Abstract
Steady-state numbers of peripheral lymphocyte are tightly controlled. For conventional T cells, signals delivered through the interaction of the T cell receptor (TCR) with antigen-loaded MHC molecules are required for the peripheral survival of naive T cells and for their homeostatic expansion in lymphopenic hosts. Cytokines, including IL-7, are also essential for survival of peripheral naive T cells. CD1d-restricted, Vα14+ natural killer (NK)-T cells are a specialized autoreactive T subset with immunoregulatory activity. The relative roles of TCR engagement and cytokine signaling in the peripheral homeostasis of Vα14+ NK-T cells were investigated. After adoptive transfer, the survival and expansion of peripheral Vα14+ NK-T cells was independent of CD1d expression in the host. In contrast, IL-15 (but not IL-7) was required for maintenance of peripheral CD1d-reactive Vα14+ T cells. Comparison of Vα14+ T cell transfers into NK-proficient vs. deficient hosts suggests that NK-T cells and NK cells compete for peripheral resources. Our results indicate that IL-15 maintains the homeostasis of peripheral Vα14+ NK-T cells. In contrast, TCR “tickling” of NK-T cells, if it occurs under steady-state conditions, does not by itself provide a sufficient signal for their peripheral survival.
Homeostasis is a self-regulating process that maintains the peripheral T cell pool at relatively constant levels throughout adulthood. Regulation of T cell numbers is achieved through different mechanisms (survival, proliferation, and apoptosis; reviewed in refs. 1 and 2) and may be important in restoring the T cell pool after insults that cause lymphopenia or in promoting or down-regulating a particular cell subset during an immune response. The specific signals that regulate T cell homeostasis differ depending on the state of cellular differentiation and include (i) cell-to-cell contact, involving interactions between the T cell receptor (TCR) and peptide/MHC complexes (TCR “tickling”), and (ii) soluble factors, including cytokines (reviewed in ref. 3). Naive αβ T cells require both TCR and IL-7 stimulation for survival: in the absence of either signal, the lifespan of naive αβ T cells appears strongly reduced (4–9). In contrast, the survival of memory αβ T cells is MHC independent (10–12), whereas memory CD8+ αβ T cells have a more stringent survival requirement for IL-7 than memory CD4+ αβ T cells (7, 8). IL-15 also acts as a proliferative factor for memory CD8+ T cells, promoting their slow turnover in normal hosts (13–15). The signals required for homeostasis of other T cell subsets (such as γδ T cells, natural killer (NK)-T cells, and CD25+-regulatory T cells) are less well defined.
NK-T cells are αβ T lymphocytes that coexpress NK cell markers, including NK1.1 and inhibitory MHC-specific receptors. NK-T cells are heterogeneous and can be categorized on the basis of their phenotype, developmental origin, selection by MHC, and distribution in peripheral organs (reviewed in refs. 16 and 17). The type I NK-T cell subset is positively selected in the thymus by CD1d/β2-microglobulin complexes expressed by CD4+8+ (double-positive) cortical thymocytes and displays a highly skewed TCR repertoire, composed of an invariant Vα14-Jα18 chain, paired with a restricted set of TCRβ chains (reviewed in ref. 18). Vα14+ NK-T cells are CD4+ or CD4−CD8− (double-negative) and, upon TCR engagement or cytokine stimulation, release large amounts of IL-4, IL-10, transforming growth factor β, and IFN-γ and transactivate conventional T, B, and NK cells (19). Although the essential physiological roles for Vα14+ NK-T cells remain poorly defined, these cells may play a role in graft-vs.-host disease, antitumor responses, and certain autoimmune conditions, including type I diabetes (reviewed in refs. 20 and 21). The ability of NK-T cells to rapidly respond under a variety of conditions suggests that they act as defense sentinels.
An instructive model of intrathymic NK-T cell development involves random rearrangement of TCRα and TCRβ chains, resulting in the formation of Vα14-Jα18/Vβ2, Vβ7, and Vβ8 TCRs; engagement of these semiinvariant TCRs by CD1d molecules then commits these double-positive thymocytes to the NK-T lineage (reviewed in refs. 16–18). By using CD1d tetramers loaded with the synthetic lipid α-galactosylceramide (CD1d/α-galactosylceramide), type I NK-T cells bearing Vα14-Jα18 paired with Vβ2, Vβ7, or Vβ8 can be identified (22). Once selected by CD1d molecules, Vα14-Jα18/Vβ2, Vβ7, or Vβ8 thymocytes are CD4+/− and NK1.1−; these Vα14+ NK-T precursors have the capacity to produce IL-4 but require additional, as-of-yet-unidentified signals, which can drive the acquisition of NK lineage markers. This NK-T cell maturation may occur intrathymically or in the periphery (23, 24). Vα14+ NK-T cells accumulate in the liver, spleen, and bone marrow and reach steady-state levels by ≈3 weeks of age. This general model of NK-T cell differentiation is consistent with previous studies of NK-T cells in common cytokine receptor γ chain (γc)-deficient mice, in which thymocytes expressing Vα14-Jα18 transcripts and having IL-4-producing capacity were identified (25). Still, these cells lacked expression of NK1.1 and inhibitory Ly49 receptors, suggesting that γc signals were required for NK-T cell maturation.
BrdUrd incorporation studies have shown that peripheral Vα14+ NK-T cells are a slowly dividing population (23, 26). However, the NK-T cell pool is labile: IL-12 or anti-CD3 antibodies can rapidly deplete hepatic and splenic NK-T cells, which replenish in a thymus-independent fashion (26). Still, the relative contribution of (i) NK-T cell redistribution (from the bone marrow, spleen, and liver), (ii) NK-T cell differentiation (from NK1.1− to NK1.1+), and (iii) homeostatic NK-T cell expansion to the maintenance of peripheral NK-T cell homeostasis is less clear. In this study, we use an adoptive transfer system to assess the roles of MHC vs. cytokine signaling in the homeostatic regulation of peripheral Vα14+ NK-T cells. A similar approach was recently used to examine the requirements for homeostasis of thymic Vα14+ NK-T cells (27).
Materials and Methods
Mouse Strains.
Alymphoid mice (B−, NK−, T−) deficient in the recombinase-activating gene (RAG)-2 and the γc (Rag°γc°, 11th backcross to C57BL/6) have been described (28). Mice deficient in MHC class I molecules associated with β2-microglobulin (β2m−/−; ref. 29) or in MHC class II molecules (I-A−/−; ref. 30) were intercrossed with Rag°γc° to generate alymphoid mice lacking MHC molecules (Rag°γc°I°II°). Mice deficient in IL-7 (31) or in IL-15 (32) were intercrossed with Rag°γc° mice to generate alymphoid hosts deficient in these cytokines. Mice transgenic for a functionally rearranged Vα14-Jα18 TCRα chain on the TCRα-deficient C57BL/6 background (Vα14Tg) have been described (33). All mice were maintained in specific pathogen-free conditions at The Pasteur Institute and were used at 4–10 weeks of age.
Isolation of Lymphoid Cells and Flow Cytometric Analysis.
Single-cell suspensions from peripheral lymph nodes, spleen, and liver were prepared and cell surface immunofluorescence was performed as described (34). Samples were analyzed by using a FACSCalibur flow cytometer with cellquest 3.3 software (Becton Dickinson). The following mAbs directly conjugated to FITC, phycoerythrin, biotin, or allophycocyanin were used: Vβ8.1/8.2 (MR5), TCRβ (H57–597), CD1d (1B1), CD5 (53-7.3), CD11c (HL3), CD19 (6D5), CD25 (PC61), CD44 (IM7.8.1), CD69 (H1.2F3), CD122 (TM-β1), CD127 (A7-R34), CD132 (TUGm2), H2Db (CTDb), I-A/I-E (114.15.2), and NK1.1 (PK136). Biotinylated antibodies were revealed with peridinin chlorophyll protein- or allophycocyanin-conjugated streptavidin (PharMingen). 5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes. RPMI medium 1640 with Glutamax, FCS, and PBS were purchased from GIBCO. CD19 microbeads and magnetic separation columns were from Miltenyi Biotec (Bergish Gladbach, Germany).
Adoptive Transfer of CFSE-Labeled Vα14+ NK-T Cells.
Lymph node suspensions from Vα14Tg mice were depleted of CD19 cells by using microbeads and then labeled with 5 μM CFSE. Cells were washed with ice-cold PBS, and 5 × 106 T cells were injected i.v. into nonirradiated Rag°γc° recipient mice or into 0.75-Gy-irradiated C57BL/6 mice. At given times after transfer, splenic and hepatic lymphocytes were prepared, and CFSE+ cell frequency and phenotype were determined. Two CD1d-restricted T cell subsets were analyzed: NK1.1+ Vα14+ T cells and Vβ8.1/8.2+ Vα14+ T cells (22). In some experiments, CFSE+ CD19− lymph node cells from Vα14Tg mice were resuspended in 200 μl of PBS containing 300 μg of CD1d mAb before i.v. injection. Recipient mice were then treated with the same amount of CD1d mAb each day until analysis. For depletion of endogenous NK cells, Rag° mice were injected i.p. with 50 μg of PK136 mAb 5 days before transfer of CFSE+ Vα14+ T cells.
Assays of NK-T Cell Effector Functions.
Adoptive transfer of total Vα14Tg lymph node cells were made into Rag°γc° mice. After 3 days, 2 μg of purified anti-CD3ɛ antibody was injected i.v. into recipient mice. Ninety minutes later, NK-T cell effector functions were assessed by (i) intracellular T cell IFN-γ staining and (ii) B cell CD69 up-regulation. For intracellular cytokine detection, total splenocyte cell suspensions were incubated for 1 h in RPMI medium 1640/5% FCS containing brefeldin A (10 μg/ml) to block cytokine secretion. Surface-stained cells (TCRβ, CD19) were fixed for 1 h in PBS containing 2% paraformaldehyde, and intracellular cytokines were detected by using a phycoerythrin-conjugated IFN-γ (XMG1.2) or control rat IgG1 in PBS containing 0.5% saponin. For cell transactivation, CD19+ cells from control or anti-CD3 mAb-injected mice were analyzed for CD69 expression.
Statistics.
P < 0.05, obtained by using the Mann–Whitney t test, was considered significant.
Results and Discussion
Analyzing Signals for Peripheral Homeostasis of CD1d-Restricted Vα14+ NK-T Cells.
Mice transgenic for a functionally rearranged Vα14-Jα18 invariant TCRα chain on a TCR Cα−/− background (Vα14Tg) have been extensively characterized (22, 33, 35, 36). These mice show an increase in the frequency of NK1.1+ T cells in the spleen, lymph nodes, and liver (refs. 33, 35, and 36 and data not shown). Previous studies using CD1d/α-galactosylceramide tetramers demonstrated bona fide generation of type I NK-T cells in Vα14Tg mice (22). CD1d-reactive T cells from Vα14Tg mice were found to have the following characteristics: (i) they were enriched in CD4−CD8− or CD4+ cells; (ii) they showed an increased frequency of Vβ2, Vβ7, and Vβ8.1/8.2 usage; and (iii) they contained both NK1.1+ and NK1.1− subsets. In contrast, the CD1d/α-galactosylceramide tetramer nonreactive T cell subset from Vα14Tg mice was NK1.1− and severely depleted in Vβ2, Vβ7, and Vβ8.1/8.2 chains. We therefore followed the fate of two partially overlapping CD1d-reactive Vα14+ T cell subsets (NK1.1+ Vα14+ T cells and Vβ8.1/8.2+ Vα14+ T cells) after adoptive transfer into various hosts. These Vα14+ T cell subsets account for the majority of the CD1d-reactive T cells present in Vα14Tg mice (22).
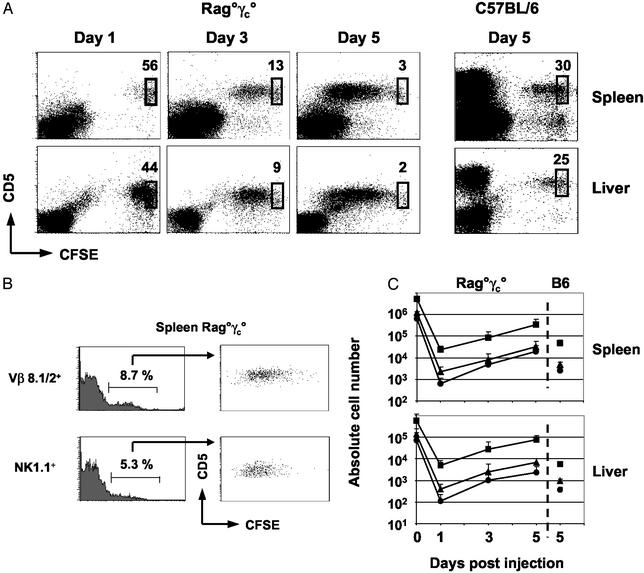
Nonirradiated alymphoid (B−, T−, NK−) Rag°γc° mice (28) were injected with CFSE+, CD19− lymph node cells from Vα14Tg mice. Total numbers of CFSE+ CD5+ T cells, Vβ8.1/8.2+ T cells, and NK1.1+ T cells were determined, and the degree of CFSE dilution in each subset was analyzed in the spleen and in the liver of the recipient mice 1, 3, and 5 days after transfer (Fig. 1). In alymphoid hosts, the following observations were made: (i) ≈0.5% of injected CFSE+ T cells from Vα14Tg mice were found in the spleens of recipient mice at day 1 after transfer, and 5-fold less were found in the liver; this recovery was similar to that observed by using transfer of monoclonal transgenic T cells or polyclonal lymph node conventional T cells (refs. 4, 7, and 9 and data not shown); (ii) Vα14+ T cells divided extensively in Rag°γc° mice; by 3 days after transfer, ≈90% of cells had divided, and, by day 5, essentially all cells had entered the cell cycle (Fig. 1A); (iii) the CD1d-reactive Vβ8.1/8.2+ and NK1.1+ Vα14+ T cell subsets divided in a similar extensive fashion (Fig. 1B); (iv) the absolute numbers of Vβ8.1/8.2+ T cells and NK1.1+ T cells increased in Rag°γc° hosts from day 1 to day 5 after transfer, resulting in a 15-fold (spleen) and 17-fold (liver) increase for Vβ8.1/8.2+ T cells and a 31-fold (spleen) and 20-fold (liver) increase for NK1.1+ Vα14+ T cells (Fig. 1C). Collectively, this short-term adoptive transfer assay demonstrates that CD1d-reactive Vα14+ T cells have the potential for extensive homeostatic proliferation.
Figure 1.
Behavior of CD1d-restricted Vα14+ T cells after adoptive transfer. (A) Kinetics of CFSE dilution in splenic and hepatic CD5+ Vα14+ T cells after transfer in nonirradiated Rag°γc° mice or 0.75-Gy-irradiated C57BL/6 mice. Percentages of nondivided cells are indicated. (B) CFSE dilution profiles of CD1d-reactive T cell subsets (NK1.1+ and Vβ8.1/8.2+) 5 days after transfer into nonirradiated Rag°γc° mice. (C) Absolute numbers of total CD5+ cells (■) or Vβ8.1/8.2+ (▴) and NK1.1+ (●) CD5+ subsets recovered from injected mice at the indicated times after transfer.
In contrast, in recent studies, Matsuda et al. (27) found that thymus-derived Vα14+ NK-T cells had a limited capacity for homeostatic expansion after transfer to irradiated wild-type C57BL/6 mice compared with conventional αβ T cells, which could divide extensively. To determine whether thymic vs. peripheral NK-T cells had different proliferative capacities, we performed adoptive transfers to irradiated C57BL/6 mice (Fig. 1 A and C). Peripheral Vα14+ T cells divided poorly in these hosts (similar to the division observed with thymic NK-T cells; ref. 27), and, by day 5 after transfer, we recovered 7- to 14-fold less Vα14+ cells compared with the nonirradiated alymphoid recipients. Because irradiation effectively reduced the endogenous T cell pool (by >95%; data not shown), our results suggest that other host factors can modify the behavior of adoptively transferred Vα14+ NK-T cells (see below).
MHC Molecules Are Not Required for Peripheral Vα14+ NKT Cell Homeostasis.
CD1d recognition is essential for the intrathymic selection of Vα14+ NK-T cells, although its role in the homeostasis of peripheral NK-T cells is unknown. MHC-deficient alymphoid mice (Rag°γc°I°II°) were generated and, as expected, had markedly reduced expression of MHC class I and background expression of class II and CD1d (data not shown). Transfers of Vα14+ T cells into Rag°γc° and Rag°γc°I°II° mice were analyzed in parallel. Surprisingly, the homeostatic expansion of Vα14+ T cells was not modified by the absence of MHC molecules (Fig. 2A). Vβ8.1/8.2+ and NK1.1+ T cells survived and divided as efficiently in the MHC-deficient Rag°γc° mice as in their MHC-competent counterparts. Absolute numbers of Vβ8.1/8.2+ and NK1.1+ T cells increased between days 3 and 5 after transfer (Fig. 2B), and the dilution profiles of CFSE in these CD1d-reactive T cell subsets were remarkably similar in both Rag°γc° and Rag°γc°I°II° recipients. The possibility that residual expression of CD1d by the injected cell cohort (by the Vα14+ T cells themselves; ref. 37) would be sufficient to compensate for the lack of host CD1d was examined. CFSE+, CD19− lymph node cells from Vα14Tg mice were transferred into Rag°γc°I°II° mice, which were then treated with anti-CD1d antibodies. Absolute numbers of CFSE+ NK1.1+ Vα14+ T cells recovered in the spleen and liver of anti-CD1d-treated mice (spleen: 600 ± 500 cells; liver: 130 ± 90 cells) and the PBS-injected controls (spleen, 1,500 ± 1,000 cells; liver, 220 ± 200 cells) were not significantly different (spleen, P = 0.3; liver, P = 0.6).
Figure 2.
Survival and proliferation of CD1d-restricted Vα14+ T cells is independent of CD1d. (A) CFSE dilution in splenic and hepatic CD5+ Vα14+ T cells 3 days after transfer in nonirradiated Rag°γc° and Rag°γc°I°II° mice. Percentages of nondivided cells are indicated. (B) Recovery of CFSE+ Vα14+ T cell subsets after injection. Results indicate the absolute numbers of CFSE+ T+ cells found in the indicated mice as a percentage of that found in control Rag°γc° mice (░⃞, day 3; □, day 5). (C) Homeostatic proliferation of CD1d-reactive Vα14+ T cells does not result in activation of effector functions. After adoptive transfer, T cells are negative for IFN-γ (Lower, solid line), and B cells have basal CD69 expression (Upper, solid line); however, CD3 stimulation results in T cell activation and B cell transactivation. Shaded histograms show staining with isotype-matched controls.
Does the transfer of Vα14+ T cells to Rag°γc° mice result in cellular activation or elicit effector functions? NK-T cell activation (by α-galactosylceramide or anti-CD3 mAb) results in cytokine release (IFN-γ, IL-4) and cross-stimulation of B, NK, and other T cells (16–21). If TCR tickling of Vα14+ T cells was occurring during their expansion in Rag°γc° mice, it might result in T cell activation and concomitant up-regulation of CD69 expression on a coinjected B cell cohort. We transferred total lymph node cells from Vα14Tg mice (or from TCRα−/− mice to control for cell-autonomous changes in B cell phenotype) into Rag°γc° recipients. No evidence of spontaneous T cell IFN-γ production or B cell transactivation was observed after adoptive transfer (Fig. 2C); in contrast, the transferred Vα14+ T cells could be stimulated in vivo by anti-CD3, resulting in IFN-γ synthesis and up-regulation of B cell CD69 expression (Fig. 2C).
These results indicate that short-term survival and homeostatic expansion of Vα14+ NK-T cells transferred into alymphoid mice does not require continuous interaction with CD1d molecules. In this respect, CD1d-reactive Vα14+ T cells are more similar to memory αβ T cells than naive αβ T cells in their requirements for MHC interactions in the periphery. Finally, it would be logical that peripheral maintenance of NK-T cell should be driven by mechanisms independent of CD1d engagement: activation of these cells should be tightly controlled considering the potent effector functions elicited by triggering their TCR.
Role of γc-Dependent Cytokines in Peripheral Vα14+ NK-T Cell Homeostasis.
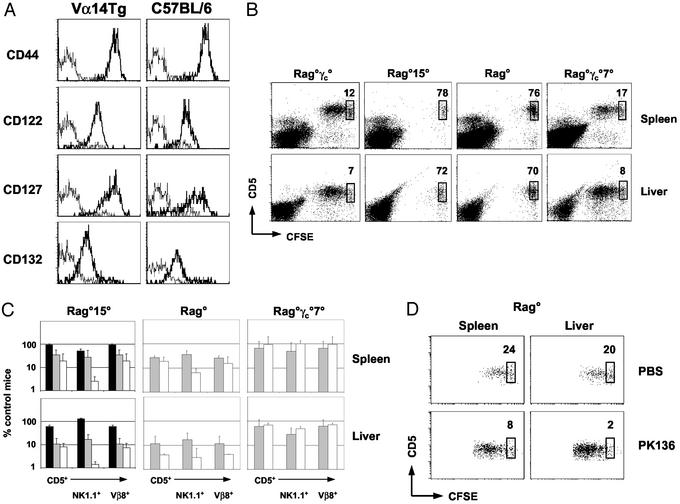
We next investigated a role for γc-dependent cytokines in Vα14+ T cell homeostasis. Peripheral NK1.1+ T cells from C57BL/6 mice and from Va14Tg mice expressed similar profiles of γc-dependent cytokine receptors: NK-T cells were positive for CD122 (IL-2Rβ), CD127 (IL-7Rα), and CD132 (γc) but were negative for CD25 (IL-2Rα; Fig. 3A and data not shown). These results indicate that peripheral Vα14+ T cells may respond to IL-7 and/or IL-15, consistent with the previously suggested roles of these cytokines in NK-T cell differentiation (27, 31, 32).
Figure 3.
Role of IL-7, IL-15, and endogenous NK cells in the behavior of adoptively transferred CD1d-restricted Vα14+ T cells. (A) Cell surface profile of splenic NK1.1+ T cells from C57BL/6 and Vα14Tg mice. Expression of indicated marker (thick line) and isotype control (dotted line) are shown. (B and C) Vα14+ T cell transfers to nonirradiated Rag°γc°, Rag°15°, Rag°, and Rag°γc°7° mice. CFSE dilution in splenic and hepatic CD5+ T cells and percentages of nondivided cells are shown 3 days after transfer. Recovery of CFSE+ Vα14+ T cell subsets are indicated (■, day 1; ░⃞, day 3; □, day 5). (D) Effect of NK depletion on Vα14+ T cell adoptive transfers. CFSE dilution profile of Vα14+ T cells in control (Upper) or PK136-treated (Lower) Rag° mice 5 days after transfer.
To assess a role for IL-15 in homeostasis of peripheral NK-T cells, CFSE+ Vα14+ T cells were transferred into Rag°15° and Rag°γc° mice, and Vβ8.1/8.2+ and NK1.1+ T cells were analyzed (Fig. 3B). Absolute numbers of Vα14+ T cells recovered 1 day after transfer to Rag°15°, and Rag°γc° mice were similar (Fig. 3C), indicating equivalent homing of the injected cells. However, by day 3 after injection, absolute numbers of splenic and hepatic Vβ8.1/8.2+ and NK1.1+ T cells were clearly reduced in the absence of IL-15, representing about 35% and 12%, respectively, of control Rag°γc° mice. The selective loss of NK1.1+ T cells in Rag°15° mice was even more striking by day 5 after transfer (NK1.1+ Rag°γc° spleen, 20,200 ± 17,000; liver, 2,300 ± 2,000; Rag°15° spleen, 600 ± 500; liver, 18 ± 11; P = 0.04 for spleen and liver). Examination of the CFSE dilution profiles in the few remaining Vα14+ T cells in Rag°15° mice demonstrated that IL-15 also appeared necessary for their proliferation (Fig. 3B). Collectively, these results identify a critical role for IL-15 in the survival and expansion of peripheral CD1d-reactive Vα14+ T cells.
IL-15 is required for NK cell development (32). Because NK cells and NK-T cells share a number of phenotypic and functional characteristics, we hypothesized that these two cell types might compete for peripheral resources, including IL-15. IL-15 availability could offer an explanation for the observations that Vα14+ T cells divide poorly in irradiated C57BL/6 mice (Fig. 1A and ref. 27): although endogenous T cells were strongly reduced in these hosts, splenic NK cells were still present in half their normal numbers (1.3 ± 0.3 × 106 cells in control C57BL/6 mice vs. 0.5 ± 0.09 × 106 cells in irradiated C57BL/6 mice). We compared the behavior of CFSE+ Vα14+ T cells after transfer into NK cell-replete Rag° mice and NK cell-deficient Rag°γc° mice (Fig. 3 B and C). We found that presence of endogenous NK cells reduced the absolute numbers of splenic and hepatic Vβ8.1/8.2+ and NK1.1+ T cells recovered after adoptive transfer. Moreover, partial depletion of NK1.1+ cells in Rag° recipients allowed for more extensive proliferation of transferred Vα14+ T cells (Fig. 3D). Similar results have been reported with thymic Vα14+ T cells (27). These results demonstrate that endogenous NK cells can affect the behavior of adoptively transferred Vα14+ T cells, which is likely mediated through competition for peripheral resources including IL-15.
To assess a role for IL-7 in the homeostasis of peripheral CD1d-reactive Vα14+ T cells, we generated NK cell-deficient mice lacking IL-7 (Rag°γc°7° mice) for use as recipients to eliminate any possible competition with endogenous NK cells. After transfer, Vβ8.1/8.2+ and NK1.1+ Vα14+ T cells survived and divided rather efficiently in IL-7-deficient Rag°γc° mice, similar to their IL-7-competent counterparts (Fig. 3 B and C). Matsuda et al. (27) reported that IL-7 played a minor role in the homeostasis of Vα14+ T cells, which could reflect the behavior of thymic vs. peripheral Vα14+ NK-T cells. Our results demonstrate that IL-7 does not play an essential role in the maintenance of peripheral CD1d-reactive Vα14+ T cells.
Concluding Remarks.
Homeostasis of the T cell pool is controlled at multiple levels and influenced by thymic production and by survival, proliferation, and death of peripheral cells. Previous studies have focused on the signals (TCR and cytokines) that influence peripheral T cell homeostasis (4–15). It has become clear that these signals are not the same for all T cell subsets. Although interactions between the TCR and peptide/MHC complexes appear necessary for naive T cell survival and proliferation, these signals are dispensable for homeostatic expansion of memory T cells and CD1d-reactive Vα14+ T cells. Cytokines are also essential for the homeostasis of conventional T cells and CD1d-reactive Vα14+ T cells, although the specific cytokine requirements differ. IL-7 promotes the survival of naive T cells and their proliferation in lymphopenic hosts (7–9). In contrast, CD1d-reactive Vα14+ T cells use a distinct cytokine, IL-15, for peripheral survival and proliferation. In this way, Vα14+ T cells more closely resemble memory CD8+ T cells and NK cells (38, 39) in their requirements for homeostasis. MHC engagement appears necessary for memory T cell effector functions (40); whether CD1d plays an analogous role for Vα14+ NK-T cells remains to be determined. How the diverse signals generated through TCR/cytokine stimulation are integrated and decoded into intracellular biochemical pathways for peripheral T cell maintenance is unclear. However, preliminary data suggest that overexpression of the antiapoptotic factor Bcl-2 alone is insufficient to replace the maintenance role played by IL-15 in Vα14+ T cells (data not shown).
Competition for peripheral resources clearly affects the homeostatic equilibrium of the T cell pool. For example, the presence of mature T cells in the host can limit the expansion capacity of adoptively transferred T cells, presumably through competition for IL-7 (reviewed in refs. 1–3). How T cells compete for environmental resources (peptide/MHC complexes, cytokines, adhesion molecules, etc.) remains unknown. Our observations highlight additional complexity by demonstrating that non-T cells (NK cells) can influence the peripheral homeostasis of certain T cell subsets. NK-T cell homeostasis may occur at the level of IL-15 availability in the host, a notion supported by our adoptive transfer experiments. Under situations in which IL-15 is limiting (wild-type or Rag° mice with a full NK cell compartment), expansion of Vα14+ NK-T cells is modest. In contrast, transfer to Rag°γc° mice (which may have higher steady-state IL-15 levels) allows for a greater degree of NK-T cell expansion. As such, changes in the absolute numbers of one of these subsets in vivo could have an effect on other IL-15-dependent lymphoid subsets. The biological consequences of lymphocyte competition for peripheral resources merits further investigation.
Acknowledgments
We thank J. Peschon (Immunex) and P. Vieira (DNAX) for IL-15- and IL-7-deficient mice and A. Freitas, D. Guy-Grand, and Francesco Colucci for stimulating discussions. This work was supported by grants from the Institut Pasteur, the Institut National de la Santé et de la Recherche Médicale, the Association pour la Recherche sur le Cancer, the Ligue National Contre le Cancer, and the Fondation de la Recherche Médicale.
Abbreviations
- TCR
T cell receptor
- NK
natural killer
- γc
common γ chain
- Rag
recombinase-activating gene
- CFSE
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Freitas A A, Rocha B. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Goldrath A W, Bevan M J. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 3.Jameson S C. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 4.Tanchot C, Lemonnier F A, Pèrarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2061. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 5.Takeda S, Rodewald H R, Arakawa H, Bluethmann H, Shimizu T. Immunity. 1996;5:217–225. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 6.Witherden D, van Oers N, Waltzinger C, Weiss A, Benoist C, Mathis D. J Exp Med. 2000;191:355–364. doi: 10.1084/jem.191.2.355. [DOI] [PubMed] [Google Scholar]
- 7.Lantz O, Grandjean I, Matzinger P, Di Santo J P. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 8.Schluns K S, Kieper W C, Jameson S C, Lefrancois L. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 9.Tan J T, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg K I, Surh C D. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murali-Krishna K, Lau L L, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1341. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 11.Garcia S, Di Santo J, Stockinger B. Immunity. 1999;11:163–168. doi: 10.1016/s1074-7613(00)80091-6. [DOI] [PubMed] [Google Scholar]
- 12.Swain S L, Hu H, Huston G. Science. 1999;286:1381–1385. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 13.Tan J T, Ernst B, Kieper W C, LeRoy E, Sprent J, Surh C D. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns K S, Williams K, Ma A, Zheng X X, Lefrancois L. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 15.Goldrath A W, Sivakumar P V, Glaccum M, Kennedy M K, Bevan M J, Benoist C, Mathis C, Butz E A. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacDonald H R. Curr Opin Immunol. 2002;14:250–254. doi: 10.1016/s0952-7915(02)00329-1. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg M, Gapin L. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A, Rivera M N, Park S H, Roark J H. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Carnaud C, Lee D, Donnars O, Park S H, Beavis A, Koezuka Y, Bendelac A. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 20.Park S H, Bendelac A. Nature. 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 21.Smyth M J, Crowe N Y, Hayakawa Y, Takeda K, Yagita H, Godfrey D I. Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 22.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 24.Pellicci D G, Hammond K J, Uldrich A P, Baxter A G, Smyth M J, Godfrey D I. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lantz O, Sharara L I, Tilloy F, Andersson A, Di Santo J P. J Exp Med. 1997;185:1395–1401. doi: 10.1084/jem.185.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberl G, MacDonald H R. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda J L, Gapin L, Sidobre S, Kieper W C, Tan J T, Ceridig R, Surh C D, Kronenberg M. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 28.Colucci F, Soudais C, Rosmaraki E, Vanes L, Tybulewicz V L J, Di Santo J P. J Immunol. 1999;162:2761–2765. [PubMed] [Google Scholar]
- 29.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 31.von Freeden-Jeffry U, Vieira P, Lucian A, McNeil T, Burdach S E, Murray R. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, et al. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehuen A, Lantz O, Beaudoin L, Laloux L, Carnaud C, Bendelac A, Bach J F, Monteiro R C. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bregenholt S, Berche P, Brombacher F, Di Santo J P. J Immunol. 2001;166:1871–1876. doi: 10.4049/jimmunol.166.3.1871. [DOI] [PubMed] [Google Scholar]
- 35.Bendelac A, Hunziker R D, Lantz O. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronet C, Mempel M, Thieblemont N, Lehuen A, Kourilsky P, Gachelin G. J Immunol. 2001;166:1755–1762. doi: 10.4049/jimmunol.166.3.1755. [DOI] [PubMed] [Google Scholar]
- 37.Hameg A, Apostolou I, Leite-De-Moraes M, Gombert J-M, Garcia C, Koezuka Y, Bach J F, Herbelin A. J Immunol. 2000;165:4917–4926. doi: 10.4049/jimmunol.165.9.4917. [DOI] [PubMed] [Google Scholar]
- 38.Cooper M A, Bush J E, Fehniger T A, VanDeusen J B, Waite R E, Liu Y, Aguila H L, Caligiuri M A. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 39. Ranson, T., Vosshenrich, C. A. J., Corcuff, E., Richard, O., Muller, W. & Di Santo, J. P. (2003) Blood, in press. [DOI] [PubMed]
- 40.Kassiotis G, Garcia S, Simpson E, Stockinger B. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]