Abstract
Objective
To investigate whether the administration of different glutamine-containing dipeptides, glycyl-l-glutamine (GLY-GLN) and l-alanyl-l-glutamine, has a differing impact on perioperative immunomodulation.
Summary Background Data
Surgery leads to transitory immunosuppression, which is associated with decreased plasma glutamine (GLN) levels and increased susceptibility to infection and sepsis. A useful tool to detect immunocompetence is the ex vivo lipopolysaccharide (LPS)-stimulated tumor necrosis factor alpha (TNF-α) secretion in whole blood.
Methods
Forty-five patients undergoing major abdominal surgery were randomized prospectively to receive 0.5 g/kg/24 h GLN dipeptides administered as GLY-GLN or as ALA-GLN or isonitrogenous Vamin (a GLN-free amino acid solution; control group) as a continuous infusion over 72 hours, starting 24 hours before surgery. Blood samples were collected before infusion, at the end of surgery, and 48 hours postoperatively to determine the TNF-α release into whole blood stimulated with LPS. Groups were compared by analysis of variance.
Results
The groups were comparable in age, gender distribution, and length of operative time. At the end of surgery a significant reduction in ex vivo LPS-stimulated TNF-α production was observed in all groups. In patients who received GLY-GLN, the induced TNF-α production was restored after 48 hours.
Conclusions
In this study perioperative infusion of GLY-GLN reduced immunosuppression. The effect of GLN-containing dipeptides seems to be different when administered in glycine or alanine form.
Impaired host defense systems following major surgery or trauma allow development of infection and sepsis. About 80% of invading microorganisms are gram-negative bacteria. Monocytes and macrophages play a key role in the first line of defense against pathogens invading the human body and are centrally involved in nonspecific and specific immune responses. Initial immunoreactions after endotoxin/lipopolysaccharide (LPS) challenge are reflected by an excessive production of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α. 1–3 Immunosuppression has been reported 4,5 following shock and trauma as well as during the course of sepsis, and the functioning of the immune system in these situations has been investigated extensively. 6–8 A diminished ex vivo LPS-stimulated TNF-α release in whole blood is considered to be a useful tool to evaluate immunocompetence. 9 TNF-α mediates essential immunologic reactions: it is chemotactic for neutrophils, stimulates phagocytic capacity and oxygen radical formation by monocytes, enhances proliferation of T cells in the absence of IL-2, and induces MHC class I and II expression. 10–12 HLA-DR, a gene product of the MHC class II complex, is highly expressed on monocytes/macrophages and plays a leading role in presentation of intracellularly processed antigen to CD4+ lymphocytes. 13 It has also been shown that monocyte HLA-DR expression is dramatically reduced during immunoparalysis. 4–6
Glutamine (GLN) is the most abundant amino acid in the blood and in the free amino acid pool of the body. GLN is synthesized in skeletal muscle, released into the bloodstream, and transported to a variety of tissues. GLN is used in the kidney, liver, gut, and cells of the immune system. 14–16 Although following trauma or surgery GLN synthesis and secretion from skeletal muscle are increased, intracellular concentrations of GLN are significantly decreased. 17 Earlier investigations have shown that the delivery of muscle GLN correlates with the severity of surgical procedures, the appearance of infections, and the mortality rate of septic patients. 18 Later we showed that the function of human monocytes is dependent on the presence of adequate amounts of GLN. 19 Lowering the GLN concentration in culture media reduced antigen-presenting capacity as well as phagocytic properties in a dose-dependent manner. In a subsequent clinical trial we demonstrated a diminished postoperative induced immunosuppression when patients received the dipeptide glycyl-glutamine (GLY-GLN) directly after surgery over a period of 48 hours. 20 In these patients a partial prevention of the decrease in HLA-DR expression on monocytes induced by surgery could be observed. This was the first study to suggest that the composition of the glutamine dipeptides (glycine or alanine) may have different physiologic effects. There are several publications showing that glycine has anti-inflammatory and anticarcinogenic properties and may also diminish the ischemia–reperfusion system. 21 On the other hand, alanine is the most important glycogenic amino acid in the human body. 22
Therefore, in the present prospective randomized trial in patients undergoing major abdominal surgery, we compared the effects of the glutamine-containing dipeptides ALA-GLN and GLY-GLN on clinically relevant immunologic parameters. We hypothesized that a continuous glutamine dipeptide infusion over a period of 72 hours starting 24 hours before surgery may compensate for the immunosuppression induced by surgery and that the effect may be different when infusing dipeptides with different amino acids.
METHODS
Patients
The study was approved by the ethics committee of the medical faculty of the University of Vienna. Informed consent was obtained from each patient. Patients were randomized into one of three groups of 15 patients each to receive glutamine-free amino acid solutions or amino acid solutions supplemented with either ALA-GLN or GLY-GLN. Patients received 1.7 g amino acids/kg/24 h (equivalent to 120 g amino acids/70 kg/24 h). Controls received the glutamine-free amino acid solution Vamin 14 EF; the experimental groups received 0.5 g/kg/24 h GLN as dipeptides administered either as GLY-GLN or ALA-GLN. Patients fasted the night before surgery. Postoperatively all patients were admitted to an intensive care unit. Amino acid solutions were infused continuously from 24 hours before surgery until 48 hours after surgery. The first postoperative day, patients received parenteral nutrition amounting to approximately 1,200 kcal/70 kg (0.5 g/kg Intralipid; Fresenius Kabi, Bad Homburg, Germany; 1.5 g/kg glucose), and the second postoperative day patients received approximately 1,700 kcal/70 kg (0.5 g/kg Intralipid; 3 g/kg glucose). First blood samples were drawn before the start of the infusion, immediately after surgery, and 48 hours after the end of surgery, but still during the infusion period.
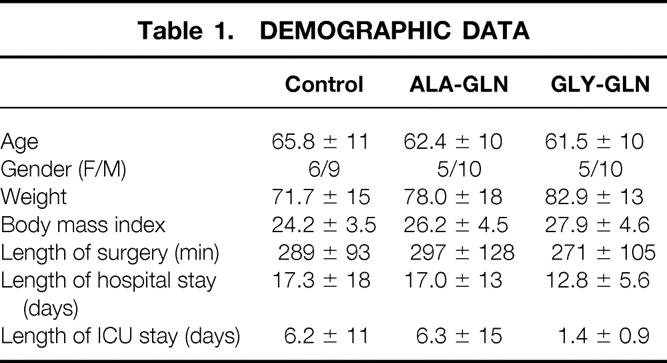
Demographic data are listed in Table 1. Patients with the following criteria were excluded from the study: acute liver failure (Normotest < 15%, signs of encephalopathy), liver cirrhosis, Child B or C type 1 diabetes, HIV infection, thyroid disorder, platelets less than 50,000/μL, leukocytes less than 2,500/μL, renal insufficiency (creatinine clearance < 25 mL/d), need for transfusion of more than two units of packed red cells/d or more than four units of fresh-frozen plasma/d, inclusion in other studies, pregnancy, and mental disorders.
Table 1.DEMOGRAPHIC DATA
LPS Stimulation Ex Vivo
Blood samples were collected into heparinized Vacutainer tubes (Becton Dickinson, San José, CA). Subsequently 1 mL of whole blood was stimulated with 1 μg/mL LPS (Escherichia coli serotype 0127:B8) and incubated for 3 hours at 37°C. Tubes were slightly rotated every 30 minutes, then centrifuged. Supernatants were immediately frozen at −70°C until analysis for TNF-α by ELISA. The assay was performed in duplicate and analyzed in a plate reader (Dynatech, Chantilly, VA).
Immunofluorescence and Flow Cytometry Measurement
Whole blood was incubated with monoclonal antibodies (mAb) for 30 minutes at room temperature in the dark. Subsequently, red blood cells were lysed and leukocytes were stabilized and fixed by Multi-Q-Prep (Coulter, Miami, FL). Finally, cells were washed three times and immediately analyzed by flow cytometry on an EPICS XL-MCL (Coulter).
To detect monocyte HLA-DR expression, cells were stained with anti-HLA-DR mAb PE (Becton-Dickinson) and with anti-CD14 mAb PC5 (Coulter). Routinely, 2,500 CD14+ gated monocytes were counted and HLA-DR was calculated as a percentage and as mean channel fluorescence intensity (MSF). Identical instrument settings were used throughout the entire study period.
E. coli Phagocytosis
Phagocytosis was determined from 100 μL heparinized whole blood and immunoglobulin-opsonized and FITC-conjugated E. coli (Phagotest, Orpegen, Heidelberg, Germany), which was incubated for 15 minutes at 37°C. Subsequently cells were cooled on ice to stop phagocytic activity. Leukocytes were additionally stained with an anti-CD14 mAb (Coulter, My4 PE) to detect monocytes. Finally, cells were washed three times and the percentage and MCF of CD14+ phagocytic cells were determined by FACS analysis.
Plasma Amino Acids
Blood was drawn into heparinized tubes, placed on ice, centrifuged, and deproteinized by the addition of 30% sulfosalicylic acid and β-thienylalanine as an internal standard. After centrifugation, one part of the sample was diluted with 99 parts of water containing 2 mmol NaN3, and clear plasma was stored at −70°C. Amino acids were analyzed using an automatic precolumn derivatization with ophthalaldehyde (OPA) and high-pressure liquid chromatography (HPLC) separation.
Statistical Analysis
Data were analyzed with the procedure MIXED (SAS statistical software). Analysis of variance was performed using the Satterthwaite approximation for unequal variances of the groups. Multiple comparisons were adjusted according to Tukey-Kramer.
RESULTS
Demographics
The demographic characteristics and clinical features of the patients are shown in Table 1. Patients showed no significant differences in age, gender, weight, body mass index, or length of surgery. Types of surgery were equally distributed between groups: 7 esophagus resections, 4 gastric resections, 3 bilary/pancreatic drainage operations, 1 pancreatic cauda resection with splenectomy, 1 liver resection, 1 retroperitoneal resection of a liposarcoma, 10 duodenopancreatectomies, 10 colon resections, and 8 rectum resections. Volume was substituted with crystalloids or colloids. If blood or coagulation substitution was necessary, it was administered.
There was a tendency for reduced development of serious complications in patients treated with ALA-GLN or GLY-GLN. Four patients developed serious complications, three in the control group (anastomosis dehiscence, massive anastomosis bleeding, and massive wound infection) and one in the ALA-GLN group (pancreatic fistula). Minor complications such as wound infection, fistula, hematoma, recurrent paresis, postoperative icterus, or cholecystitis occurred in four patients treated with GLY-GLN, in five patients in the ALA-GLN group, and in one patient in the control group.
There was no significant difference in the amount of days spent in the intensive care unit or the length of hospital stay, but there was a tendency for a reduced stay in the intensive care unit (control 6.2 ± 11, ALA-GLN 6.3 ± 15, GLY-GLN 1.4 ± 0.9) and a diminished length of total hospital stay of GLY-GLN patients (control 17.3 ± 18, ALA-GLN 17.0 ± 13, GLY-GLN 12.8 ± 5.6).
Plasma Amino Acid Levels
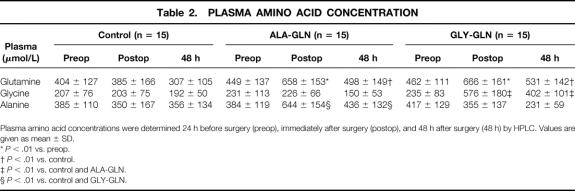
There were no significant differences between groups in the plasma amino acid concentrations before the start of the infusion (Table 2). Significant increases in GLN plasma levels were detectable in the GLN groups. Postoperatively, plasma GLN levels were significantly higher than the preoperative values in the GLY-GLN group (44%;P < .01) and in the ALA-GLN group (47%;P < .01). Forty-eight hours after surgery, GLN levels were significantly higher in the two medication groups (GLY-GLN 73%;P < .01; ALA-GLN 62%;P < .01) than in the controls. As expected, in the GLY-GLN group, GLY levels were significantly higher than in the ALA-GLN group immediately (144%;P < .01) and 48 hours after surgery (71%;P < .01). Similarly, in the ALA-GLN group, plasma ALA levels were significantly higher than in the GLY-GLN group postoperatively (68%;P < .01) and 48 hours after surgery (13%;P < .05).
Table 2.PLASMA AMINO ACID CONCENTRATION
Plasma amino acid concentrations were determined 24 h before surgery (preop), immediately after surgery (postop), and 48 h after surgery (48 h) by HPLC. Values are given as mean ± SD.
*P < .01 vs. preop.
†P < .01 vs. control.
‡P < .01 vs. control and ALA-GLN.
§P < .01 vs. control and GLY-GLN.
TNF Release Following LPS Stimulation
Immediately after surgery, TNF-α secretion of whole blood following LPS stimulation was significantly lower than preoperative values in all groups (Fig. 1). However, the LPS-stimulated TNF-α release 48 hours after the end of surgery was significantly higher (94% of preoperative levels, P = .02) in the GLY-GLN group than in the control group or the ALA-GLN group.
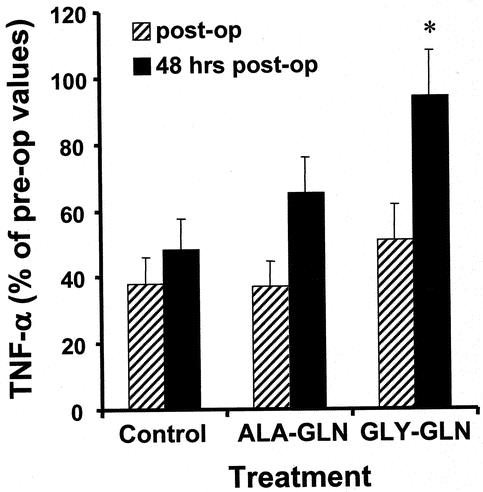
Figure 1. The ex vivo LPS-stimulated TNF-α production into whole blood was measured 24 hours before surgery, immediately after surgery, and 48 hours after surgery. TNF-α secretion is given as percentage of the changes in comparison to preoperative values. Level of significance P = .02; GLY-GLN vs. control and GLY-GLN vs. ALA-GLN.
Monocyte HLA-DR Expression
After surgery there was a significant reduction of monocytic HLA-DR expression, which was extended 48 hours postoperatively in all groups (Table 3). There were, however, no significant differences between groups.
Table 3.MONOCYTE HLA-DR EXPRESSION
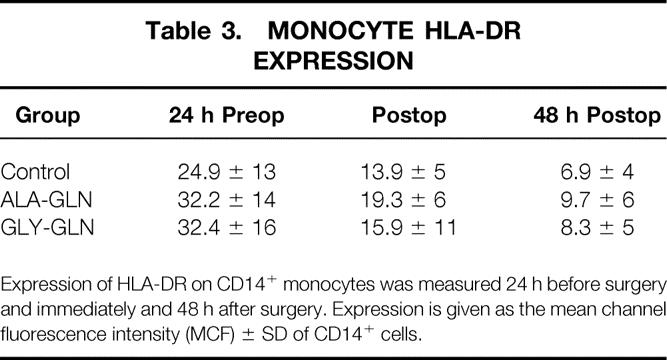
Expression of HLA-DR on CD14+ monocytes was measured 24 h before surgery and immediately and 48 h after surgery. Expression is given as the mean channel fluorescence intensity (MCF) ± SD of CD14+ cells.
Phagocytosis of E. coli
As shown in Figure 2, the GLY-GLN group showed a greater amount of phagocytosis of E. coli by monocytes after 48 hours than did the ALA-GLN group. However, this difference was not significant.
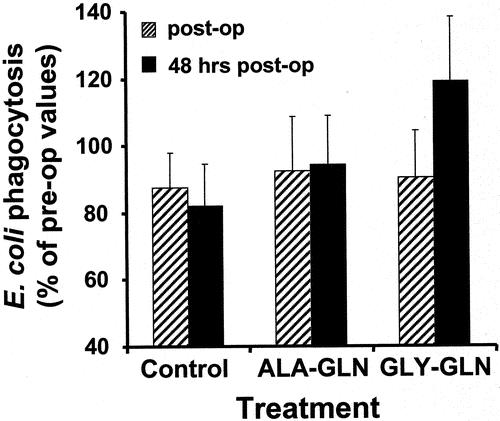
Figure 2. The phagocytic capacity of monocytes in whole blood was measured 24 hours before surgery, immediately after surgery, and 48 hours after surgery. Phagocytosis of groups is given as percentage of the changes in comparison to preoperative values.
DISCUSSION
The immunostimulating effects of GLN have been demonstrated by various in vitro studies. GLN has an important role as a regulative amino acid: it stimulates the proliferation of lymphocytes and expression of heat-shock molecules, reduces apoptosis, and influences cell size. 23–28 In a previous study we showed that reduced glutamine levels diminish monocytic cell surface marker expression. This reduction is paralleled by a reduced capacity to present antigen and to phagocytize. 19 Moreover, in animal studies, glutamine improved survival in rats after cecal ligation and puncture and improved nitrogen balance and attenuated the loss of glutamine from the skeletal muscle intracellular pool. 29 Recently it has been shown that oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. 30 In humans GLN is normally considered to be a nonessential amino acid. However, previous studies provided evidence that GLN may become “conditionally essential” during inflammatory processes such as infection and injury. A meta-analysis of 14 randomized trials including 737 patients has shown that the administration of GLN to surgical and critically ill adult patients reduces infectious complications and shortens length of hospital stay without any adverse effect on mortality. 31 In surgical patients a reduction of plasma GLN levels directly after surgery has been paralleled with a higher infection rate. In our study patients receiving the GLN-free control solution showed a reduction of plasma GLN levels directly after surgery, which was further reduced after 48 hours. In patients who received GLY-GLN or ALA-GLN, these levels were elevated.
To detect immunosuppression in various clinical situations, an in vitro LPS-stimulated TNF-α secretion test system was developed. TNF-α is mainly produced from monocytes/macrophages on stimulation with endotoxin and has been shown to be one of the leading cytokines in the initial inflammatory immune response. During the later course of sepsis, there is a dramatically reduced LPS-stimulated TNF-α secretion into whole blood. 9 In the present study we also saw transitory immunosuppression directly after surgery in all groups. However, in patients who received the dipeptide GLY-GLN, TNF-α secretion was almost completely restored after 48 hours. This was not the case in patients who received the control solution. In patients who received the dipeptide ALA-GLN, LPS-induced TNF-α secretion after 48 hours was intermediate, but this effect was not significant. We cannot explain the divergent reactivity of the two dipeptides from our data. However, in 1992 Vincent et al. demonstrated that glycine caused a dose-dependent increase in the uptake of glutamine from the cell culture medium by isolated rat liver cells. 32 They concluded that glycine did not stimulate net glutamine removal from the medium by isolated rat hepatocytes by a direct stimulatory effect on glutaminase, but rather by causing hepatocyte swelling, which is known to stimulate glutamine metabolism. Similar experiments by this group with alanine failed to stimulate an increased GLN uptake of hepatocytes. Recently it has been shown that increasing the glutamine concentration in the cell culture medium results in an increased TNF-α production of murine macrophages and human monocytes following LPS stimulation. 33 When we consider that the administration of glycine enhances glutamine uptake in human monocytes and an increased intracellular glutamine level causes elevated TNF-α secretion, our data possibly explain why ALA-GLN was not significantly effective in our setting. In the present study it appears that the impact was not so much attributable to the provision of glutamine, but rather to the administration of glycine and the relationship between glycine and glutamine. We also believe that a more catabolic group, such as patients with polytrauma, might benefit to a greater extent from the administration of GLY-GLN dipeptide. However, at the moment we have no evidence for this hypothesis.
We have already demonstrated that HLA-DR surface antigens on monocytes are downregulated immediately at the onset of anesthesia and remain low during the first days after surgery. 34 The reason for the marked reduction of HLA-DR expression on monocytes is unknown. In a preceding clinical trial we showed that postoperative GLY-GLN infusion partially reduces the surgery-induced decrease in HLA-DR expression. 20 However, these results could not be confirmed in the current investigation. We observed the expected reduction of HLA-DR expression directly after surgery, which was prolonged until 48 hours after surgery, but was not influenced by GLN. One explanation could be that the two studies were different in the mode and time of onset of dipeptide administration. In the present study GLN was administered at a dose of 0.5 g/kg/24 h and 24 hours before surgery, whereas in the previous study it was administered at a dose of 0.25 g/kg/24 h postoperatively. Possibly the effect of GLY-GLN on perioperative HLA-DR expression is time- and dose-dependent.
We also investigated the phagocytic properties of monocytes. It has already been shown that supplementation of whole blood with GLN in vitro in postoperative patients augments phagocytosis by neutrophils and monocytes. 35 In this study phagocytosis was detected by fluorescence-activated cell sorting of incorporated fluorescence-labeled beads. In our study plasma GLN levels were elevated in the GLN-treated groups, although monocyte phagocytosis was not significantly different between groups after 48 hours. However, there was a tendency for an increased E. coli incorporation 48 hours after surgery in the GLY-GLN group.
In summary, perioperative GLY-GLN infusion helps to enhance the restoration of immune reactions that have been depressed by surgery, as shown by ex vivo LPS-stimulated TNF-α production into whole blood as a marker of immune state. This is possibly due to increased GLY-mediated cellular plasma GLN uptake. ALA-GLN and GLY-GLN may exert different physiologic effects.
Footnotes
Supported in part by Fresenius Kabi, Homburg/Saar, Germany.
Correspondence: Andreas Spittler, MD, Surgical Research Laboratories, AKH, University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria.
E-mail: a.spittler@akh-wien.ac.at
Accepted for publication March 21, 2002.
References
- 1.Cerami AC, Beutler B. Role of cachectin TNF in endotoxic shock and cachexia. Immunol Today 1988; 9: 28–31. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Lowry SF. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol 1990; 55: 157–170. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Hirano T, Taga T, et al. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF). FASEB J 1990; 4: 2860–2867. [PubMed] [Google Scholar]
- 4.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR expression characterizes clinical outcome in the trauma patient. Br J Surg 1990; 77: 204–207. [DOI] [PubMed] [Google Scholar]
- 5.Kono K, Sekikawa T, Matsumoto Y. Influence of surgical stress on monocytes and complications of infection in patients with esophageal cancer: Monocyte HLA-DR antigen expression and respiratory burst capacity. J Surg Res 1995; 58: 275–280. [DOI] [PubMed] [Google Scholar]
- 6.Doecke WD, Randow F, Syrbe U, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nature Med 1997; 3: 678–681. [DOI] [PubMed] [Google Scholar]
- 7.Faist E, Storck M, Hultner L, et al. Functional analysis of monocyte activity through synthesis patterns of proinflammatory cytokines and neopterin in patients in surgical intensive care. Surgery 1992; 112: 562–572. [PubMed] [Google Scholar]
- 8.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol 1993; 53: 602–618. [DOI] [PubMed] [Google Scholar]
- 9.Ertel W, Kremer JP, Kenney J, et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 1995; 85: 1341–1347. [PubMed] [Google Scholar]
- 10.Beutler B, Cerami A. The biology of cachectin/TNF-α primary mediator of the host response. Ann Rev Immunol 1989; 7: 625–655. [DOI] [PubMed] [Google Scholar]
- 11.Stenger S, Sollbach W, Röllinghoff M, et al. Cytokine interactions in experimental cutaneous leishmaniasis. II. Endogenous tumor necrosis factor-α production by macrophages is induced by the synergistic action of interferon (IFN)-γ and interleukin (IL) 4 and accounts for the antiparasitic effect mediated by IFN-γ and IL-4. Eur J Immunol 1991; 21: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 12.Spittler A, Willheim M, Leutmezer F, et al. Effects of 1α,25-dihydroxyvitamin D3 and cytokines on the expression of MHC antigens, complement receptors and other antigens on human blood monocytes and U937 cells: role in cell differentiation, activation and phagocytosis. Immunology 1997; 90: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germain RN. MHC-associated antigen processing, presentation, and recognition: adolescence, maturity and beyond. Immunologist 1995; 3: 185–190. [Google Scholar]
- 14.Van der Hulst RRWJ, van Kreel BK, von Meyenfeldt MF, et al. Glutamine and the preservation of gut integrity. Lancet 1993; 341: 1363–1365. [DOI] [PubMed] [Google Scholar]
- 15.Chang WK, Yang KD, Shaio MF. Lymphocyte proliferation modulated by glutamine: involved in the endogenous redox reaction. Clin Exp Immunol 1999; 117: 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziegler TR, Young LS, Benfell K, et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. Ann Intern Med 1992; 116: 821–828. [DOI] [PubMed] [Google Scholar]
- 17.Roth E, Karner J, Ollenschläger G, et al. Alanylglutamine reduces muscle loss of alanine and glutamine in postoperative anaesthetized dogs. Clin Sci 1988; 75: 641–648. [DOI] [PubMed] [Google Scholar]
- 18.Roth E, Funovics J, Mühlbacher F, et al. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1982; 1: 25–29. [DOI] [PubMed] [Google Scholar]
- 19.Spittler A, Winkler S, Gotzinger P, et al. Influence of glutamine on the phenotype and function of human monocytes. Blood 1995; 86: 1564–1569. [PubMed] [Google Scholar]
- 20.Spittler A, Sautner T, Gornikiewicz A, et al. Postoperative glycyl-glutamine infusion reduces immunosuppression: partial prevention of the surgery induced decrease in HLA-DR expression on monocytes. Clin Nutr 2001; 20: 37–42. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler MD, Ikejema K, Enomoto N, et al. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci 1999; 56: 843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin L, Gevers W, Jardine L, et al. Serum amino acids in weight-losing patients with cancer and tuberculosis. Eur J Cancer Clin Oncol 1983; 19: 711–715. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths M, Keast D. The effect of glutamine on murine splenic leukocyte response to T and B cell mitogens. Immunol Cell Biol 1990; 68: 405–408. [DOI] [PubMed] [Google Scholar]
- 24.Yaqoob P, Calder PC. Glutamine requirement of proliferating T-lymphocytes. Nutrition 1997; 13: 646–651. [DOI] [PubMed] [Google Scholar]
- 25.Oehler R, Pusch E, Dungel P, et al. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr 2001; 86: 1–6. [DOI] [PubMed] [Google Scholar]
- 26.Wischmeyer PE, Kahana M, Wolfson R, et al. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol 2001; 90: 2403–2410. [DOI] [PubMed] [Google Scholar]
- 27.Exner R, Weingartmann G, Munk Eliasen M, et al. Glutamine deficiency renders human monocytic cells more susceptible to specific apoptosis triggers. Surgery 2002; 131: 75–80. [DOI] [PubMed] [Google Scholar]
- 28.Häussinger D, Roth E, Lang F, et al. Cellular hydration state: an important determinant of protein catabolism in health and disease. Lancet 1993; 341: 1330–1334. [DOI] [PubMed] [Google Scholar]
- 29.Ardawi MSM. Effects of glutamine-enriched total parenteral nutrition on septic rats. Clin Sci 1991; 81: 215–222. [DOI] [PubMed] [Google Scholar]
- 30.Manhart N, Vierlinger K, Spittler A, et al. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. Ann Surg. 2001; 234: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak F, Heyland DK, Avenell A, et al. Glutamine supplementation in critically ill adults: a meta-analysis. Clin Nutr 2001; 20: 54. [Google Scholar]
- 32.Vincent N, Martin G, Baverel G. Glycine, a new regulator of glutamine metabolism in isolated rat-liver cells. Biochim Biophys Acta 1992; 1175: 13–20. [DOI] [PubMed] [Google Scholar]
- 33.Newsholme P. Why is l -glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 2001; 131: 2515S–2522S. [DOI] [PubMed] [Google Scholar]
- 34.Hiesmayr MJ, Spittler A, Lassnigg A, et al. Alterations in the number of circulating leukocytes, phenotype of monocyte and cytokine production in patients undergoing cardiothoracic surgery. Clin Exp Immunol 1999; 115: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furukawa S, Saito H, Inoue T. Supplemental glutamine augments phagocytosis and reactive oxygen intermediate production by neutrophils and monocytes from postoperative patinets in vitro. Nutrition 2000; 16: 458–459. [DOI] [PubMed] [Google Scholar]