Abstract
Objective
To compare patterns in mortality and the use of subsequent biliary drainage interventions (surgical, endoscopic, and percutaneous) associated with the different types of biliary bypass.
Summary Background Data
Surgical palliation of obstructive jaundice due to pancreatic cancer is often accomplished with an intestinal bypass to either the gallbladder or the bile duct. It is not known whether a gallbladder bypass, which is a simpler operation and more amenable to laparoscopic surgery, performs as well as a bypass to the bile duct.
Methods
The authors conducted a retrospective cohort study of 1,919 patients 65 years of age or older who had a surgical biliary bypass for pancreatic cancer diagnosed between 1991 and 1996 using Medicare claims data and the Surveillance, Epidemiology and End Results (SEER) database.
Results
At 1, 2, and 5 years, 7.5%, 17.4%, and 26.0% of 945 patients initially treated with a gallbladder bypass had additional biliary interventions, as compared with 2.9%, 11.0%, and 13.3% of 974 patients initially treated with a bile duct bypass. Patients who initially had a gallbladder bypass were 4.4 times as likely to have additional biliary surgery and 2.9 times as likely to have any subsequent biliary intervention as were patients who initially had a bile duct bypass. Median survival was longer following bile duct bypass. The adjusted hazard ratio for death associated with gallbladder bypass was 1.2.
Conclusions
Compared to patients whose initial biliary bypass was to the bile duct, the risk of having one or more additional surgical, endoscopic, or percutaneous biliary drainage procedures is substantially greater in patients whose initial bypass was to the gallbladder.
Jaundice caused by obstruction of the bile duct occurs in the majority of patients with pancreatic cancer. 1 Reversal of malignant biliary obstruction provides palliation of jaundice even when a pancreatic tumor is unresectable. 2 Although endoscopic stenting of the bile duct can relieve biliary obstruction, 3–6 surgical bypass is done in many cases because of an inability to access the bile duct using endoscopic methods, patient or physician preference, or failure of nonoperative interventions. A biliary bypass may also be done when a pancreatic tumor proves to be unresectable during an operation intended to remove the tumor.
Operative bypass of the biliary system requires establishing continuity between the gastrointestinal tract and a portion of the biliary tree. If the gallbladder is diseased or has been removed, the anastomosis between the intestine and biliary system must be made to the bile duct (the hepatic duct or common bile duct). However, when the gallbladder is intact and continuous with the proximal biliary tree, the surgeon has the choice of fashioning an anastomosis either to the gallbladder or to the bile duct. Creating an anastomosis to the gallbladder is technically easier than bypassing to the bile duct and is more amenable to a laparoscopic approach. 7,8 In contrast, an anastomosis to the bile duct may provide more durable palliation of jaundice. 9 It is not clear whether the choice of biliary bypass influences survival.
Previous studies have included too few subjects to provide precise estimates of the relative effectiveness of gallbladder and bile duct bypass. The only randomized trial comparing the two procedures included 31 subjects, of whom only 71% had malignant biliary obstruction. 9 We used Medicare claims data and a population-based cancer registry to study patients with pancreatic cancer 65 years of age or older who initially had a biliary enteric bypass to either the gallbladder or the bile duct without removal of the pancreatic tumor. Our objectives were to determine the relative frequency of the different types of bypass, and to study patterns of mortality and the use of subsequent biliary drainage procedures.
METHODS
Data Sources
We used data from the Surveillance, Epidemiology, and End Results (SEER) program and from Medicare claims files. The SEER program actively acquires data from several population-based cancer registries in various geographic regions of the United States that represent 13.9% of the total population. Detailed cancer data including tumor site, stage, histology, and treatment are abstracted from a number of sources for up to 10 incident cancers per person residing in a SEER area. Vital status is updated annually from a public use file from the National Center for Health Statistics. 10 The Medicare file we used contains records for up to 10 surgical procedures and up to 10 medical diagnoses as International Classification of Diseases, ninth revision, Clinical Modification 11 (ICD-9-CM) procedure and diagnosis codes for each hospitalization. To protect the confidentiality of the Medicare beneficiaries, physician and hospital identifiers were omitted from the Medicare files. Medicare claims have been successfully linked to 93.8% of SEER records using a unique identification number. 12 These linked files permit longitudinal follow-up of individuals using Medicare claims data, combined with detailed, population-based oncologic information from the SEER file, and have been used to study surgical treatment for different types of cancers. 13–15 The analysis and interpretation of these data are the sole responsibility of the authors and do not represent the views of either the National Cancer Institute or the Centers for Medicare and Medicaid Services.
Study Subjects
The study cohort consisted of Medicare beneficiaries 65 years of age or older who resided in SEER areas and received a new diagnosis of pancreatic cancer (International Classification of Diseases for Oncology, second edition 16 [ICD-O-2] codes 25.0–25.3 and 25.7–25.9) between 1991 and 1996, who had a palliative intestinal bypass to either the gallbladder or bile duct (with or without a gastric bypass), and never had a procedure to resect or ablate the pancreatic tumor.
Measurement of Exposures and Outcomes
The initial biliary bypass operation was classified as a gallbladder or bile duct bypass according to the ICD-9-CM procedure code (see the appendix). Any of these procedures was also considered to constitute a subsequent biliary drainage operation if it occurred during a hospitalization following hospital discharge for the initial procedure. Appropriate ICD-9-CM procedure codes were used to identify subsequent biliary drainage operations (in addition to bypass procedures), concomitant gastric bypass, pancreatic resection, endoscopic biliary drainage procedures, and percutaneous biliary drainage procedures.
Comorbid Medical Conditions
To model comorbid medical conditions, we used a code-based modification of the Charlson comorbidity index 17,18 that has been used to adjust for comorbidity in other studies using SEER-Medicare data. 13,15 The index was further modified by excluding the categories for any malignancy and metastatic tumor. 15 We used the ICD-9-CM diagnosis codes present on the hospital admission for the initial biliary bypass to calculate the comorbidity score.
Statistical Analysis
Proportions of categorical variables were analyzed using the chi-square test. The unadjusted and stratified relative risk of binary outcomes was estimated using Mantel- Haenszel methods. 19 All reported P values are two-sided. We calculated survival for each subject from the date of the initial biliary bypass operation until the date of death for patients who died, or until December 31, 1998, for patients who had not died by this date. For the analysis of subsequent biliary interventions, the follow-up time began on the date of the initial bypass operation and ended on the date of the first subsequent biliary intervention, death, or on December 31, 1998, whichever came first. A subsequent biliary intervention was defined as one or more of a subsequent biliary bypass operation, a biliary drainage operation, an endoscopic biliary intervention, or a percutaneous biliary intervention. The cumulative risk of death and of having additional biliary interventions was estimated using Kaplan-Meier survival curves. 20 Differences between curves were assessed using the log-rank test, and Greenwood’s formula was used to estimate the standard error of the Kaplan-Meier estimate of the survival function. 21
The effects of exposures on the risk of experiencing an outcome over time were estimated using Cox proportional hazards regression. 22 We assumed that the proportional hazards assumption was reasonable for a covariate if the log-log survival curves for different levels of the covariate were approximately parallel. The relative hazard of an outcome associated with each exposure was expressed as the hazard ratio and 95% confidence interval. Multivariate and stratified regression models were fit to estimate the independent effects of exposures.
The choice of whether to perform a gallbladder or bile duct bypass is frequently up to the discretion of an individual surgeon. It is likely that a surgeon’s choice is influenced by patient factors, such as the tumor stage or the presence of medical comorbidities, which are also strongly associated with the risk of treatment failure or death. We used propensity score methods to reduce bias and imbalances in prognostic factors between treatment groups. 23 A propensity score is a measure of the probability that a subject was exposed to a treatment of interest given his or her values for a set of characteristics, regardless of the actual treatment received. Stratification on the propensity score reduces bias in estimating treatment effects in observational studies. 23
We estimated the conditional probability of having had a gallbladder bypass, independent of the actual treatment received, for each subject given his or her values for the following set of covariates: age, sex, race, comorbidity score, concomitant gastric bypass, tumor location, tumor size, tumor grade, and nodal involvement and extent at diagnosis, according to the categorization listed in Table 1. Predicted probabilities were estimated using a logistic regression analysis of the entire cohort, where the response variable was having a gallbladder bypass as the initial operation, and the predictor variables were all the other exposure variables listed. The c statistic for the logistic regression model was 0.62. The cohort was then evenly divided into quintiles according to the probability of having been treated initially with a gallbladder bypass. We confirmed that stratification on the propensity score eliminated imbalances in the covariates between treatment groups within propensity strata by testing, for each covariate, the significance of the F statistic for the treatment main effect term in a two-way analysis of variance that included the main effects for propensity score and initial type of biliary bypass, and an interaction term. 23 We adjusted for the propensity to have had a gallbladder bypass in proportional hazards regression models by stratifying the analysis by propensity quintile. In the stratified models, a single set of regression coefficients was fit to the entire dataset, but the baseline hazard function was allowed to vary between propensity strata. SAS version 8.01 software (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Table 1.PATIENT CHARACTERISTICS
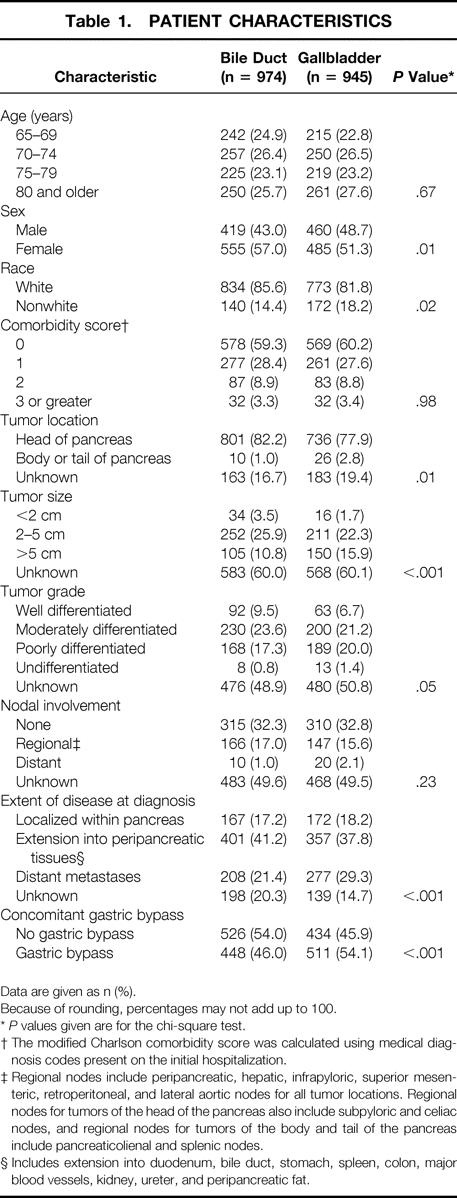
Data are given as n (%).
Because of rounding, percentages may not add up to 100.
*P values given are for the chi-square test.
† The modified Charlson comorbidity score was calculated using medical diagnosis codes present on the initial hospitalization.
‡ Regional nodes include peripancreatic, hepatic, infrapyloric, superior mesenteric, retroperitoneal, and lateral aortic nodes for all tumor locations. Regional nodes for tumors of the head of the pancreas also include subpyloric and celiac nodes, and regional nodes for tumors of the body and tail of the pancreas include pancreaticolienal and splenic nodes.
§ Includes extension into duodenum, bile duct, stomach, spleen, colon, major blood vessels, kidney, ureter, and peripancreatic fat.
RESULTS
Study Subjects
We identified 1,919 patients aged 65 years and over who were newly diagnosed with pancreatic cancer between 1991 and 1996 and underwent an operative bypass of their biliary system without having a resection of their primary pancreatic tumor. Of these, 974 (50.8%) underwent a bypass to the bile duct and 945 (49.2%) had a bypass to the gallbladder. Of the bile duct bypasses, 911 (93.5%) were to the common bile duct, 48 (4.9%) were to the hepatic duct, and 15 (1.5%) were unspecified.
The characteristics of all 1,919 subjects are listed in Table 1. Compared with persons having an initial bile duct bypass, those who had a gallbladder bypass were more likely to be male (48.7% vs. 43.0%, P = .01), to have a tumor larger than 5 cm (15.9% vs. 10.8%, P < .001), to have distant metastases at diagnosis (29.3% vs. 21.4%, P < .001), and to have a gastric bypass at the time of their initial biliary bypass (54.1% vs. 46.0%, P < .001). Patients having a bile duct bypass were more likely to be nonwhite (18.2% vs. 14.4%, P = .02) and to have a tumor located in the head of the pancreas (82.2 vs. 77.9%, P = .01).
Short-Term Outcomes of Surgery
Overall, 227 subjects died within 30 days of surgery. The probability of death within this time was 14.1% for gallbladder bypass and 9.7% for bile duct bypass (P < .01). The relative risk of death within 30 days of surgery associated with gallbladder bypass compared with bile duct bypass was 1.5 (95% confidence interval [CI], 1.1–1.9). When stratified according to the propensity to have had a gallbladder bypass, the relative risk was 1.2 (95% CI, 1.0–1.6, P = .08). The mean length of the hospital stay for the initial procedure was 15.6 days for patients who had a gallbladder bypass and 15.7 days for patients who had a bile duct bypass (mean difference −0.1, 95% CI, −0.8–0.9).
Subsequent Biliary Drainage Procedures
After the initial biliary bypass, one or more additional procedures to drain the bile duct were performed in 76 patients, 23 of whom initially had a bypass to the bile duct and 53 of whom had a bypass to the gallbladder. Using the composite end point of any surgical, endoscopic, or percutaneous biliary intervention, the cumulative risk of a subsequent procedure was substantially higher in patients who had a bypass to the gallbladder compared with those who had a bypass to the bile duct (Fig. 1). Within 1 year of the initial biliary bypass, 2.9% of patients who had a bile duct bypass required an additional intervention, compared with 7.5% of patients who had a gallbladder bypass. The corresponding values at 2 years for patients with a bile duct bypass compared to a gallbladder bypass were 11.0% and 17.4%; at 5 years they were 13.3% and 26.0%, respectively. When stratified according to the propensity of the patient to have had a gallbladder bypass, those who had a bypass to the gallbladder were 4.4 times as likely to require additional biliary surgery than patients who had a bypass to the bile duct (95% CI, 1.6–12.0) and were 2.9 times as likely to require any additional biliary intervention (95% CI, 1.8–4.8, Table 2).
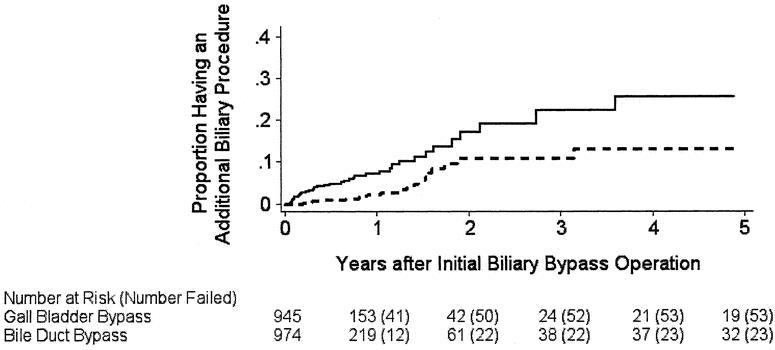
Figure 1. Estimated probability of one or more additional biliary drainage interventions after a biliary bypass operation in 1,919 Medicare beneficiaries 65 years of age or older newly diagnosed with pancreatic cancer between 1991 and 1996, according to the type of initial biliary bypass procedure performed. Solid line, gallbladder bypass as initial procedure; broken line, bile duct bypass as initial procedure. Numbers in parentheses indicate the cumulative number of subjects who had an additional procedure. The curves were truncated at 5 years. P value for the comparison of the curves was <.001 by the log-rank test.
Table 2.RELATIVE HAZARD OF HAVING ONE OR MORE SUBSEQUENT BILIARY DRAINAGE PROCEDURES ASSOCIATED WITH INITIAL GALLBLADDER BYPASS COMPARED WITH BILE DUCT BYPASS
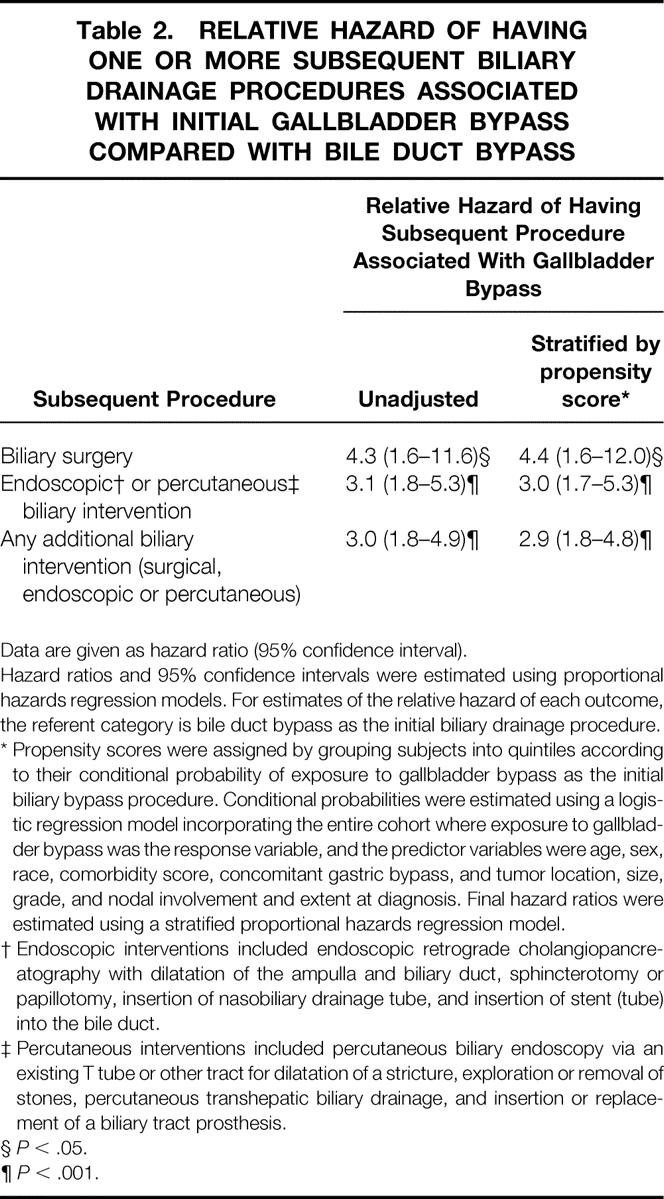
Data are given as hazard ratio (95% confidence interval).
Hazard ratios and 95% confidence intervals were estimated using proportional hazards regression models. For estimates of the relative hazard of each outcome, the referent category is bile duct bypass as the initial biliary drainage procedure.
* Propensity scores were assigned by grouping subjects into quintiles according to their conditional probability of exposure to gallbladder bypass as the initial biliary bypass procedure. Conditional probabilities were estimated using a logistic regression model incorporating the entire cohort where exposure to gallbladder bypass was the response variable, and the predictor variables were age, sex, race, comorbidity score, concomitant gastric bypass, and tumor location, size, grade, and nodal involvement and extent at diagnosis. Final hazard ratios were estimated using a stratified proportional hazards regression model.
† Endoscopic interventions included endoscopic retrograde cholangiopancreatography with dilatation of the ampulla and biliary duct, sphincterotomy or papillotomy, insertion of nasobiliary drainage tube, and insertion of stent (tube) into the bile duct.
‡ Percutaneous interventions included percutaneous biliary endoscopy via an existing T tube or other tract for dilatation of a stricture, exploration or removal of stones, percutaneous transhepatic biliary drainage, and insertion or replacement of a biliary tract prosthesis.
§P < .05.
¶P < .001.
Survival
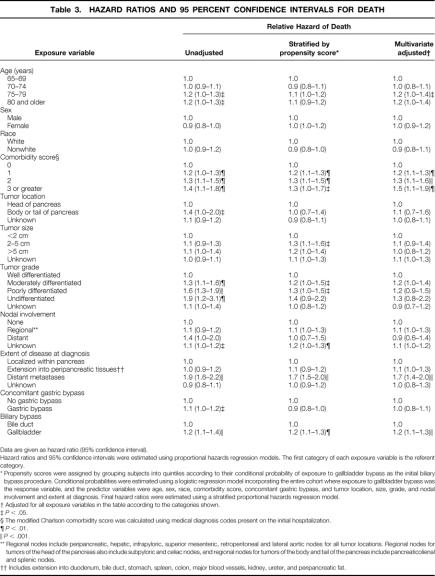
During the observation period, 1,845 persons died (96%). Kaplan-Meier survival estimates are shown in Figure 2. The median survival of patients who had a bypass to their bile duct was substantially longer than those who had a bypass to the gallbladder (6.3 months vs. 4.4 months, P < .001). Estimates of the relative hazard of death associated with various exposure variables are listed in Table 3. The results of different analyses are shown, including an unadjusted analysis, an analysis stratified by the propensity to have been exposed to gallbladder bypass, and an analysis adjusted for multiple potentially confounding variables. In general, the results did not differ substantially according to the method of analysis.
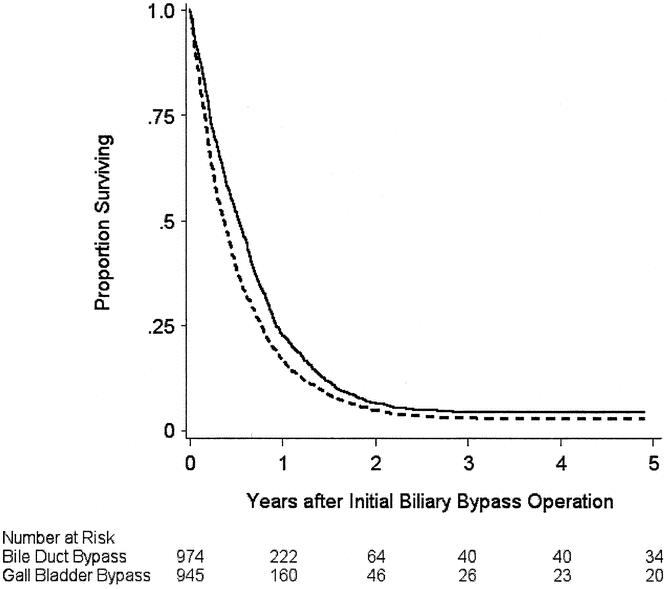
Figure 2. Estimated probability of survival after a biliary bypass operation in 1,919 Medicare beneficiaries 65 years of age or older newly diagnosed with pancreatic cancer between 1991 and 1996, according to the type of initial biliary bypass procedure performed. Solid line, bile duct bypass as initial procedure; broken line, gallbladder bypass as initial procedure. The curves were truncated at 5 years. P value for the comparison of the curves was <.001 by the log-rank test.
Table 3.HAZARD RATIOS AND 95 PERCENT CONFIDENCE INTERVALS FOR DEATH
Data are given as hazard ratio (95% confidence interval).
Hazard ratios and 95% confidence intervals were estimated using proportional hazards regression models. The first category of each exposure variable is the referent category.
* Propensity scores were assigned by grouping subjects into quintiles according to their conditional probability of exposure to gallbladder bypass as the initial biliary bypass procedure. Conditional probabilities were estimated using a logistic regression model incorporating the entire cohort where exposure to gallbladder bypass was the response variable, and the predictor variables were age, sex, race, comorbidity score, concomitant gastric bypass, and tumor location, size, grade, and nodal involvement and extent at diagnosis. Final hazard ratios were estimated using a stratified proportional hazards regression model.
† Adjusted for all exposure variables in the according to the categories shown.
‡P < .05.
§ The modified Charlson comorbidity score was calculated using medical diagnosis codes present on the initial hospitalization.
¶P < .01.
∥P < .001.
** Regional nodes include peripancreatic, hepatic, infrapyloric, superior mesenteric, retroperitoneal and lateral aortic nodes for all tumor locations. Regional nodes for tumors of the head of the pancreas also include subpyloric and celiac nodes, and regional nodes for tumors of the body and tail of the pancreas include pancreaticolienal and splenic nodes.
†† Includes extension into duodenum, bile duct, stomach, spleen, colon, major blood vessels, kidney, ureter, and peripancreatic fat.
An increased risk of death was associated with older age, a greater burden of comorbidity, tumor grade (moderately or poorly differentiated vs. well differentiated), the presence of distant metastases at diagnosis, and the use of the gallbladder for the initial biliary bypass. When stratified by propensity score, the relative hazard of death associated with gallbladder bypass was 1.2 (95% CI, 1.1–1.3) compared with bile duct bypass.
DISCUSSION
In a population-based retrospective cohort study of adults with pancreatic cancer who underwent a palliative biliary bypass, we found that additional biliary interventions were done more frequently following a gallbladder bypass than a bile duct bypass. There was a small survival advantage for patients treated initially with bile duct bypass. We did not find evidence that the short-term outcomes of bile duct bypass were appreciably worse than gallbladder bypass. These findings are consistent with previous observations suggesting that bile duct bypass provides more durable palliation of obstructive jaundice than gallbladder bypass. 9,24,25
Despite the strong advocacy of influential surgeons that bypass to the bile duct is superior to gallbladder bypass for malignant biliary obstruction, 24,25 gallbladder bypass continues to be performed frequently in the United States. About half of the subjects in our cohort had their initial bypass to the gallbladder. From an anatomic point of view, a bile duct bypass is a more sensible procedure. Most patients with periampullary cancer have an obstructed cystic duct, a diseased gallbladder, or a tumor that extends close to the junction of the cystic duct and the bile duct. 26 The reasons for the continued popularity of gallbladder bypass among practicing surgeons are not clear. Possible explanations include the technical ease with which gallbladder bypass can be accomplished compared with bile duct bypass, and the impression among those who treat patients with pancreatic cancer that survival is so poor, and the risk of additional procedures following gallbladder bypass low, that gallbladder bypass remains a reasonable option.
Our study has potential limitations. We do not know how many patients who had a bile duct bypass had a previous cholecystectomy or a diseased gallbladder, which obviously precludes having a gallbladder bypass. Undoubtedly some of the patients who had a bile duct bypass did not have a suitable gallbladder; therefore, it is likely that gallbladder bypasses are actually performed more frequently than bile duct bypasses in patients who have a normal gallbladder. The data sources we used lacked surgeon- and hospital-specific data. However, it is unlikely that detailed provider or institution information would have affected our conclusions regarding the relative effectiveness of the procedures.
We used data contained in administrative and tumor registry databases, which are subject to measurement error. We were not able to detect detailed clinical information, such as whether patients were jaundiced before the initial biliary bypass or at the time that subsequent interventions were done. We could not detect whether patients had recurrent biliary obstruction but died without having another intervention. We could not determine some important operative details, such as whether an intestinal bypass was configured as an end-to-side or side-to-side anastomosis. However, it is unlikely that these shortcomings affected our main findings, because any measurement error is probably nondifferential with respect to treatment. Although we used an observational study design, we do not believe that our results can be explained solely by bias or confounding, since the principal results persisted despite the use of various strategies to reduce bias and confounding.
What, then, is the role of gallbladder bypass in the surgical palliation of jaundice due to pancreatic cancer? Although our data suggest that bile duct bypass is more durable and may be associated with longer survival than gallbladder bypass, we believe that a gallbladder bypass may be appropriate in some circumstances. When portal hypertension or portal or superior mesenteric vein occlusion is unexpectedly found at operation in a patient who requires biliary bypass surgery, a gallbladder bypass is safer than a bile duct bypass. 24 The choice is further complicated by the fact that a different surgical approach may be used for the different types of biliary bypass. A gallbladder bypass can readily be done using laparoscopic surgery. 7,8 The additional technical complexity of a bile duct bypass makes this procedure difficult, but not impossible, to accomplish using minimally invasive techniques. Since the absolute risk of adverse events following gallbladder bypass is still relatively small, some surgeons who can perform a gallbladder bypass but not a bile duct bypass using laparoscopic surgery may feel that the excess risk of a laparotomy outweighs any shortcomings of a gallbladder bypass.
In conclusion, a gallbladder bypass is associated with a substantially greater risk of subsequent biliary drainage procedures than a bile duct bypass in patients with pancreatic cancer. Surgeons must trade off any potential short-term benefits of a gallbladder bypass, such as the ability to perform a laparoscopic operation, against the higher risk of subsequent interventions and the possibility of a small decrease in survival.
Acknowledgments
The authors thank Dr. John Marshall and Dr. Stacey L. Urbach for their thoughtful comments on earlier versions of this manuscript, and Ms. Cindy Muller and Mrs. Olukemi Quinn for their assistance. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Information Services, and the Office of Strategic Planning, CMS; Information Management Systems (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
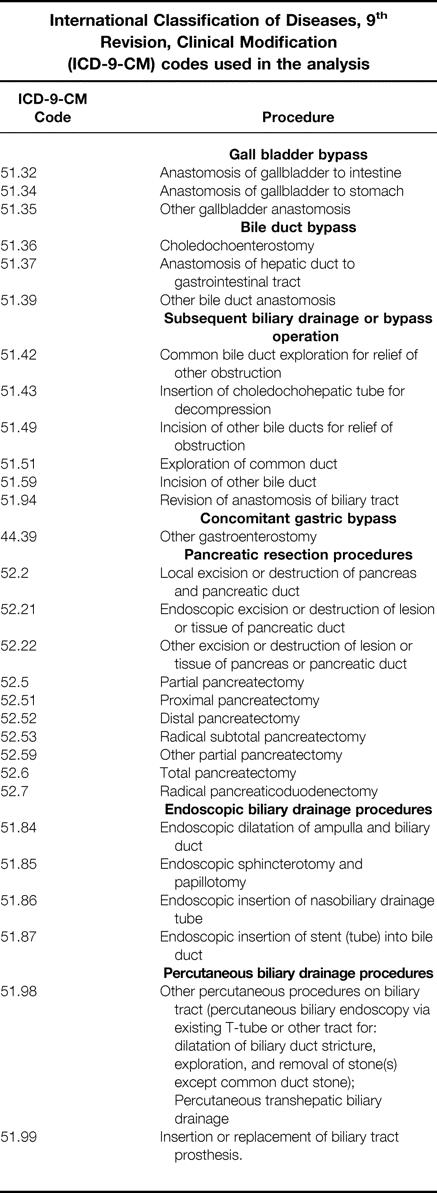
APPENDIX
International Classification of Diseases, ninth Revision, Clinical Modification (ICD-9-CM) codes used in the analysis
Table. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes used in the analysis.
Footnotes
Dr. Bell is supported by Clinician-Scientist Awards from the Canadian Institutes of Health Research and the Department of Medicine at the University of Toronto.
Correspondence: David R. Urbach, MD, Toronto General Hospital, 200 Elizabeth Street, Room 9EN-236A, Toronto, ON M5G 2C4, Canada.
E-mail: david.urbach@uhn.on.ca
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Accepted for publication March 26, 2002.
References
- 1.Reber HA. Pancreas. In Schwartz SI, Shires GT, Spencer FC, et al, eds. Principles of surgery, 6th ed. New York: McGraw-Hill Inc.; 1994: 1401–1432.
- 2.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–465. [DOI] [PubMed] [Google Scholar]
- 3.Bornman PC, Harries-Jones EP, Tobias R, et al. Prospective controlled trial of transhepatic biliary endoprosthesis versus bypass surgery for incurable carcinoma of head of pancreas. Lancet 1986; 8472: 69–71. [DOI] [PubMed] [Google Scholar]
- 4.Speer AG, Cotton PB, Russell RC, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet 1987; 8550: 57–62. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd HA, Royle G, Ross AP, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg 1988; 75: 1166–1168. [DOI] [PubMed] [Google Scholar]
- 6.Andersen JR, Sorensen SM, Kruse A, et al. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut 1989; 30: 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimi S, Banting S, Cuschieri A. Laparoscopy in the management of pancreatic cancer: endoscopic cholecystojejunostomy for advanced disease. Br J Surg 1992; 79: 317–319. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher DR, Jones RM. Laparoscopic cholecystojejunostomy as palliation for obstructive jaundice in inoperable carcinoma of the pancreas. Surg Endosc 1992; 6: 147–149. [DOI] [PubMed] [Google Scholar]
- 9.Sarfeh IJ, Rypins EB, Jakowatz JG, et al. A prospective, randomized clinical investigation of cholecystoenterostomy and choledochoenterostomy. Am J Surg 1988; 155: 411–414. [DOI] [PubMed] [Google Scholar]
- 10.SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 2000, 1973.
- 11.1999 Hospital, Payor ICD-9-CM. International classification of diseases, 9th rev., clinical modification, 5th ed. Salt Lake City: Medicode; 1998.
- 12.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care 1993; 31: 732–748. [PubMed] [Google Scholar]
- 13.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999; 341: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 14.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001; 345: 181–188. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 16.International classification of diseases for oncology, 2nd ed. Geneva: World Health Organization; 1990.
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 18.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol 1993; 46: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 19.Modern epidemiology, 2d ed. Philadelphia: Lippincott-Raven; 1998.
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley; 1980.
- 22.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972; 34: 187–220. [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 24.Lillemoe KD, Kal Sauter P, Pitt HA, et al. Current status of surgical palliation of periampullary carcinoma. Surg Gynecol Obstet 1993; 176: 1–10. [PubMed] [Google Scholar]
- 25.Watanapa P, Williamson RCN. Surgical palliation for pancreatic cancer: Developments during the past two decades. Br J Surg 1992; 79: 8–20. [DOI] [PubMed] [Google Scholar]
- 26.Tarnasky PR, England RE, Lail LM, et al. Cystic duct patency in malignant obstructive jaundice. An ERCP-based study relevant to the role of laparoscopic cholecystojejunostomy. Ann Surg 1995; 221: 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]