Abstract
Objective
To test the hypothesis that laparoscopic staging improves outcome in patients with peripancreatic carcinoma compared to standard radiology staging.
Summary Background Data
Diagnostic laparoscopy of peripancreatic malignancies has been reported to improve assessment of tumor stage and to prevent unnecessary exploratory laparotomies in 10% to 76% of patients.
Methods
Laparoscopy and laparoscopic ultrasound were performed in 297 consecutive patients with peripancreatic carcinoma scheduled for surgery after radiologic staging. Patients with pathology-proven unresectable tumors were randomly allocated to either surgical or endoscopic palliation. All others underwent laparotomy.
Results
Laparoscopic staging detected biopsy-proven unresectable disease in 39 patients (13%). At laparotomy, unresectable disease was found in another 72 patients, leading to a detection rate for laparoscopic staging of 35%. In total, 145 of the 197 patients classified as having “possibly resectable” disease after laparoscopic staging underwent resection (74%). Average survival in the group of 14 patients with biopsy-proven unresectable tumors randomly allocated to endoscopic palliation was 116 days, with a mean hospital-free survival of 94 days. The corresponding figures were 192 days and 164 days in the 13 patients allocated to surgical palliation.
Conclusions
Because of the limited detection rate for unresectable metastatic disease and the likely absence of a large gain after switching from surgical to endoscopic palliation, laparoscopic staging should not be performed routinely in patients with peripancreatic carcinoma.
Diagnostic laparoscopy of peripancreatic malignancies has been reported to improve the assessment of tumor stage and to prevent unnecessary exploratory laparotomies. 1–12 Laparoscopy enables the detection of small superficial metastases at the liver surface and the peritoneum that are easily missed with radiologic staging techniques and often first encountered during laparotomy. Diagnostic laparoscopy can be combined with laparoscopic ultrasound, which has been reported to be sensitive for the detection of small intrahepatic metastases and for the evaluation of enlarged lymph nodes and tumor ingrowth in vascular structures surrounding peripancreatic tumors. 8–23
As laparoscopy is the final staging procedure before surgery, the eventual benefits of laparoscopic staging apply to patients already selected for resection by radiologic imaging techniques. In recent years radiologic imaging techniques have been improved. New staging methods have been introduced, such as helical computed tomography, endoscopic ultrasonography, and intravascular ultrasonography, affecting patient selection for resection and increasing resectability rates. 24–26 It is likely that the improved accuracy of radiologic staging techniques will limit the additional value of laparoscopic staging. Two centers have reported a decreased benefit of laparoscopic staging compared to their initial results. 1,2,11,27
Any evaluation of a diagnostic technique would be incomplete without an assessment of the subsequent improvement in patient outcome. The benefits of laparoscopic staging have been extensively described for many different gastrointestinal tumors, including peripancreatic carcinoma. 1–3,8,11,16–23 Laparoscopic staging of peripancreatic carcinoma has been reported to prevent exploratory laparotomies in 15% to 82% of patients. 1–3,8,11,16–23
Patients with unresectable peripancreatic tumors are candidates for nonsurgical palliative treatment with an endoprosthesis in the common bile duct, which relieves the obstructive jaundice. 28 Endoscopic palliation is associated with a shorter hospital stay and lower morbidity. Between 8% to 30% of these patients develop symptoms of duodenal obstruction and later require a gastric bypass operation. Insertion of an endoprosthesis in the duodenum is not yet routinely performed. 28–32
Surgical palliation is an alternative. It generally consists of a biliary bypass (hepaticojejunostomy) and a gastric bypass (gastroenterostomy). Such a double bypass has been shown to prevent recurrent biliary and duodenal obstruction at the expense of increased early morbidity and mortality after surgery and a prolonged hospital stay. 33–37
If a surgical procedure is the preferred form of palliative treatment for patients with unresectable tumors, there is little need for laparoscopic staging to prevent unnecessary laparotomies. Routine laparoscopic staging in patients with peripancreatic carcinoma will therefore improve patient outcome only if two conditions are satisfied: first, laparoscopic staging is able to identify patients with unresectable tumors and, second, endoscopic palliation is at least as effective as surgical palliation.
The study reported here was designed to test the hypothesis that laparoscopic staging improves outcome in patients with peripancreatic carcinoma compared to standard radiologic staging. We performed laparoscopy and laparoscopic ultrasound in a consecutive series of patients with peripancreatic carcinoma scheduled for surgery. Patients with histopathology-proven unresectable tumors detected by laparoscopic staging were randomly allocated to surgical or endoscopic palliation. All others underwent laparotomy.
METHODS
The study was performed in the Academic Medical Center (AMC), Amsterdam, and Erasmus Medical Center (EMC), Rotterdam, both university hospitals specializing in hepatopancreatobiliary diseases.
Patients with peripancreatic tumors (pancreatic head, papillary or distal common bile duct tumors) considered fit for surgery were eligible for this study. Exclusion criteria were venous occlusion or encasement of the portal or superior mesenteric vein, artery, or celiac trunk, duodenal obstruction, considerable impairment in normal functioning (Karnofsky < 80), and serious comorbidity (Greenfield Index of Disease Severity > 2). 38,39
Standardized prelaparoscopic staging was performed with transabdominal ultrasonography and helical computed tomography scan. Biopsies were taken only from lesions suspected of being metastases. The primary tumor was never punctured for diagnosis. Patients with obstructive jaundice were treated with a biliary endoprosthesis placed prelaparoscopically during endoscopic retrograde cholangiopancrea-tography.
Diagnostic Laparoscopy
The diagnostic procedures performed in this study have been described in detail elsewhere. 10,11,27,29 Diagnostic laparoscopy combined with laparoscopic ultrasonography was performed as a separate procedure under general anesthesia. After the Veress needle was inserted just below the umbilicus, a CO2 pneumoperitoneum was installed and three trocars were inserted. A structured inspection of the peritoneal cavity was performed. The surgeon introduced a 7.5-mHz linear array probe (Aloka) for laparoscopic ultrasonography. A radiologist performed or assisted the surgeon with the ultrasonography. The liver and enlarged lymph nodes were visualized, as were the vascular structures near the tumor. Finally, biopsies of suspected lesions were taken with the biopsy forceps (superficial lesions) or with Rotex or Tru-cut needles (deeper lesions) under guidance of the laparoscope or laparoscopic ultrasound, respectively.
After pathologic examination of the biopsy samples, the findings of diagnostic laparoscopy were summarized as “possibly resectable” (no metastases or tumor ingrowth were visualized during diagnostic laparoscopy), “probably irresectable” (tumor ingrowth or a metastasis was highly suspected but could not be proven with a biopsy sample), or “definitely irresectable” (irresectable tumor due to metastatic disease or local tumor ingrowth proven with a biopsy).
Randomization
Patients with a biopsy-proven unresectable tumor, identified by diagnostic laparoscopy, were informed about the randomized part of the study. The study was approved by the local ethical committees of the AMC and EMC. Consenting patients were randomly allocated to either endoscopic or surgical palliation. Randomization was performed centrally using a computer program, with stratification for center, metastases, and papillary tumors. 40
Surgical palliation consisted of a double-bypass procedure (retrocolic gastroenterostomy and Roux-en-Y side-to-side hepaticojejunostomy) performed in combination with a celiac plexus block, as described previously. 41 A plexus block can be performed relatively easily during the operation and is known to be an effective pain treatment. 41
Patients randomly allocated to endoscopic palliation received a Wallstent (Schneider, Benelux). These patients received a percutaneous celiac plexus block only in case of intractable pain or insufficient pain relief from oral medication.
All randomized patients were followed up regularly until death. The duration of any hospital readmission was recorded.
Analysis
The primary analysis focused on the percentage of patients undergoing laparoscopy who were classified as having “definitely irresectable” and “probably irresectable” disease and the number of times these classifications proved to be correct after exploratory laparotomy. The detection rate of laparoscopic staging was calculated; it was defined as the percentage of patients with unresectable disease identified as such after laparoscopic staging.
The second analysis focused on the randomized comparison of endoscopic versus surgical palliation in patients with unresectable tumors on an intention-to-treat basis. We calculated the survival and hospital-free survival in both groups and calculated the differences and associated 95% confidence intervals. We also used the log-rank test statistic to test for significant survival differences.
The number of patients to be included in the study was based on an estimated 33% of patients with biopsy-proven unresectable disease after laparoscopic staging and a gain of 3 weeks (SD 5) in hospital-free survival from switching to endoscopic palliation in this subgroup. Aiming at a power of 90%, with a significance level of 5%, 300 patients had to be included in the study.
RESULTS
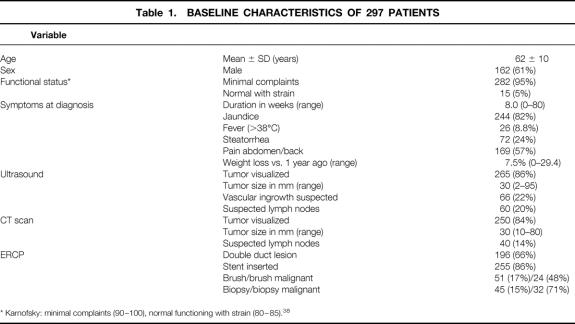
A total of 297 patients were included, 198 in the AMC and 99 in the EMC. Patient characteristics and results of prelaparoscopic staging are summarized in Table 1. Preoperative biliary drainage was performed with an endoprosthesis in most patients with obstructive jaundice; the others had received a papillotomy or percutaneous transhepatic drainage. Staging using ultrasound and spiral CT revealed a median tumor size of 30 mm.
Table 1.BASELINE CHARACTERISTICS OF 297 PATIENTS
* Karnofsky: minimal complaints (90–100), normal functioning with strain (80–85). 38
Laparoscopic Staging
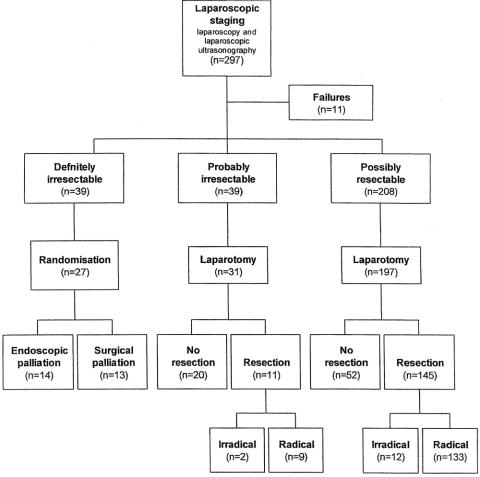
A flow chart of the patients is given in Figure 1. For the diagnostic laparoscopy, patients were hospitalized from 2 to 26 days (average 4.3). Mean operative time for laparoscopy was 55 minutes (SD 16). In 11 patients the diagnostic laparoscopy could not be performed. Nine patients had adhesions from previous abdominal surgery, one patient had a biloma after PTC, and one patient needed a conversion to laparotomy to stop bleeding from a biopsy site. Complications of diagnostic laparoscopy occurred in 11 patients, with two major ones: a small bowel perforation and a stomach perforation.
Figure 1. Flow chart of patients included for laparoscopic staging.
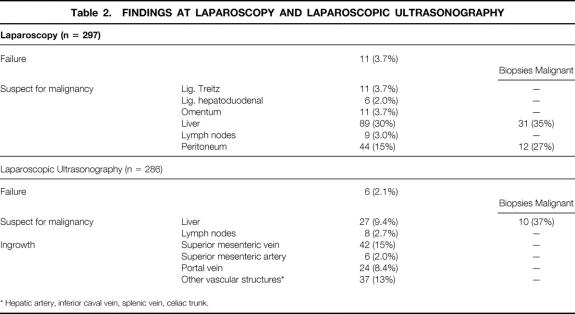
Table 2 summarizes the findings at laparoscopy and laparoscopic ultrasonography. Suspected lesions were laparoscopically detected on the liver surface in 89 (30%) and on the peritoneum in 44 (15%) patients; of these, biopsy samples were positive in 31 and 12, respectively.
Table 2.FINDINGS AT LAPAROSCOPY AND LAPAROSCOPIC ULTRASONOGRAPHY
* Hepatic artery, inferior caval vein, splenic vein, celiac trunk.
Failure of laparoscopic ultrasonography was caused by adhesions in the abdomen (n = 6). Laparoscopic ultrasound showed possible tumor ingrowth in the superior mesenteric vein in 15% and in the portal vein in 8.4%; this was not confirmed on biopsy. Laparoscopic ultrasound detected 27 (9.6%) suspect liver lesions and 8 (2.9%) suspect lymph nodes; 10 and 0, respectively, were histologically confirmed.
In summary, biopsy-proven unresectable disease was found in 39 of 297 patients after laparoscopic staging (13%; 95% confidence interval 9.3–17%). Probably unresectable disease that could not be confirmed on biopsy was found in another 39 patients (13%): 28 patients with vascular tumor ingrowth, 5 patients with metastases that were punctured but negative for tumor, and 6 patients with both vascular tumor ingrowth and unproven metastases.
Exploratory Laparotomy
Patients with probably unresectable disease and patients with possibly resectable disease were scheduled for an exploratory laparotomy 1 to 5 weeks after laparoscopic staging. Nineteen of these patients did not undergo a laparotomy: nine patients were included in a neoadjuvant trial with 5-fluoruracil and radiotherapy, three had rapidly progressive disease, in five a tumor was not visualized, and two did not want to undergo an operation. A laparotomy was performed in the remaining 228 patients, followed by a pylorus-preserving pancreaticoduodenectomy in 156 patients and no resection in 72 patients.
After exploratory laparotomy, 145 of 197 (74%; 95% confidence interval 67–80%) patients classified with “possibly resectable” disease after laparoscopic staging were found to have resectable tumors (Table 3) 74% (95% confidence interval 67–80%). In addition, 11 of 31 patients classified with “probably irresectable” disease had resectable tumors (35%; 95% confidence interval 19–55%).
Table 3.RESULTS OF LAPAROSCOPIC STAGING
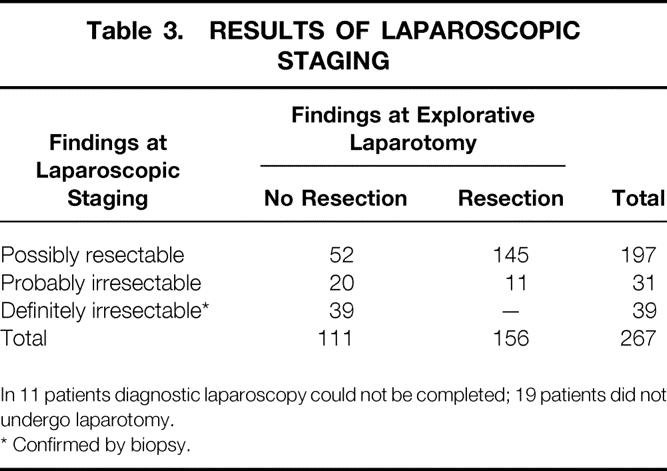
In 11 patients diagnostic laparoscopy could not be completed; 19 patients did not undergo laparotomy.
* Confirmed by biopsy.
In total, 111 patients did receive a final classification of “irresectable disease” after laparoscopy and exploratory laparotomy. Of these, laparoscopic staging had been able to detect 39 (detection rate 35%; 95% confidence interval 26–44%).
Endoscopic Versus Surgical Palliation
The 39 patients identified with unresectable disease by diagnostic laparoscopy were invited to participate in the randomized comparison of endoscopic versus surgical palliation. Eleven patients declined. One patient died of progressive disease before he could be randomized. Fourteen patients were randomly allocated to endoscopic palliation and 13 to surgical palliation.
Five randomized patients could not be treated according to the protocol. One patient refused to undergo laparotomy, one patient was treated in another hospital, one patient had a gallbladder empyema and did not receive a bypass during exploration, one patient died before he received a bypass, and in one patient stent insertion failed. All were included in the analysis. Six patients allocated to endoscopic palliation had complications during follow-up compared to four in the group with surgical palliation (Table 4). There was no procedure-related mortality. The procedure-related morbidity was 1/14 (7%) for patients after an endoscopic palliation and 1/13 (8%) after bypass surgery. Nine patients had to be readmitted in the endoscopic group (for a total of 14 admissions) compared to 7 in the surgical group (for a total of 11 admissions).
Table 4.COMPLICATIONS OF TREATMENT, COMPLICATIONS DURING FOLLOW-UP AND HOSPITAL ADMISSIONS OF PATIENTS RANDOMIZED TO RECEIVE A WALLSTENT OR DOUBLE BYPASS
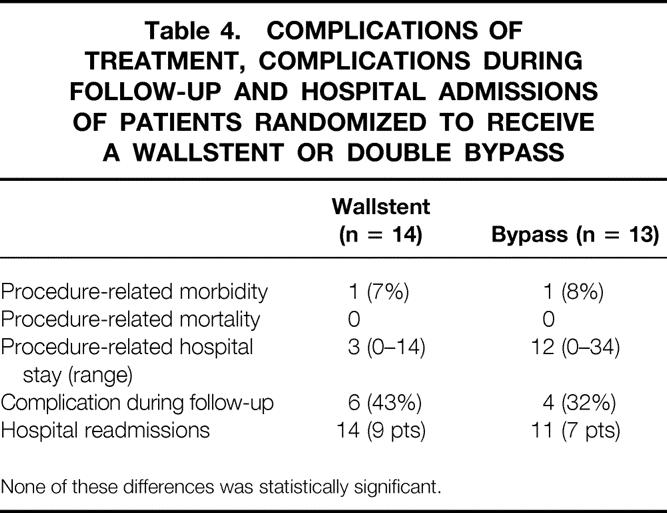
None of these differences was statistically significant.
Average survival in the patients allocated to endoscopic palliation was 116 days, with a mean hospital-free survival of 94 days (median 99). In patients allocated to surgical palliation, average survival was 192 days, with a mean hospital-free survival of 164 days (median 154). The difference in survival for the group treated with endoscopic palliation, compared to surgical palliation, was −70 days (95% confidence interval −15–10). The difference in hospital-free survival was −76 days (95% confidence interval −159–7).
Figure 2 depicts survival in both groups. The log-rank test statistic for survival was 3.8 (P = .05). This figure shows the initial hospital stay to be longer in patients treated with surgical palliation, with more patients with prolonged survival.
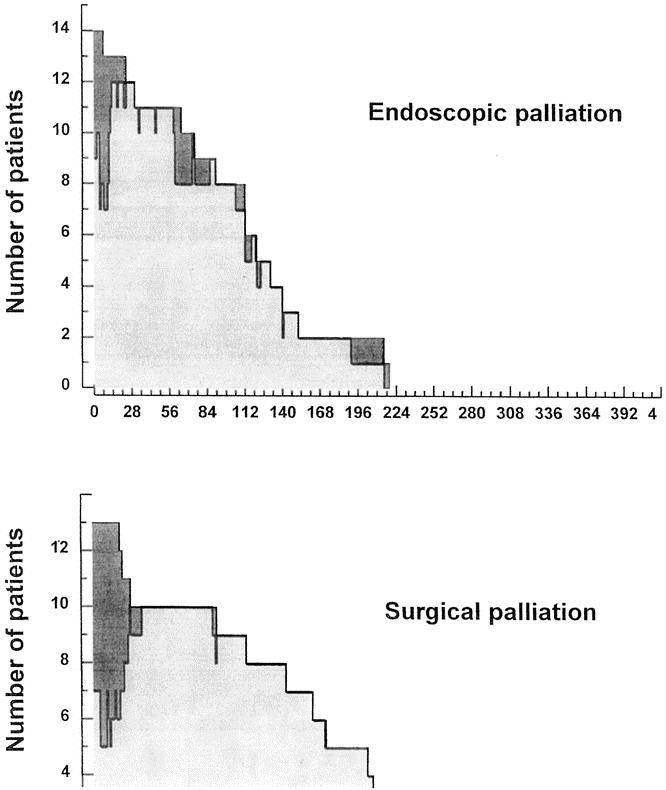
Figure 2. Survival in patients with unresectable tumors allocated to endoscopic palliation (top) and surgical palliation (bottom).
DISCUSSION
In this prospective study, the benefits of laparoscopy and laparoscopic ultrasonography for staging peripancreatic malignancies were found to be lower than expected. 11 Laparoscopic staging identified biopsy-proven unresectable disease in 13% of the 297 patients, with a detection rate of 35%. Only 74% of the patients classified as having “possibly respectable” disease after laparoscopic staging underwent resection. In the randomized comparison of endoscopic versus surgical palliation in patients with biopsy-proven unresectable disease, no benefits could be observed from switching to stenting, but the number of randomized patients was low. As a consequence, an overall gain in hospital-free survival is highly unlikely from the routine introduction of laparoscopic staging and subsequent stenting in patients with biopsy-proven unresectable disease.
Our study design was based on a standard diagnostic strategy in consecutive patients. This was supplemented by a randomized comparison of two forms of palliation in the subgroup of patients with unresectable disease identified by laparoscopic staging. The number of randomized patients was very small, but the low irresectability rate of laparoscopic staging by itself limits its potential overall beneficial effect. An alternative design would have been a randomized comparison of laparoscopic staging versus radiologic staging only. Although this is preferred in principle, such a trial would have required a huge sample size to obtain comparable estimates of overall effectiveness. 40
Our findings contrast with those from earlier studies, as reported by Warshaw et al. 1,2 It is unlikely that the design of the study can be held responsible for the low biopsy-proven irresectability rate identified by diagnostic laparoscopy. In both academic centers, experienced clinicians performed the procedure. A more plausible explanation is the quality of preceding radiologic staging, especially the introduction of spiral or helical CT scanning, which has affected the selection of patients eligible for laparoscopic staging. 24 Vascular tumor ingrowth, for example, can be visualized with preoperative helical CT scanning. If such ingrowth is suspected during laparoscopic ultrasonography, histopathologic confirmation is difficult. All 39 patients in whom this was the case underwent surgical exploration; of these, 11 underwent resection.
The overall resection rate after laparoscopy was relatively low (74%). An explanation could be that histology findings were used as the gold standard in this study. Therefore, all patients with suspected metastases or local ingrowth without pathologic proof underwent exploration. Most patients had local tumor ingrowth that could not be proven by biopsy during laparoscopy. In other series, these findings could have been interpreted as unresectable disease, or patients are even not referred for surgical treatment.
Medical tests cannot escape the growing demand for proof of effectiveness before being introduced into clinical practice. In these evaluations, health outcomes are quintessential. As diagnostic laparoscopy opens up the possibility of preventing unnecessary laparotomies and endoscopic palliation as an alternative to surgical palliation, an evaluation of this diagnostic modality should also include the results of subsequent nonsurgical palliation. Because of the disappointing detection rate of diagnostic laparoscopy, the limited number of randomized patients in the present study does not allow us to make firm recommendations about the preferred form of palliative treatment. Yet our results are not compatible with a substantial gain in hospital-free survival from endoscopic palliation. A possible explanation is the celiac plexus block, which all surgically palliated patients received during operation. Lillemoe et al have described a pain benefit for patients who underwent a celiac or splanchnic plexus block and a survival benefit for a subgroup of patients. 41 Based on a meta-analysis, Eisenberg et al described pain relief until death in 70% to 90% of patients, with pancreatic cancer patients having the same results as patients with other intra-abdominal malignancies. 42
The less favorable outcome in previous trials in patients receiving palliation for peripancreatic tumors by a bypass procedure has led others to recommend endoscopic palliation. 35–37,43 In contrast, the outcome in patients who underwent a bypass procedure in this trial was relatively good. This difference is probably due to patient selection. In our study, all patients were thought to have resectable tumors after conventional radiologic staging and were considered fit for a major surgical procedure, whereas most previous studies have included patients with more extensive disease as well as those with a poor general condition. 35–37,43 However, one should also realize that all patients included for randomization had proven metastatic disease.
Future developments may influence the implications of our findings. The limited detection rate of unresectable local disease offers room for improvement using new developments in imaging. If these occur, adequate nonsurgical management strategies can make the timely detection of patients with unresectable disease a more attractive option. In this study, diagnostic laparoscopy was performed as a separate procedure for logistical reasons. No attempt was made at laparoscopic palliation by a laparoscopic gastrojejunostomy or cholecystoenterostomy. Preliminary results reported with these new procedures are encouraging but better-designed studies are warranted. 44
Diagnostic laparoscopy as performed in this study is associated with an additional hospital stay, with inevitable discomfort for the patient and an increase in healthcare costs. These can be justified only by good chances of an improvement in health outcome, due to a likely reduction in unnecessary laparotomies. Based on the low detection rate of unresectable disease, it is unlikely that such a major health gain can be expected from routine diagnostic laparoscopy in patients with peripancreatic malignancies. At present, the additional value of laparoscopic staging in terms of patient benefit does not seem to warrant a rapid diffusion in this well-selected group of patients.
Footnotes
Supported by a grant from the Dutch National Health Service.
Correspondence: Dirk J. Gouma, Academic Medical Center, University of Amsterdam, Department of Surgery, G.4-116, P.O. Box 22700, 1100 DE Amsterdam, The Netherlands.
E-mail: d.j.gouma@amc.uva.nl
Accepted for publication April 2, 2002.
References
- 1.Warshaw AL, Tepper JE, Shipley WU. Laparoscopy in the staging and planning of therapy for pancreatic cancer. Am J Surg 1986; 151: 76–80. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Further experience with laparoscopy and peritoneal cytology in the staging of pancreatic cancer. Br J Surg 1995; 82: 1127–1129. [DOI] [PubMed] [Google Scholar]
- 3.Cuschieri A, Hall AW, Clark J. Value of laparoscopy in the diagnosis and management of pancreatic carcinoma. Gut 1978; 19: 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babineau TJ, Lewis WD, Jenkins RL, et al. Role of Staging Laparoscopy in the Treatment of Hepatic Malignancy. Am J Surg 1994; 167: 151–155. [DOI] [PubMed] [Google Scholar]
- 5.Stein HJ, Kraemer SJM, Feussner H, et al. Clinical value of diagnostic laparoscopy with laparoscopic ultrasound in patients with cancer of the esophagus or cardia. J Gastrointestinal Surg 1997; 1: 167–173. [DOI] [PubMed] [Google Scholar]
- 6.Hunerbein M, Rau B, Schlag PM. Laparoscopy and laparoscopic ultrasound for staging of upper gastrointestinal tumours. Eur J Surg Oncol 1995; 21: 50–55. [DOI] [PubMed] [Google Scholar]
- 7.Vargas C, Jeffers LJ, Bernstein D, et al. Diagnostic laparoscopy: a 5-year experience in a hepatology training program. Am J Gastro 1995; 90: 1258–1262. [PubMed] [Google Scholar]
- 8.John TG, Greig JD, Carter DC, et al. Carcinoma of the pancreatic head and peripancreatic region. Tumor staging with laparoscopy and laparoscopic ultrasonography. Ann Surg 1995; 221: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John TG, Greig JD, Crosbie JL, et al. Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg 1994; 220: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romijn MG, van Overhagen H, Spillenaar Bilgen EJ, et al. Laparoscopic and laparoscopic ultrasonography in staging of oesophageal and cardial carcinoma. Br J Surg 1998; 85: 1010–1012. [DOI] [PubMed] [Google Scholar]
- 11.Bemelman WA, de Wit LT, van Delden OM, et al. Diagnostic laparoscopy combined with laparoscopic ultrasonography in staging of cancer of the pancreatic head region. Br J Surg 1995; 82: 820–824. [DOI] [PubMed] [Google Scholar]
- 12.Bemelman WA, van Delden OM, van Lanschot JJ, et al. Laparoscopy and laparoscopic ultrasonography in staging of carcinoma of the esophagus and gastric cardia. J Am Coll Surg 1995; 181: 421–425. [PubMed] [Google Scholar]
- 13.Van Delden OM, de Wit LT, Nieveen van Dijkum E, et al. Value of laparoscopic ultrasonography in staging of proximal bile duct tumors. J of Ultrasound in Med 1997; 16: 7–12. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen MB, Scheelhincke JD, Madsen MR, et al. Combined endoscopic ultrasonography and laparoscopic ultrasonography in the pretherapeutic assessment of resectability in patients with upper gastrointestinal malignancies. Scand J Gastroenterol 1996; 31: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 15.Goletti O, Buccianti P, Chiarugi M, et al. Laparoscopic sonography in screening metastases from gastrointestinal cancer: comparative accuracy with traditional procedures. Surg Lap Endosc 1995; 5: 176–182. [PubMed] [Google Scholar]
- 16.Hann LE, Conlon KC, Dougherty E, et al. Laparoscopic sonography of peripancreatic tumors: preliminary experience. AJR Am J Roentgenol 1997; 169: 1257–1362. [DOI] [PubMed] [Google Scholar]
- 17.Minnard EA, Conlon KC, Hoos A, et al. Laparoscopic ultrasound enhances standard laparoscopy in the staging of pancreatic cancer. Ann Surg 1998; 228: 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conlon KC, Dougherty E, Klimstra DS, et al. The value of minimal access surgery in the staging of patients with potentially resectable peripancreatic malignancy. Ann Surg 1996; 223: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sand J, Marnela K, Airo I, et al. Staging of abdominal cancer by local anesthesia outpatient laparoscopy. Hepato-Gastroenterology 1996; 43: 1685–1688. [PubMed] [Google Scholar]
- 20.Pietrabissa A, Di Candio G, Giulianotti PC, et al. Operative technique for the laparoscopic staging of pancreatic malignancy. Minimally Invasive Ther 1996; 5: 274–280. [Google Scholar]
- 21.Cuschieri A. Laparoscopy for pancreatic cancer: does it benefit the patient? Eur J Surg Oncol 1988; 14: 41–44. [PubMed] [Google Scholar]
- 22.Murugiah M, Paterson-Brown S, Windsor JA, et al. Early experience of laparoscopic ultrasonography in the management of pancreatic carcinoma. Surg Endosc 1993; 7: 177–181. [DOI] [PubMed] [Google Scholar]
- 23.Meduri F, Diana F, Merenda R, et al. Implication of laparoscopy and peritoneal cytology in the staging of early pancreatic cancer. Zentr Path 1994; 140: 243–246. [PubMed] [Google Scholar]
- 24.Freeny PC, Traverso LW, Ryan JA. Diagnosis and staging of pancreatic adeno-carcinoma with dynamic computed tomography. Am J Surg 1993; 165: 600–606. [DOI] [PubMed] [Google Scholar]
- 25.Rosch T, Braig C, Gain T, et al. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology 1992; 102: 188–199. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko T, Nakao A, Inoue S, et al. Intraportal endovascular ultrasonography in the diagnosis of portal vein invasion by pancreatobiliary carcinoma. Ann Surg 1995; 222: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieveen van Dijkum E, de Wit LT, van Delden OM, et al. Staging laparoscopy and laparoscopic ultrasonography in over 400 patients with upper gastrointestinal carcinoma. J Am Coll Surg 1999; 189:5: 459–465. [DOI] [PubMed] [Google Scholar]
- 28.Nieveen van Dijkum EJM, de Wit LT, van Delden OM, et al. The efficacy of laparoscopic staging in patients with upper gastrointestinal tumors. Cancer 1997; 79: 1315–1319. [PubMed] [Google Scholar]
- 29.Lillemoe KD, Pitt HA. Palliation; surgical, otherwise. Cancer 1996; 78: 605–614. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt AW, Vida F, Kozarek RA, et al. Expandable metallic prostheses for malignant obstructions of gastric outlet and proximal small bowel. Gastrointest Endosc 1998; 47: 271–276. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda Y, Shimakura K, Akamatsu T. Factors affecting the patency of stents in malignant biliary obstructive disease: univariate and multivariate analysis. Am J Gastroenterol 1991; 86: 843–849. [PubMed] [Google Scholar]
- 32.Davids PHP, Groen AK, Rauws EAJ, et al. Randomized trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet 1992; 340: 1488–1492. [DOI] [PubMed] [Google Scholar]
- 33.Van Wagensveld BA, Coene PPLO, Gulik TM, et al. Biliary and gastric bypass surgery in the palliation of pancreatic head carcinoma; Outcome of 126 patients. Br J Surg 1997; 84: 1402–1406. [PubMed] [Google Scholar]
- 34.Lillemoe KD, Sauter PK, Pitt HA, et al. Current status of surgical palliation of peripancreatic carcinoma. Surg Gynecol Obstet 1993; 176: 1–10. [PubMed] [Google Scholar]
- 35.Smith AC, Dowsett JF, Russell RC, et al. Randomized trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet 1994; 344: 1655–1660. [DOI] [PubMed] [Google Scholar]
- 36.Andersen JR, Sorensen SM, Kruse A, et al. Randomized trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut 1989; 30: 1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd HA, Royle G, Ross AP, et al. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. Br J Surg 1988; 75: 1166–1168. [DOI] [PubMed] [Google Scholar]
- 38.Karnofsky DA, Abelmann WH, Craver LF, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948; 1: 634–656. [Google Scholar]
- 39.Greenfield S, Apolone G, McNeil BJ, et al. The importance of co-existent disease in the occurrence of postoperative complications and one-year recovery in patients undergoing total hip replacement. Comorbidity and outcomes after hip replacement. Med Care 1993; 31: 141–154. [DOI] [PubMed] [Google Scholar]
- 40.Bossuyt PMM, Lijmer JG, Mol BWJ. Randomised comparisons of medical tests: Sometimes invalid, not always efficient. Lancet 2000; 356: 1844–1847. [DOI] [PubMed] [Google Scholar]
- 41.Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg 1993; 217: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995; 80: 290–295. [DOI] [PubMed] [Google Scholar]
- 43.Bornman PC, Harries-Jones EP, Tobias R, et al. Prospective controlled trial of transhepatic biliary endoprosthesis versus bypass surgery for incurable carcinoma of head of pancreas. Lancet 1986; 1: 69–71. [DOI] [PubMed] [Google Scholar]
- 44.Giraudo G, Kazemier G, van Eijck CH, et al. Endoscopic palliative treatment of advanced pancreatic cancer: thoracoscopic splanchnicectomy and laparoscopic gastrojejunostomy. Ann Oncol 1999; 10: 278–280. [PubMed] [Google Scholar]