Abstract
Objective
To determine whether temporary occlusion of the main pancreatic duct with human fibrin glue decreases the incidence of intra-abdominal complications after pancreatoduodenectomy (PD) or distal pancreatectomy (DP).
Summary Background Data
To the authors’ knowledge, there are no randomized studies comparing outcomes after pancreatic resection with or without main pancreatic duct occlusion by injection of fibrin glue. Of three nonrandomized studies, two reported no fistulas after intracanal injection and ductal occlusion with fibrin glue after PD with immediate pancreatodigestive anastomosis, while another study reported no protective effect of glue injection.
Methods
This prospective, randomized, single-blinded, multicenter study, conducted between January 1995 and January 1999, included 182 consecutive patients undergoing PD followed by immediate pancreatic anastomosis or DP, whether for benign or malignant tumor or for chronic pancreatitis. One hundred two underwent pancreatic resection followed by ductal occlusion with fibrin glue (made slowly resorbable by the addition of aprotinin); 80 underwent resection without ductal occlusion. The main end point was the number of patients with one or more of the following intra-abdominal complications: pancreatic or other digestive tract fistula, intra-abdominal collections (infected or not), acute pancreatitis, or intra-abdominal or digestive tract hemorrhage. Severity factors included postoperative mortality, repeat operations, and length of hospital stay.
Results
The two groups were similar in pre- and intraoperative characteristics except that there were significantly more patients in the ductal occlusion group who were receiving octreotide, who had reinforcement of their anastomosis by fibrin glue, and who had fibrotic pancreatic stumps. However, the rate of patients with one or more intra-abdominal complications, and notably with pancreatic fistula, did not differ significantly between the two groups. There was still no significant difference found after statistical adjustment for these patient characteristic discrepancies, confirming the inefficacy of fibrin glue. The rate of intra-abdominal complications was significantly higher in the presence of a normal, nonfibrotic pancreatic stump and main pancreatic duct diameter less than 3 mm, whereas reinforcement of the anastomosis with fibrin glue or use of octreotide did not influence outcome. In multivariate analysis, however, normal pancreatic parenchyma was the only independent risk factor for intra-abdominal complications. No significant differences were found in the severity of complications between the two groups.
Conclusions
Ductal occlusion by intracanal injection of fibrin glue decreases neither the rate nor the severity of intra-abdominal complications after pancreatic resection.
Morbidity and mortality after partial pancreatectomy are essentially secondary to intra-abdominal complications (pancreatic, biliary, or digestive tract fistula, acute pancreatitis) and their consequences (abscess, hemorrhage); rates range from 20%1 to 40%2 and from 0%1 to 15%, 3 respectively, and cannot be underestimated. The main cause of these complications is anastomotic or suture leakage or fistula arising from the pancreatic stump. 1,2 The rate of the latter ranges between 7.5%4 and 38%. 5
Several controlled studies have attempted to reduce the incidence of pancreatic fistula. Octreotide was shown to be effective in five 5–9 of seven 5–11 controlled studies. Ligation of the main pancreatic duct, combined with fish-mouth pancreatic closure and reinforcement of the sutures with fibrin glue after distal pancreatectomy (DP), 12 proved positive, while two other trials studying reinforcement of the digestive anastomosis by fibrin glue 13 or pancreatogastrostomy compared with pancreatojejunostomy 14 after pancreatoduodenectomy (PD) were negative.
Other procedures used in an attempt to reduce the incidence of pancreatic fistula, not yet tested in randomized trials, can be divided into two categories: those involving the technique of pancreatoenteric anastomosis, 15–19 and those decreasing pancreatic secretion, including preoperative 16 or intraoperative 13 radiation therapy; ligation of the main pancreatic duct, associated or not with closure of the divided pancreatic surface, usually performed without anastomosis;20,21 and ductal occlusion by glue with 22–24 or without 25–28 anastomosis.
Ductal occlusion with neoprene or prolamine, both nonresorbable glues, was used most often without anastomosis after PD but has been abandoned because permanent occlusion induces pancreatic atrophy and complete loss of exocrine function. 28 Following that came the idea of using resorbable glues, 22,23,25,27,29 limiting the action of pancreatic proteases while waiting for the pancreatodigestive tract anastomosis or the pancreatic stump to heal after (proximal or distal) pancreatectomy. With the intention of increasing its efficacy, aprotinin, which delays the dissolution of the glue, was added. 22 Until now, only nonrandomized or uncontrolled studies have been reported. Of these, no postoperative pancreatic fistulas were observed in 80 22 and 12 23 patients undergoing PD for carcinoma or for chronic pancreatitis, respectively, followed by immediate pancreatodigestive anastomosis. However in a prospective, comparative, but not randomized trial involving 80 patients undergoing PD (40 for cancer and 40 for chronic pancreatitis), half of each group either had ductal occlusion with fibrin glue or not, 24 no significant protective effect was afforded by the use of glue. Moreover, among the 40 patients with cancer, there were twice as many complications in patients who underwent ductal occlusion compared with those who did not (P = .2). 24
We therefore undertook this prospective randomized multicenter trial to verify whether ductal occlusion with human fibrin glue could decrease morbidity after PD with pancreatodigestive anastomosis or after DP.
METHODS
Patients
Between January 1995 and January 1999 (4 years), 15 centers (10 university hospitals and 5 general hospitals) enrolled 182 consecutive patients (102 men, 80 women), mean age 56 ± 13 years (range 17–81), undergoing pancreatic resection for either benign or malignant (pancreatic or extrapancreatic [biliary, ampullary, or duodenal]) tumors or chronic pancreatitis. Patients undergoing total pancreatectomy, tumorectomy or enucleation, or internal drainage for pseudocyst, those in whom the pancreatic stump was not anastomosed after PD, those who underwent side-to-side pancreaticojejunostomy without resection, and those whose resection was performed for acute pancreatitis or trauma were not included.
Not all centers started or finished the study at the same time. The median number of inclusions per center was 11 (range 2–32).
Surgical Technique
PD was performed according to each surgeon’s preferences, but it was mandatory that the pancreatic stump be anastomosed either to the jejunum or the stomach. The pylorus could be preserved or not and vagotomy could be performed when the pylorus was removed. Excluded from this study were patients who underwent PD without pancreatodigestive tract anastomosis. 25,27,29,30
DP could be either caudal or corporeocaudal, with preservation or not of the spleen. The stump and the main pancreatic duct were either closed or anastomosed to a Roux-en-Y jejunal loop.
Resections extended to nearby organs (colon, liver, mesenteric portal confluence, kidney, adrenals, diaphragm) as well as resection of nearby organs extended to the pancreas were allowed (Tables 1 and 2). All patients had intravenous antibiotic prophylaxis at anesthetic induction.
Table 1.COMPARABILITY OF GROUPS: PREOPERATIVE CHARACTERISTICS
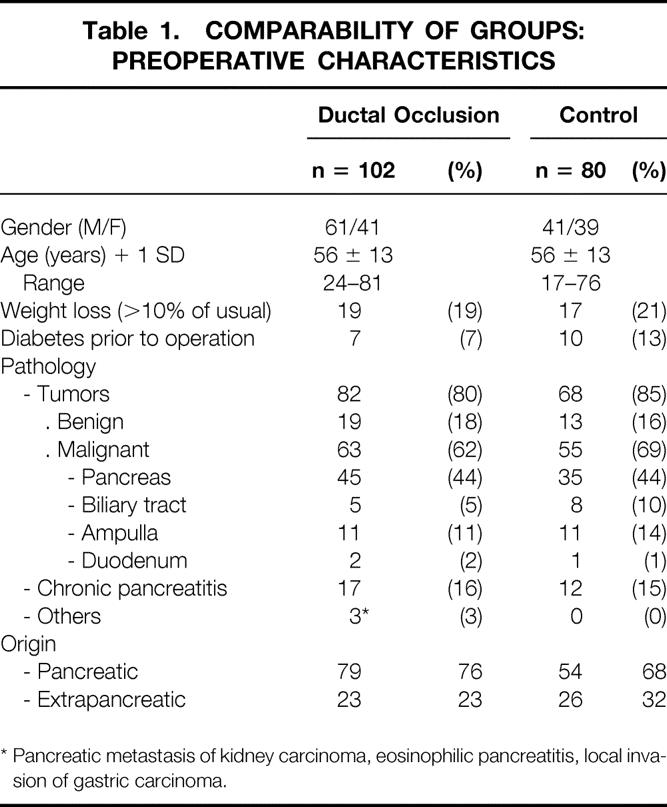
* Pancreatic metastasis of kidney carcinoma, eosinophilic pancreatitis, local invasion of gastric carcinoma.
Table 2.COMPARABILITY OF GROUPS: INTRAOPERATIVE CHARACTERISTICS
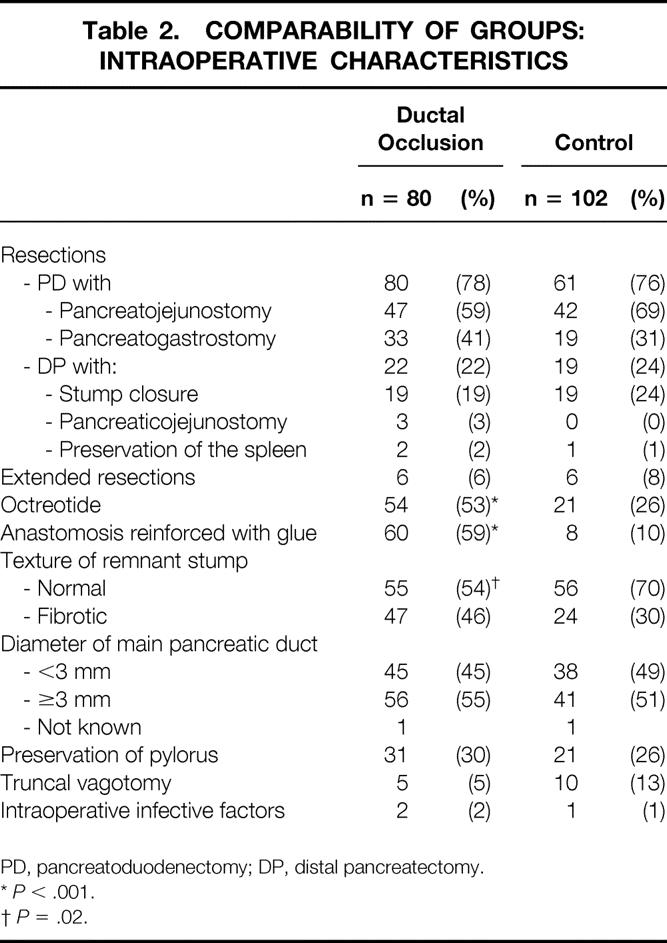
PD, pancreatoduodenectomy; DP, distal pancreatectomy.
*P < .001.
†P = .02.
Three to 5 mL of rapidly acting fibrin glue (Tissucol) containing 500 IU thrombin (Immuno France) was used to occlude the main pancreatic duct by intracanal injection through a double-barreled syringe connected to an Y-shaped catheter (Duploject, Tissucol Kit), mixing the two active products just before application. Aprotinin delays degradation of Tissucol by pancreatic enzymes;22 in this study, 10,000 UIK/mL of aprotinin (Antagosan, Behring Laboratories) was used instead of the usual 3,000 UIK/mL of aprotinin furnished with Tissucol. For PD, the catheter was pushed toward the tail of the pancreas. For DP, the catheter was inserted toward the head, avoiding injury to the papilla. In both instances, the catheter was withdrawn while injecting the glue until a fibrin plug was obtained at the end of the duct.
Postoperative drainage and other associated preventive measures such as use of octreotide, omentoplasty, or reinforcement of the anastomosis with fibrin glue were left to the discretion of the surgeon. Drainage of the main pancreatic duct was not allowed. 17
End Points
The main end point was the number of patients with one or more postoperative intra-abdominal complications, 6 as diagnosed during the postoperative period (entire hospital stay and 30 days after discharge for patients leaving the hospital within 1 month), during reoperation, and at autopsy. These complications included pancreatic, biliary, or digestive tract fistula, intra-abdominal collection (either infected [abscess] or not), acute pancreatitis, and intra-abdominal or digestive tract hemorrhage. Pancreatic fistula was defined either chemically 10 as fluid obtained through drains or percutaneous aspiration containing at least four times normal serum values of amylase for 3 days, irrespective of the amount of output and the date of appearance, or clinically and radiologically as anastomotic leaks demonstrated by fistulography, or by hydrosoluble contrast studies in the case of pancreatogastrostomy. Biliary fistulas were diagnosed by the distinctive color of discharge containing bilirubin or by fistulography. Gastrojejunal fistulas were diagnosed by fistulography, contrast follow-through studies, or contrast-enhanced CT scan. Intra-abdominal collections were diagnosed by CT scan, CT-guided needle aspiration, and cultures and amylase levels of the contents. Acute pancreatitis was defined as increased values of serum amylase (fourfold normal), lipase (threefold normal), and urine amylase (fourfold normal), assessed every other day during 8 days and confirmed by CT scan. Digestive tract hemorrhage was defined as blood exiting through the nasogastric tube and confirmed on fibroendoscopy or arteriography. Intra-abdominal hemorrhage was confirmed by exteriorization of blood through drains, on arteriography, operation, or any combination thereof.
Severity of intra-abdominal complications was evaluated by the mortality rate, rate of reoperations and/or percutaneous drainage for collections, and length of hospital stay.
Risk Factors
Patients were randomized into four strata according to two variably combined risk factors: type of resection (PD or DP) 6 and type of pathology (tumor or chronic pancreatitis). 5,7
The other three risk factors studied were the consistency of the pancreatic remnant parenchyma (“normal” parenchyma was defined as healthy, soft, or friable pancreatic tissue) or “fibrotic”; the diameter of the main pancreatic duct, as measured with a ruler on the severed surface after pancreatic resection (≥3 mm or <3 mm 31); and the type of pancreaticoenteric anastomosis after pancreatoduodenectomy (pancreatojejunostomy or pancreatogastrostomy). 11,21
Randomization
To minimize the number of patients withdrawn secondarily after randomization because the proposed operation was deemed impractical, random assignment was performed in the operating room once the surgeon had checked both the inclusion and exclusion criteria and was sure that both therapeutic arms were feasible, after resection but before restoration of digestive tract continuity or closure of the remnant stump in DP. Treatment with ductal occlusion or not, as generated by computerized random-number tables, was allotted through a telephone call to the coordinating center, which has been recommended as the best method of randomization. 32 Random allotment was balanced every four patients within each stratum and at each center.
Ethical Issues
The ethics committee of the coordinating center approved this study, and informed consent was obtained preoperatively for all patients. Immuno France provided secretarial assistance for this trial.
Blinding
Patients as well as the nursing staff were not aware of the treatment arm to which the patients were allotted, but the surgeon performing the operation, obviously, was (single-blind study without placebo). Postoperative complications, however, were assessed by a physician who was unaware of the allotted treatment.
Number of Patients
Based on the expectation of decreasing the rate of patients with one or more postoperative intra-abdominal complications from 40%2 to 20%, 1 with a 5% alpha risk and 80% power in one-tailed explanatory analysis, 64 patients were required in each group, for a total of 128 patients. 33,34 The number was incremented by 20% for the smaller group to compensate for eventual a posteriori exclusions (i.e., 154 patients).
Quality Control
The validity of data was checked randomly (1/5 patients) by a quality control officer (surgical resident student in Applied Sciences Research).
Statistical Analysis
Percentages were compared either with the chi-square test or with Fisher exact test, as appropriate. Discrete variables were compared with the Student t test, analysis of variance, or the Mann-Whitney test, as appropriate. The Mantel-Haenszel adjustment test 35 was used to check whether statistically significant differences in patient characteristics influenced outcome. The center effect was analyzed. Risk factors were analyzed according to univariate and multivariate analysis using the stepwise logistic regression model on variables with a P ≤ .25 in univariate analysis.
RESULTS
Of the 182 patients randomized, 102 underwent ductal occlusion and 80 served as controls. There were no protocol violations, especially as concerned randomization. No patient was withdrawn from analysis after randomization, and there were no crossovers after allocation.
The two groups were similar in pre- and intraoperative characteristics (27 items) except that there were significantly more patients in the ductal occlusion group who received octreotide (53% vs. 26%;P < .001), who had reinforcement of their anastomosis by fibrin glue (59% vs. 10%;P < .001), or who had fibrotic pancreatic stumps (46% vs. 30%;P = .02). Randomization was discontinued when the minimum number of patients in each arm (n = 77) was attained, without taking into account the uncompleted randomization blocks of four in each center and in each stratum.
Main End Point
There were fewer patients with one or more intra-abdominal complications, and in particular with deep collections, in the ductal occlusion group (24 and 15 [24% and 15%], respectively) than in the control group (21 and 19 [26% and 24%], respectively), but neither of these differences were statistically significant (P < .2 for both) (Table 3). The individual rates of the other complications, in particular pancreatic fistula (15% vs. 17%), did not differ significantly. Even after statistical adjustment 35 for significantly different patient characteristics (administration of octreotide, consistency of pancreatic parenchyma, reinforcement of the anastomosis with fibrin glue), these differences remained nonsignificant.
Table 3.RESULTS: POSTOPERATIVE INTRA-ABDOMINAL COMPLICATIONS AND MORTALITY
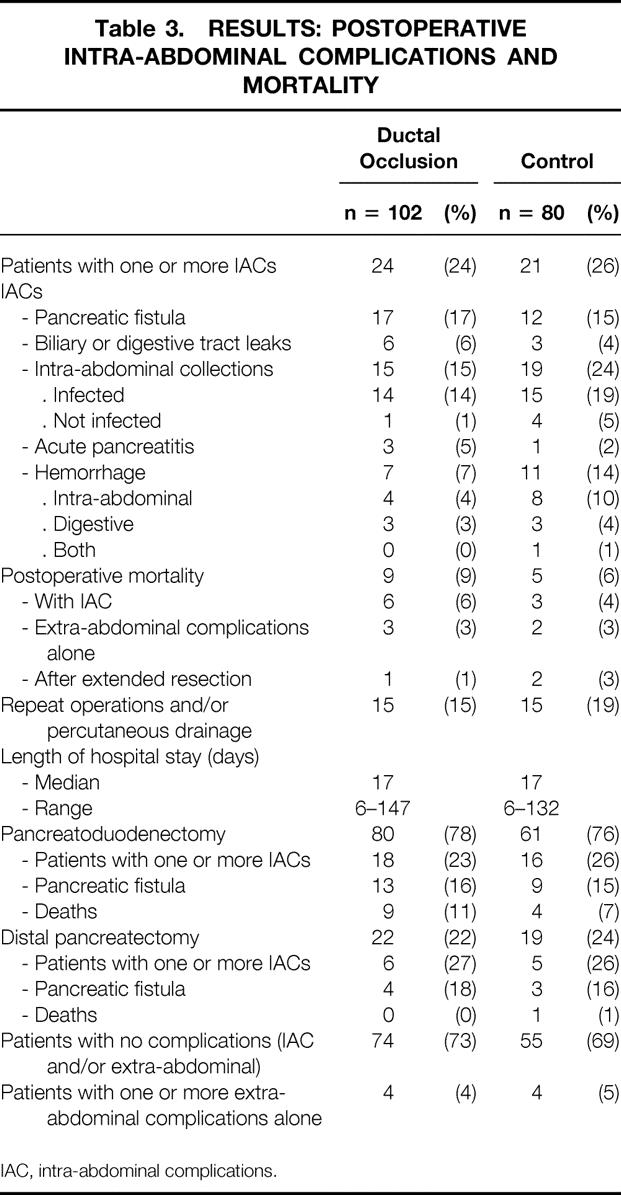
IAC, intra-abdominal complications.
Severity Criteria
No significant differences were found between the two groups in terms of postoperative mortality, reoperations, or length of hospital stay (see Table 3).
Fourteen patients died (8%), 10 (6%) after standard resection, 3 (25%) of 12 patients undergoing extended resection (see Tables 2 and 3). Nine patients (5%) died of intra-abdominal causes (six pancreatic fistula, one multiple fistula, one biliary fistula after associated hepatic resection, and one hemorrhage) and five (3%) from extra-abdominal causes (two heart failure, two pleuropulmonary infection, and one acute hepatic failure due to isoflurane). Mortality was higher in PD (n = 13 [9%]) than in DP (n = 1 [2%]), but this difference was not significant (P = .30).
Thirty patients (17%), 19% in the control group and 15% in the ductal occlusion group (see Table 3), underwent reoperation or percutaneous drainage for the following reasons: intra-abdominal or digestive tract hemorrhage alone (n = 10), infected collections (abscess) alone (n = 10), associated hemorrhage and infected collections (n = 4), generalized peritonitis (n = 5), and intestinal obstruction (n = 1).
No significant difference was found in the median length of postoperative hospital stay (median 17 days in both groups) (see Table 3).
Risk Factors
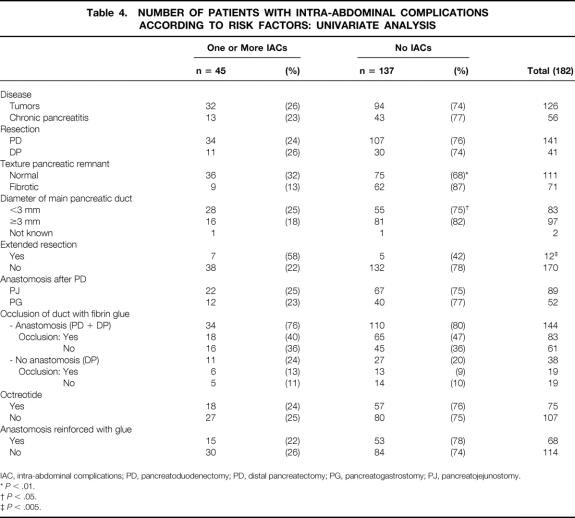
On univariate analysis, three factors were found to significantly influence the onset of intra-abdominal complications (Table 4): normal parenchyma (P < .01), diameter of the main pancreatic duct less than 3 mm (P < .05), and extended resection (P = .005). On multivariate analysis, only normal pancreatic parenchyma remained an independent risk factor (P < .01, confidence interval 1.3–8.17; odds ratio = 3.23). The other factors studied (in Table 4) were not found to differ significantly, including specifically octreotide and anastomotic reinforcement by fibrin glue, which were performed significantly more often in the occlusion group.
Table 4.NUMBER OF PATIENTS WITH INTRA-ABDOMINAL COMPLICATIONS ACCORDING TO RISK FACTORS: UNIVARIATE ANALYSIS
IAC, intra-abdominal complications; PD, pancreatoduodenectomy; PD, distal pancreatectomy; PG, pancreatogastrostomy; PJ, pancreatojejunostomy.
*P < .01.
†P < .05.
‡P < .005.
No adverse effects could be directly attributed to the use of fibrin glue or its injection.
Center Effect
Although results differed from one center to another, no significant center effect was found.
DISCUSSION
To the best of our knowledge, this is the first controlled, randomized trial testing the effects of ductal occlusion with fibrin glue (Tissucol). Our results show that this technique did not significantly decrease the rate of intra-abdominal complications, notably pancreatic fistula, or their severity after pancreatic resection.
The lopsided difference in the number of patients in each arm (102 ductal occlusion vs. 80 controls) was because randomization was performed according to four strata and balanced every four patients per stratum and per center; some centers entered fewer than four patients overall and/or in each of the strata. The study was closed before any equilibrium was obtained for each of the strata in each center.
As in the report by Montorsi et al, 6 our primary end point was the number of patients with one or more intra-abdominal complications, not simply the rate of pancreatic fistula or the crude overall number of complications. 11 This was chosen for several reasons. First, pancreatic fistulas can promote, be confounded by, or be associated with other complications; for example, intra-abdominal hemorrhage can be secondary to pancreatic fistula. Tabulation of all intra-abdominal complications ensures that no atypical manifestation of pancreatic fistula is ignored. Second, this criterion is better than the overall complication rate, including extra-abdominal complications, because most of the latter can be generated by intra-abdominal complications. 7 Third, there is no consensus as to the exact definition of pancreatic fistula, 36 rendering any comparison with other series confusing or impossible.
Our overall rate of intra-abdominal complications was 25%, close to those reported elsewhere (23%6–8 and 27%10). In two other trials, the rate was higher: 37%11 because of inclusion of delayed gastric emptying, which was excluded from other studies, and 44%5 because of inclusion of duodenum-preserving resections, well known for their higher rate of intra-abdominal complications. 37 In our series, fibrin glue injection did not significantly decrease the rate of intra-abdominal complications (23.5% vs. 26%; see Table 3).
Pancreatic Fistula
The rate of pancreatic fistula in our series (16%) was not decreased by ductal occlusion (see Table 3). However, this rate was lower than those found in most other randomized trials testing the efficacy of octreotide, 5–8,10,13 ranging from 19%7 to 38%5 in the control arm and from 18%7 to 25%10 in the octreotide arm. In two controlled trials from the same center, one comparing pancreatogastrostomy versus pancreatojejunostomy 14 and the other comparing the use of octreotide or not, 11 the fistula rates were lower at 12% and 10%, respectively. The most likely explanation for this discrepancy remains, as suggested above, the differing definitions of pancreatic fistula. 36,37
Failure of fibrin glue ductal occlusion to act on the pancreatic fistula rate can be explained in several ways. First, blockage of the main pancreatic duct, even transiently, might artificially increase the secretion of pancreatic juice in the severed secondary canals 38 or on the suture line. Second, occlusion of the main pancreatic duct might be incomplete 39 or might not last long enough in humans. Experimentally, glue absorption is inversely proportional to the concentration of aprotinin. 22 In this study we used 10,000 UIK/mL of aprotinin, which might not have been high enough. Both Waclawicek et al 22 and Cavallini et al 27 advocated using at least 20,000 UIK/mL. In these two retrospective studies, no fistulas were observed in the 80 cases of the former, but five of six patients still developed fistula in the latter. The effectiveness of higher doses of aprotinin to maintain occlusion for more than 8 days remains to be tested.
As there were significantly fewer potentially high-risk patients in the ductal occlusion group (i.e., fewer patients not receiving octreotide, without anastomotic reinforcement by fibrin glue, or with normal parenchyma), this should have favored the group with ductal occlusion. When we began this study in 1995, there was no consensus as to the prophylactic use of octreotide despite two published randomized trials. 5,7 Today, five 5–9 of seven 5–11 controlled studies have shown a significant action of octreotide in the prevention of pancreatic fistula or intra-abdominal complications. In our study, the number of patients receiving octreotide or not was not evenly distributed between the two groups by random sampling: significantly more patients received octreotide in the occlusion group (53% vs. 26%;P < .001)(see Table 2). This should have led to fewer fistulas and/or intra-abdominal complications in the occlusion group, but this was not the case. To prove this, however, an adjustment technique for statistical analysis 35 was necessary. After adjustment, the rate of pancreatic fistula and/or intra-abdominal complications did not improve, underscoring the absence of action of ductal occlusion. Moreover, administration of octreotide, a nonrandomized factor, did not appear to be an independent protective factor on univariate (see Table 4) or multivariate analysis.
There were significantly more patients with anastomotic reinforcement in the fibrin group (60 [59%] vs. 8 [10%]; (P < .001), probably for economic reasons (once fibrin glue had been allocated, the surgeon chose to use it to reinforce the anastomosis as well). In the only controlled study to date, however, anastomotic reinforcement with fibrin glue 13 did not affect outcome.
Moreover, if ductal occlusion associated with anastomotic reinforcement by fibrin glue had been effective, there should have been fewer fistulas in this group; this was not the case. After adjustment, 35 this factor did not change outcome and was not an independent protective factor on univariate or multivariate analysis.
Normal consistency of pancreatic parenchyma was significantly less often encountered in the ductal occlusion group (54% vs. 70%;P < .02)(see Table 2). In several reports, normal pancreatic parenchyma has been found to promote pancreatic fistula and intra-abdominal complications. 5–9,11 In our study as well, normal parenchymal consistency was an independent risk factor for pancreatic fistula on univariate and multivariate analysis (see Table 4). This should have once again favored the ductal occlusion group, leading to fewer pancreatic fistula and/or intra-abdominal complications, but this did not occur. After statistical adjustment for this unbalanced characteristic, 35 there was still no significant effect noted on the rate of pancreatic fistula and/or intra-abdominal complications in the ductal occlusion group.
Ductal occlusion did not decrease the severity of postoperative complications in our study, as attested by the nonsignificant differences in mortality, reoperations, or length of postoperative hospital stay.
Our overall mortality rate was 8%, higher than reported in other controlled studies (0.4%, 11 0.8%, 10 1%, 14 3%, 7 4%, 5 and 7%6), but unlike these same reports, 12 of the patients in our study had undergone extended pancreatectomy (well known for increased morbidity and mortality 21; see Table 2). If the deaths caused by extended pancreatectomy were eliminated, our mortality rate would have been 6.5%, close to the 7% found in one controlled series on octreotide. 6 Another reason for this apparently higher mortality might be the multicenter character of our study, which included low-volume as well as high-volume centers. Inclusion of only high-volume centers, 10,11 to which highly selected, low-risk patients are referred, 40 can lead to selection bias and better outcome.
Reoperations
In our series, the rate of reoperations and/or percutaneous drainage was similar in the two groups (19% for control vs. 15% for occlusion; see Table 3). Only two other controlled studies 10,11 studied this without showing any significant difference. The other controlled trials 5–9 did not analyze this factor.
Length of Stay
Four trials 5,8,10,11 studied the length of hospital stay in addition to ours. Similar to our results (17 days in both groups; see Table 3), the difference between the two groups was not significant. Comparison with other studies is difficult, however, because some studies used means rather the median, 5,8 and the difference in length of hospital stay might have been due to other unrelated factors such as differences in cultural, payer, 41 or local 11 organization.
Given that fibrin ductal occlusion was not efficient, other techniques proposed to decrease the complication rate still remain to be tested by controlled studies, notably those decreasing juice pancreatic outflow through the anastomosis: drainage of the main duct 15,17,18 and preoperative 16 or intraoperative 10 radiation therapy, particularly in pancreatic cancer, associated or not with octreotide.
Acknowledgments
The following surgeons participated in the study: Jean-Pierre Arnaud, MD (Angers), Loïc de Calan, MD, Pierre Bourlier, MD (Tours), Jean-Marie Hay, MD, Guy Zeitoun, MD, Yves Flamant, MD, Simon Msika, MD (Colombes), Jean Escat, MD, Giles Fourtanier, MD, Bertrand Suc, MD (Toulouse), Jean-Luc Gouzi, MD, Bernard Pradère, MD (Toulouse), Pierre-Louis Fagniez, MD, Daniel Cherqui, MD, Nelly Rotman, MD (Créteil), André Elhadad, MD (Aulnay-sous-Bois), Philippe Vicq, MD, Jean-Louis André, MD (Paris), Yves Laborde, MD (Pau), Abe Fingerhut, MD (Poissy), Jean-Claude Le Néel, MD (Nantes), Jean-Pierre Lenriot, MD, Jean-Christophe Paquet, MD (Longjumeau), Bernard Descottes, MD (Limoges), Alain Gainant, MD, Pierre Cubertafond, MD, Muriel Mathonnet, MD (Limoges), Bernard Sastre, MD (Marseille).
Footnotes
Correspondence: Abe Fingerhut, MD, FACS, FRCS, Hôpital Léon Touladjian, Possy-St Germain, 78303 France.
E-mail: abefinger@aol.com
Accepted for publication October 25, 2001.
References
- 1.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993; 217: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trede M, Schwall G. The complications of pancreatectomy. Ann Surg 1988; 207: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hertner FP, Cooperman AM, Ahlborn TN, et al. Surgical experience with pancreatic and peri-ampullary cancer. Ann Surg 1982; 195: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez Del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–300. [DOI] [PubMed] [Google Scholar]
- 5.Buchler M, Friess H, Klempa I, et al. Role of octreotide in the prevention of postoperative complications following pancreatic resection. Am J Surg 1992; 163: 125–131. [DOI] [PubMed] [Google Scholar]
- 6.Montorsi M, Zago M, Mosca F, et al. Efficacy of octreotide in the prevention of complication fistula after elective pancreatic resections. A prospective, controlled randomized trial. Surgery 1995; 117: 26–31. [DOI] [PubMed] [Google Scholar]
- 7.Pederzoli P, Bassi C, Falconi M, et al. Efficacy of octreotide in the prevention of complications of elective pancreatic surgery. Br J Surg 1994; 81: 265–269. [DOI] [PubMed] [Google Scholar]
- 8.Friess H, Beger HG, Sulkowski U, et al. Randomized controlled multicentre study of the prevention of complications by octreotide in patients undergoing surgery for chronic pancreatitis. Br J Surg 1995; 82: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 9.Suc B, Msika S, Piccinini M, et al, the French Associations for Surgical Research. Octreotide in the prevention of intra-abdominal complications following elective pancreatic resection. A prospective, multicenter, randomized clinical trial. (Submitted) [DOI] [PubMed]
- 10.Lowy AM, Lee JE, Pisters PWT, et al. Prospective, randomized trial of octreotide to prevent pancreatic fistula after pancreaticoduodenectomy for malignant disease. Ann Surg 1997; 226: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Cameron JL, Lillemoe KD, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg 2000; 232: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki Y, Kuroda Y, Morita A, et al. Fibrin glue sealing of pancreatic injuries, resections, and anastomoses. Arch Surg 1995; 130: 952–955. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea AA, Costantino V, Speri C, et al. Human fibrin sealant in pancreatic surgery: is it useful in preventing fistulas? A prospective randomized study. Ital J Gastroenterology 1994; 26: 283–286. [PubMed] [Google Scholar]
- 14.Yeo CJ, Cameron JL, Maher MM, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreatico-duodenectomy. Ann Surg 1995; 222: 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanaka Y, Suzuki T. Total pancreatic duct drainage for leakproof pancreatojejunostomy. Surgery 1994; 115: 22–26. [PubMed] [Google Scholar]
- 16.Ishikawa O, Higashi H, Imaoka S, et al. Concomitant benefit of preoperative irradiation in preventing pancreas fistula formation after pancreato-duodenectomy. Arch Surg 1991; 126: 885–889. [DOI] [PubMed] [Google Scholar]
- 17.Roder JD, Stein HJ, Böttcher KA, et al. Stented versus nonstented pancreaticojejunostomy after pancreaticoduodenectomy. A prospective study. Ann Surg 1999; 229: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tashiro S, Murata E, Hiraoka T, et al. New technique for pancreaticojejunostomy using a biological adhesive. Br J Surg 1987; 74: 392–394. [DOI] [PubMed] [Google Scholar]
- 19.Kingsnorth AN. Duct-to-mucosa isolated Roux loop pancreaticojejunostomy as an improved anastomosis after resection of the pancreas. Surg Gynecol Obstet 1989; 169: 451–453. [PubMed] [Google Scholar]
- 20.Brunschwig A. Resection of the head of pancreas and duodenum for carcinoma-pancreatoduodenectomy. Surg Gynecol Obstet 1937; 65: 681–685. [Google Scholar]
- 21.Papachristou DN, Fortner JG. Pancreatic fistula complicating pancreatectomy for malignant disease. Br J Surg 1981; 68: 238–240. [DOI] [PubMed] [Google Scholar]
- 22.Waclawiczek HW, Boechl O. Pancreatic duct occlusion with fibrin sealant for the protection of the pancreatic digestive anastomosis following resection of the pancreatic head (experimental and clinical study). In: General and Abdominal Surgery. Berlin: Springer Verlag, 1994; 88: 106. [Google Scholar]
- 23.Van Gulik TM, Van Berge Henegouwen MI, Gabeler EEE, et al. Leakage of the pancreatic anastomosis in 224 Whipple’s resections. Incidence and prevention using systemic somatostatin or fibrin glue for temporary occlusion of the pancreatic duct. European I.H.P.B.A. Congress “Athens 95.” Bologna: Monduzzi, 1995: 539–542.
- 24.Lorenz D, Wolff H, Waclawiczek H. Die Pankreasgangocclusion in der Resektionsbehandlung der chronischen Pankreatitis und des pankreaskophcarcinoms. Eine 3 jährige Nachbeobachtungsstudie. Chirurgie 1988; 59: 90–95. [PubMed] [Google Scholar]
- 25.Itoh T, Idezuki Y, Konishi T, et al. Ethibloc-occlusion for prevention of pancreatic fistula after distal pancreatectomy. J Jpn Soc Clin Surg 1989; 50: 1526–1531. [Google Scholar]
- 26.Little JM, Lauer C, Hoog J. Pancreatic duct obstruction with an acrylate glue: a new method for producing pancreatic exocrine atrophy. Surgery 1977; 81: 243–249. [PubMed] [Google Scholar]
- 27.Cavallini M, Tallerini A, Stipa F. Pancreatic duct occlusion with fibrin sealant and pylorus preserving technique after duodenopancreatectomy for ampullary carcinoma. Minerve Ch 1991; 46: 733–739. [Google Scholar]
- 28.Di Carlo V, Chiera R, Pontiroli AE, et al. Pancreatoduodenectomy with occlusion of the residual stump by neoprene injection. World J Surg 1989; 13: 105–111. [DOI] [PubMed] [Google Scholar]
- 29.Marczell AP. Indications for fibrin sealing in pancreatic surgery with special regard to occlusion of a non anastomosed stump with fibrin sealant. 2nd World Congress International Hepatopancreaticobiliary Association. Bologna, Italy, June 2–6, 1996; 1229–1235.
- 30.Miedema BW, Sarr MG, Van Heerden JA, et al. Complications following pancreaticoduodenectomy: current management. Arch Surg 1992; 127: 945–950. [DOI] [PubMed] [Google Scholar]
- 31.Hamanaka Y, Nishihara K, Hamasaki T, et al. Pancreatic juice output pancreatoduodenectomy in relation to pancreatic consistency, duct size and leakage. Surgery 1996; 119: 281–287. [DOI] [PubMed] [Google Scholar]
- 32.Cancer Research Campaign Working Party. Trials and tribulations: thoughts on the organization of multicentre clinical studies. Br Med J 1980; 281: 918–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz D, Flamant R, Lellouch J. Clinical trials. London: Academic Press, 1980:93.
- 34.Murray GD. Statistical aspects of research methodology. Br J Surg 1991; 78: 777–781. [DOI] [PubMed] [Google Scholar]
- 35.Mantel N. Chi-square tests with one degree of freedom: extension of the Mantel-Haenszel procedure. J Am Stat Assoc 1963; 58: 690–700. [Google Scholar]
- 36.Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001; 88: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 37.Beger HG, Büchler M, Bittner R, et al. Duodenum-preserving resection of the head of the pancreas in severe chronic pancreatitis. Ann Surg 1989; 209: 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohwada S, Ogawa T, Tanamashi, et al. Fibrin glue sandwich prevents pancreatic fistula following distal pancreatectomy. World J Surg 1998; 22:494–498. [DOI] [PubMed]
- 39.Gall FP, Gebhardt C, Meister R, et al. Severe chronic cephalic pancreatitis. Use of partial duodenopancreatectomy with occlusion of the pancreatic duct in 289 patients. World J Surg 1989; 13: 809–817. [DOI] [PubMed] [Google Scholar]
- 40.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg 1998; 228: 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salcedo-Wasicek MD, Thirlby RC. Postoperative course after herniorrhaphy: a case-controlled comparison of patients receiving worker’s compensation vs. patients with commercial insurance. Arch Surg 1995; 130: 29–32. [DOI] [PubMed] [Google Scholar]