resulted in a significant reduction in mDO2 to 50% of baseline. Postburn treatment with IL-1α abrogated this deleterious impact of LPS, and mDO2 showed an increase to 121% of baseline. Correspondingly, post-LPS diminished mVO2 was attenuated in animals receiving IL-1α (79% vs. 136% of baseline).
Lactulose and Mannitol Assay
No differences were found between groups in baseline measurements of the L/M ratio. The L/M ratio for burned animals began to increase 6 hours after burn, reaching a sevenfold elevation 12 hours after burn in the burn/LPS group, compared with only a threefold increase in the treatment group. The differences between groups were significant at this time point. LPS administration to burned animals caused a clear deterioration in the mucosal permeability, as assessed by the L/M ratio. The L/M excretion ratio was 10- and eightfold increased in the burn/LPS group at 12 and 18 hours after LPS administration, respectively. On the contrary, animals treated with IL-1α showed only a four- and threefold increase at these time points. Differences between groups were significant (Fig. 7).
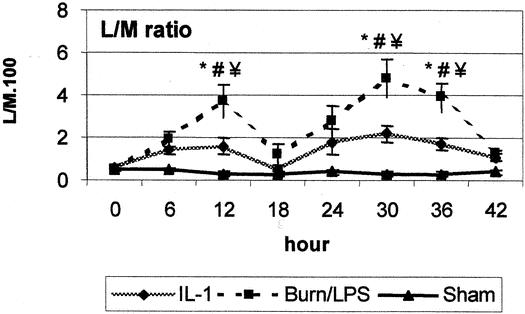
Figure 7. Intestinal permeability, as assessed by the lactulose/mannitol (L/M) excretion ratio, was significantly increased after burn (0 hour) and endotoxin (18 hours). IL-1α administration significantly reduced intestinal permeability. P < .05 *vs. baseline, #vs. sham, ¥vs. IL-1α.
Quantitative Bacteriologic Culture of Tissue Samples
Tissue cultures tested positive for enteric bacteria in all animals in the burn/LPS group. In contrast, only one of seven animals in the sham group showed positive tissue cultures (P < .05). IL-1α treatment yielded a significant reduction in the rates of positive tissue cultures: only two of seven animals in this group tested positive (P < .05 vs. burn/LPS).
Only tissue cultures with enteric bacteria of the same biotype as that found in the intestine of the corresponding animal were interpreted as evidence of bacterial translocation (Table 1).
Table 1.RATES, TYPES, AND ORIGINS OF TISSUE ISOLATES FROM THE THREE STUDY GROUPS
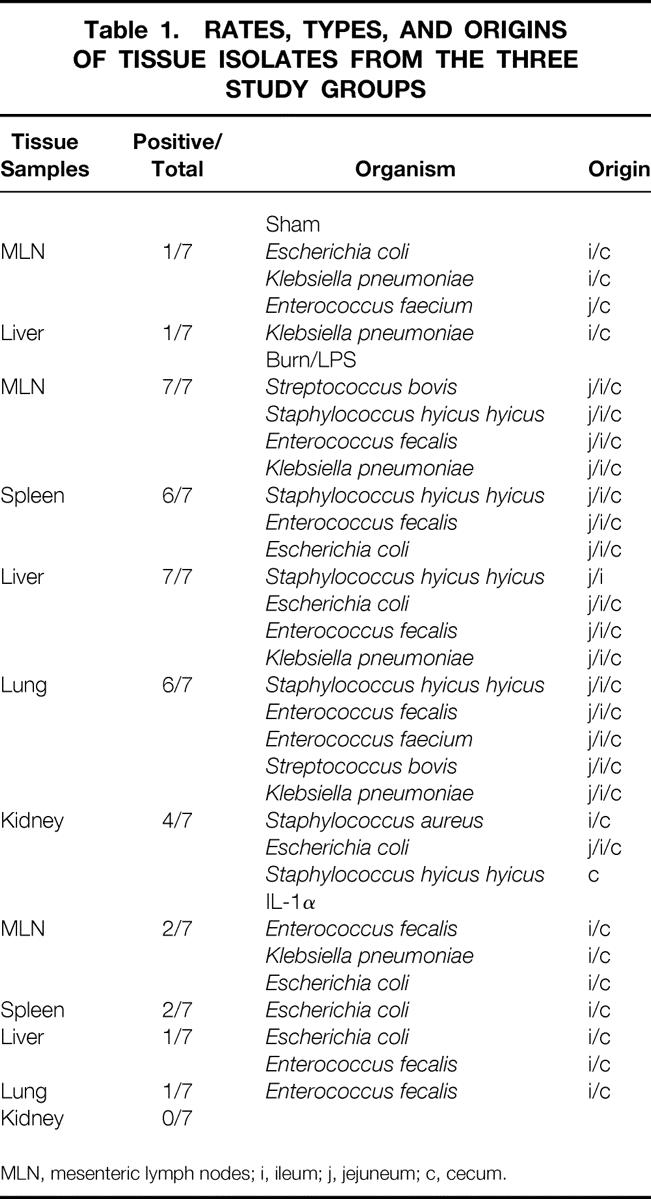
MLN, mesenteric lymph nodes; i, ileum; j, jejuneum; c, cecum.
In the burn/LPS group, 100% of the animals showed bacterial translocation to the mesenteric lymph nodes and liver, 86% to the spleen and lung, and 57% to the kidney (Fig. 8). On the contrary, only 28% of the treated animals showed bacterial translocation to the nodes and spleen (P < .05), 14% to the liver and lung (P < .05), and none to the kidney.
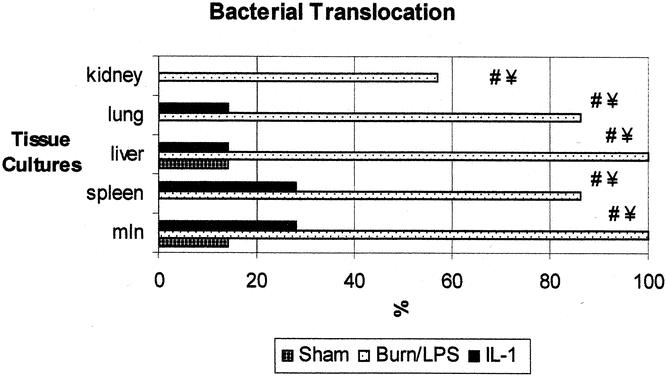
Figure 8. Incidence of bacterial translocation to remote organs was significantly increased in the burn and endotoxin group. IL-1α treatment yielded a significant reduction in the rates of positive tissue cultures with enteric bacteria. Data are presented as percentages of harvested tissue samples. P < .05 #vs. sham, ¥vs. IL-1α.
DISCUSSION
The immense inflammatory focus incited by the burn causes the release of numerous cytokines and inflammatory mediators that have many systemic effects and may ultimately result in MODS. Recently, the role of the gut as a cytokine-generating organ in the postinjury inflammatory response has been studied. 12 As the causal relation of bacterial translocation or gut-derived cytokines to the development of sepsis and MODS has become more apparent and more complex, the need to identify the mechanisms involved in the pathophysiology of postinjury gut dysfunction is increasing. 13 We recently reported on possible mediators in this complex process. 7 Among the molecular mediators relevant to the pathogenesis of burn injury-associated changes, cytokines have attracted special attention. IL-1 is presumed to be a key component of the inflammatory mediator cascade, directing the host response to infection, injury, or inflammation. 14
Since IL-1α has multiple effects on the elements of the immune system, we examined in this study the ability of IL-1α to reduce bacterial translocation in a porcine model of burn and endotoxemia. Although the potential role of bacteria/endotoxin translocation and its clinical relevance remain controversial, many lines of evidence support the concept that early gut hypoperfusion sets the stage for progressive gut dysfunction, such that the gut becomes a reservoir for pathogens and toxins that contribute to SIRS and MODS. 15 The consequences of bacterial translocation for the host were not addressed in this study, but rather the pathophysiology of this process. Our animal model is based on the “two-hits” concept, 16,17 referring to the episodes of endotoxemia to which burned patients are frequently exposed. 18 IL-1α was administered as a single dose (1,000 ng/kg) directly after a major thermal injury to evaluate the action of IL-1α as a treatment modality. The efficacy of this treatment scheme in improving survival in a burn and sepsis animal model has been documented in a previous study. 9
The results of this experiment show that postburn administration of IL-1α significantly reduces bacterial translocation. In contrast to a rate of 100% in animals with combined burn and LPS insults, bacterial translocation was observed in only 28.5% of animals receiving burn and LPS and treated with IL-1α. These findings suggest several explanations. IL-1α may have enhanced bacterial clearance capacities in treated animals. Also, IL-1α treatment may have altered local or systemic factors involved in the pathophysiologic pathways of the bacterial translocation process. There is evidence in the literature to support the first theory. IL-1α has been reported to decrease rates of positive blood cultures and increase the absolute neutrophil counts in a murine model of burn wound sepsis. 9 In addition, IL-1α could have increased the animal’s ability to kill translocated bacteria via its influence on hematopoietic growth factors known to enhance the proliferation and functional capacity of neutrophils and macrophages. 19
The second hypothesis regarding the possible impact of IL-1α on potential initiators of bacterial translocation was the subject of our study. A direct or indirect action of IL-1α at the cellular level could have interacted with the intestinal mucosal integrity, leading to decreased translocation of enteric bacteria. To examine whether IL-1α interferes with intestinal permeability, we measured the L/M excretion ratio. The clinical relevancy of this assessment method has been shown in previous studies 20,21 in which an association between increased L/M ratio and postburn infections was observed in humans. Furthermore, an increased L/M ratio was found to correlate with oxidant-induced damage and bacterial translocation in our combined burn and sepsis model. 22 Burn yielded in our study a sevenfold increase in intestinal permeability during the first 12 hours after injury, as indicated by the L/M ratio. This detrimental impact of thermal trauma on intestinal permeability was augmented after the administration of endotoxin. During this postinjury toxemic phase, the L/M ratio was 10-fold elevated during the first 12 hours after LPS infusion. Eighteen hours post-LPS, intestinal permeability was still eightfold increased. These results confirm the data of previous investigators (including ourselves). 22,23 Our data show that postburn administration of IL-1α can counteract the negative effects of both challenges (i.e., the initial burn injury and the secondary endotoxemia). Intestinal permeability in animals treated with IL-1α was significantly reduced to three- and fourfold after burn and endotoxin, respectively. The beneficial effect of IL-1α on intestinal permeability might be the result of a direct pathway by which IL-1α protects the intestinal integrity. IL-1 has been shown to increase intestinal crypt cell mitoses and intestinal villus height, leading to improving intestinal cytoarchitecture and reducing bacterial translocation after a 32% total body surface area burn in mice. 24 A similar effect could explain the impact of IL-1α in our study; however, we have not explored this possibility in the current study by means of histologic studies of intestinal samples. The hypothesis that IL-1α indirectly influences the intestinal permeability by interacting with other factors or pathways, such as mucosal perfusion, was the subject of this study.
Ischemia and reperfusion injury to the intestinal mucosa, caused by burn and endotoxin, and its detrimental impact on intestinal permeability and bacterial translocation have been profoundly illustrated in previous studies from our laboratory. 7,22 The current data confirm our previous findings of the occurrence of mesenteric ischemia and reperfusion injury following burn and endotoxin, despite the presence of adequate resuscitation status. The observation of a significant mesenteric vasoconstriction (decreased SMA blood flow and increased mesenteric vascular resistance) during the early phase after thermal injury, while the animals were showing a hyperdynamic status (increased CO and decreased systemic vascular resistance), demonstrates the selective negative impact of thermal injuries on mesenteric circulation. Administration of IL-1α directly postburn led to moderate alterations in the systemic hemodynamic parameters. In contrast, a significant improvement of the mesenteric blood supply was noticed after IL-1α postburn treatment. This beneficial effect of IL-1α treatment on intestinal blood supply in burned animals could be explained by an interaction of IL-1α with vasodilators, such as some of the prostanoids. IL-1 has been documented to cause an alteration in prostaglandin concentrations, increasing prostaglandin E (PGE2) and prostacyclin (PGI2). 14 In another study IL-1α was found to act directly on endothelial cells, inducing the production of vasodilators such as PGI2. 25 A similar impact of IL-1α on systemic and mesenteric circulations was observed during the postburn septic phase (i.e., after administration of LPS). IL-1α was found to significantly reduce mesenteric vascular resistance and improve SMA blood flow. The effect on systemic hemodynamic parameters was noticeable only during the first 4 hours after LPS infusion.
Although systemic oxygen delivery and consumption were increased following thermal injury, mesenteric oxygen supply was significantly reduced early postburn. The discrepancy between the changes measured in both systemic and mesenteric oxygenation status after burn demonstrates the independence of the impact of thermal injury on the mesenteric oxygenation status, as previously seen. 22 During the same period, mesenteric oxygen consumption became dependent on mesenteric oxygen delivery, with a significant reduction, indicating the inability of the gut to compensate for inadequate oxygen delivery by increasing oxygen extraction, resulting in tissue hypoxia. Early hypoxia in the splanchnic region has been implicated in the process of developing secondary organ failure after major injuries or sepsis. 26 Postburn administration of IL-1α resulted in a significant improvement in mesenteric oxygen delivery. Mesenteric hypoxia, as indicated by decreased mesenteric oxygen consumption, was not noticed in animals in the treatment group. The enhancement in oxygen supply to meet the increased oxygen demand was observed only in the mesenteric circulation, and no significant differences were found between treated and untreated burned animals with respect to systemic oxygen delivery or consumption. During the postburn septic phase, a typical biphasic response was observed in mesenteric oxygen delivery and consumption, with a more pronounced flow-dependent mesenteric hypoxic period lasting approximately 8 hours. On the contrary, LPS had only moderate effects on systemic oxygen delivery and consumption. LPS alone has been shown to cause mesenteric hypoperfusion, yielding intestinal mucosal acidosis and increased permeability. 27 After the second insult (LPS), burned animals receiving IL-1α showed a significant improvement in their mesenteric oxygenation status compared with nontreated animals.
The data of this study show that postburn administration of IL-1α attenuates the mesenteric ischemia and reperfusion injury induced by thermal injury and postburn endotoxemia. Our data are in correspondence with data from other studies documenting the beneficial effects of IL-1α in other organs against ischemia and reperfusion injury. The results of those studies have indicated that low doses of IL-1α can be used as a therapeutic agent to precondition the heart against ischemia and reperfusion injury 28 and to decrease the onset of myocardial ischemia and reperfusion injury. 10 However, this particular effect of IL-1α seems to be of a selective nature and is probably organ-specific, as IL-1 was found to be of unlikely benefit in the recovery of renal function after ischemia. 29 The mechanisms by which IL-1 interacts with other mediators and modulators in the cascade of ischemia and reperfusion injury induced by burn and sepsis and the consequential effects of such actions on different organs have not been fully elucidated. Therefore, further studies on this field are warranted.
CONCLUSIONS
The cytokine IL-1α appears to play an important role in intestinal perfusion and oxygenation after burn and sepsis. Postburn treatment with IL-1α reduces intestinal permeability and bacterial translocation, most likely by attenuating mesenteric ischemia and reperfusion injury.
Footnotes
Correspondence: Tamer Tadros, MD, Department of Surgery, University Hospital Rotterdam, P.O Box 2040, 3000 CA Rotterdam, The Netherlands.
E-mail: tadros@hlkd.azr.nl
Accepted for publication March 21, 2002.
References
- 1.Huang YS, Yang ZC, Liu XS, et al. Serial experimental and clinical studies on the pathogenesis of multiple organ dysfunction syndrome (MODS) in severe burns. Burns 1998; 24: 706–716. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA, Rutan R, Waymack JP. Trauma, shock, and gut translocation. New Horiz 1996; 4: 289–299. [PubMed] [Google Scholar]
- 3.Magnotti LJ, Xu DZ, Lu Q, et al. Gut-derived mesenteric lymph: a link between burn and lung injury. Arch Surg 1999; 134: 1333–1341. [PubMed] [Google Scholar]
- 4.Kelly JL, O’Sullivan C, O’Riordain M, et al. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury? Ann Surg 1997; 225: 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston DH, Mosenthal AC, Deitch EA. Sepsis and multiple organ dysfunction syndrome: a clinical-mechanistic overview. New Horiz 1995; 3: 257–266. [PubMed] [Google Scholar]
- 6.Fisher CJ Jr., Zheng Y. Potential strategies for inflammatory mediator manipulation: retrospect and prospect. World J Surg 1996; 20: 447–453. [DOI] [PubMed] [Google Scholar]
- 7.Tadros T, Traber DL, Herndon DN. Mediators and intervention modalities in injury and sepsis-induced intestinal ischemia. In Faist E, Schildberg FW, eds. The immune consequences of trauma, shock and sepsis mechanisms and therapeutic approaches, Vol. 1. Legerich, Germany: Pabst Science Publishers, 1996;:153–162.
- 8.Liu XS, Yang ZC, Luo ZH, et al. Clinical significance of the change of blood monocytic interleukin-1 production in vitro in severely burned patients. Burns 1994; 20: 302–306. [DOI] [PubMed] [Google Scholar]
- 9.Silver GM, Gamelli RL, O’Reilly M, et al. The effect of interleukin 1 alpha on survival in a murine model of burn wound sepsis. Arch Surg 1990; 125: 922–925. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM, White CW, Terada LS, et al. Interleukin 1 pretreatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci USA 1990; 87): 5026–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming SC, Kapembwa MS, Laker MF, et al. Rapid and simultaneous determination of lactulose and mannitol in urine, by HPLC with pulsed amperometric detection, for use in studies of intestinal permeability. Clin Chem 1990; 36: 797–799. [PubMed] [Google Scholar]
- 12.Deitch EA, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock 1994; 1: 141–145. [DOI] [PubMed] [Google Scholar]
- 13.Mainous MR, Ertel W, Chaudry IH, et al. The gut: a cytokine-generating organ in systemic inflammation? Shock 1995; 4: 193–199. [PubMed] [Google Scholar]
- 14.Kaplan E, Dinarello CA, Gelfand JA. Interleukin-1 and the response to injury. Immunol Res 1989; 8: 118–129. [DOI] [PubMed] [Google Scholar]
- 15.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg 1999; 178: 449–453. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg 1992; 216: 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am 1995; 75: 257–277. [DOI] [PubMed] [Google Scholar]
- 18.Dobke MK, Simoni J, Ninnemann JL, et al. Endotoxemia after burn injury: effect of early excision on circulating endotoxin levels. J Burn Care Rehabil 1989; 10: 107–111. [DOI] [PubMed] [Google Scholar]
- 19.Kampschmidt RF. Infection, inflammation, and interleukin 1. Lymphokine Res 1983; 2: 97–102. [PubMed] [Google Scholar]
- 20.Ziegler TR, Smith RJ, O’Dwyer ST, et al. Increased intestinal permeability associated with infection in burn patients. Arch Surg 1988; 123: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 21.LeVoyer T, Cioffi WG Jr., Pratt L, et al. Alterations in intestinal permeability after thermal injury. Arch Surg 1992; 127: 26–30. [DOI] [PubMed] [Google Scholar]
- 22.Tadros T, Traber DL, Heggers JP, et al. Angiotensin II inhibitor DuP753 attenuates burn- and endotoxin-induced gut ischemia, lipid peroxidation, mucosal permeability, and bacterial translocation. Ann Surg 2000; 231: 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery 1990; 107: 411–416. [PubMed] [Google Scholar]
- 24.Schindel D, Maze R, Liu Q, et al. Interleukin-11 improves survival and reduces bacterial translocation and bone marrow suppression in burned mice. J Pediatr Surg 1997; 32: 312–315. [DOI] [PubMed] [Google Scholar]
- 25.Fleisher-Berkovich S, Danon A, Steen MB, et al. IL-1α but not IL-1β-induced prostaglandin synthesis is inhibited by corticotropin-releasing factor. Differential effect of corticotropin releasing factor on interleukin-1α and interleukin-1β-induced prostaglandin synthesis in endothelial cells and fibroblasts. Spontaneous activation of endothelial cells: a central role for endogenous IL-1α. Cytokine 1999; 11: 239–243. [DOI] [PubMed] [Google Scholar]
- 26.Arvidsson D, Rasmussen I, Almqvist P, et al. Splanchnic oxygen consumption in septic and hemorrhagic shock. Surgery 1991; 109: 190–197. [PubMed] [Google Scholar]
- 27.Fink MP. Adequacy of gut oxygenation in endotoxemia and sepsis. Crit Care Med 1993; 21: S4–8. [DOI] [PubMed] [Google Scholar]
- 28.Maulik N, Engelman RM, Wei Z, et al. Interleukin-1 alpha preconditioning reduces myocardial ischemia reperfusion injury. Circulation 1993; 88: II387–394. [PubMed] [Google Scholar]
- 29.Haq M, Norman J, Saba SR, et al. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 1998; 9: 614–619. [DOI] [PubMed] [Google Scholar]


