Abstract
Objective
To analyze prognostic factors influencing pancreatic cancer survival following curative resection, using prospectively collected, population-based data.
Summary Background Data
Several studies have analyzed the determinants of long-term survival in postresection pancreatic cancer patients, but the majority of these have been single-institutional chart reviews yielding inconsistent results.
Methods
This retrospective cohort study examined 396 Medicare-eligible patients over age 65 who were diagnosed with nonmetastatic pancreatic adenocarcinoma and who underwent surgical resection with curative intent while residing in one of the 11 Survival, Epidemiology, and End Results (SEER) registries between January 1991 and December 1996. Linked Medicare data provided information on treatment and comorbidity, while linked census tract data supplied sociodemographic characteristics.
Results
Median survival for the overall study population was 17.6 months, with 1- and 3-year survival rates of 60.1% and 34.3%, respectively. Survival appears to be gradually improving over time, concomitant with a rise in the proportion of patients undergoing surgery in teaching centers. Prognostic variables significantly diminishing survival on univariate analysis included African American race, treatment not in a teaching hospital, lack of adjuvant chemoradiation therapy, as well as histopathologic factors that included tumor size larger than 2 cm in diameter, moderate to poor histologic grade, and positive lymph node metastases. Higher socioeconomic status was associated both with an increased likelihood of receiving adjuvant therapy and improved overall survival. Multivariate analyses indicated the strongest predictors of survival were adjuvant combined chemoradiotherapy, small tumors (<2 cm in diameter), negative lymph nodes, well-differentiated histology, undergoing surgery in a teaching hospital, and high socioeconomic status.
Conclusions
Although biologic characteristics remain important predictors of survival for patients with resected pancreatic cancer, the most powerful determinant is postoperative adjuvant chemoradiation therapy. An interesting finding that warrants further investigation is the effect of socioeconomic status on both the likelihood of receiving adjuvant treatment and subsequent survival, indicating a possible relationship between the quality of care delivered and outcomes.
Pancreatic cancer is a devastating disease. In 1999, incidence and mortality rates were identical, accounting for 28,600 deaths that year, and making it the fourth leading cause of cancer death for both men and women in the United States. 1 Based on nationwide statistics, less than 5% of all patients diagnosed with pancreatic cancer can expect to live for more than 5 years. 1 Unfortunately, due to the late presentation of symptoms, only 10% to 20% of these individuals are candidates for surgical resection, which remains the only viable chance for cure of this highly aggressive and lethal disease. 2–5
According to data from the National Cancer Data Base, pancreaticoduodenectomy, also known as the Whipple procedure, was the most commonly performed cancer-directed operation, but it resulted in a 5-year survival rate of only 3% in 1985. 6 This slim chance for cure is further borne out by the historical results of this procedure. As recently as a decade ago, patients who underwent resection for cure had survival outcomes that were approximately equivalent to those who received palliative bypass procedures. 7–10 Recent studies from centers of excellence specializing in the care of cancer patients, however, have reported improved rates of long-term survival for patients with adenocarcinoma of the head of the pancreas after pancreaticoduodenectomy, with 5-year survival rates exceeding 20%. 11–18 While some of this improved success may be attributed to increased experience with the procedure and advances in perioperative care, decrease in procedure-related mortality cannot be the sole explanation for this increase in 5-year survival from less than 5% to greater than 20%.
In an effort to seek other explanations for the improved survival rates, several studies have analyzed the determinants of long-term survival in postresection pancreatic cancer patients. Factors found to be potentially associated with survival outcomes have included: 1) demographic factors, such as age, gender, and race; 2) perioperative factors, such as type of resection, estimated blood loss, packed red blood cell transfusions, operative time, surgeon’s experience, decade of resection, and adjuvant therapy; and 3) histopathologic factors, such as tumor size, status, and DNA ploidy, histologic grade, blood vessel or neural invasion, lymph node status, and resection margins. However, there is controversy around most of these, and the majority of prior studies have been small, single-institution experiences, limiting the power and generalizability of the results. To overcome these limitations, we undertook a cohort study combining prospectively collected, population-based registry data with linked administrative claims files and census data to evaluate the prognostic factors influencing survival after potentially curative resection of pancreatic cancer.
METHODS
Data Sources
Eleven tumor registries participate in the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program: San Francisco/Oakland, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle/Puget Sound, Utah, Atlanta, San Jose/Monterey, and Los Angeles. These registries prospectively collect uniform information on all cancers diagnosed within their geographic regions, capturing approximately 97% of all incident cases in these areas. 19 Approximately 14% of the U.S. population reside in the geographic areas covered by the SEER registries, which are therefore fairly representative of the nation’s population. 20 The registries also record patients’ demographic characteristics, such as age, gender, and race/ethnicity. Disease-related data collected by the tumor registries include the cancer site, tumor size, tumor grade, lymph node involvement, date of diagnosis, and date and cause of patient death. Census data at the level of each patient’s census tract or ZIP code of residence have been linked to the SEER registry files, providing information on levels of education, per capita income, and wealth.
The Health Care Finance Administration (HCFA) Medicare database includes files for inpatient and outpatient care, as well as physician and laboratory billings. Each patient in the SEER and Medicare databases has a unique case identification number, which permits matching and merging of the different files. The linkage of cases between the databases results in a 94% successful match rate. 21 The Medicare data enable analysis of perioperative factors such as type of resection, blood transfusions, and the use of adjuvant chemoradiotherapy, in addition to the histopathologic factors that are already available in the SEER database alone, and the sociodemographics provided by census data.
Cohort Assembly
Patients were eligible if they were over age 65 and resided in one of the 11 SEER catchment areas during a 6-year period between January 1, 1991 and December 31, 1996, as patients diagnosed in 1996 are the most recent with linked SEER and Medicare data. All patients were diagnosed with nonmetastatic pancreatic adenocarcinoma and underwent surgical resection with curative intent.
Exclusion criteria included the following: (1) enrollment in a health maintenance organization (HMO) at any time during the study period (19%), since these entities do not report claims detailing specific diagnoses and procedures to HCFA; (2) untrustworthy observations, such as the dates of death differing by more than 2 months between the SEER and Medicare databases (4.0%); and (3) ineligibility for either part of Medicare (6.4%).
Curative resection was determined using the International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) Principal Procedure code for the operation defined as “radical pancreaticoduodenectomy,” or Whipple procedure (ICD-9-CM code 52.7). ICD-9 codes for related pancreatic resection operations that aim to cure, not palliate, the patient with pancreatic cancer were also included in the analysis, such as “total pancreatectomy” (52.6), “radical subtotal pancreatectomy” (52.53), “proximal pancreatectomy” (52.51), “partial pancreatectomy of neck” (52.59), and “distal pancreatectomy” (52.52).
Definitions of Potential Prognostic Factors
Potential prognostic factors evaluated in this study included demographic, perioperative, and histopathologic factors. The demographic factors analyzed were age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or Asian), and socioeconomic status (SES). SES was categorized as being either above or below the median, according to the following hierarchy, depending on the availability of information: 1) unadjusted median household income by census tract (94.9% of patients); 2) median household wealth by census tract (3.0%); and 3) median per capita income in the census tract (2.0%). Consequently, 99.9% of the patients were classified with census-level data, while only 0.1% of the patients could not be classified by SES status. The utility of census-level SES classification, although not as precise as patient-level data, has been previously validated. 22
The perioperative factors analyzed were type of resection (partial, non-Whipple pancreatectomy, total pancreatectomy, or Whipple), packed red blood cell transfusions, and adjuvant therapy. The partial, non-Whipple pancreatectomy category included proximal (ICD-9-CM code 52.51), neck (52.59), and distal (52.52) pancreatectomy cases; the total pancreatectomy category included total pancreatectomy (52.6) and radical subtotal pancreatectomy (52.53) procedures; and the Whipple category included radical pancreaticoduodenectomy (52.7) cases. Data regarding presence or absence and quantity of units of blood transfused during the perioperative period were collected from the Medicare database.
SEER collects information on initial cancer-directed surgery and radiation, defined as those treatments applied within 4 months of diagnosis. However, the NCI does not release chemotherapy treatment data because outpatient chemotherapy usage may be missed by SEER chart abstractions. 21 Therefore, we identified chemotherapy use from Medicare claims using the International Classification of Disease Version 9 (ICD-9) diagnostic code V58.1 and procedure code 9925, and the DRG code 410 for chemotherapy administration in the inpatient setting. For the outpatient files and physician billing claims, HCFA Common Procedure Coding System (HCPCS) codes for chemotherapy administration (Q0083-Q0085, J7150, or J8999-J9999), Current Procedural Terminology (CPT) codes (96400, 96408, 96410, 96412, 96414, and 96545), as well as the relevant revenue center codes (0331, 0332, and 0335), were used where applicable. Radiation was identified either from the SEER registry, or if ICD-9 diagnostic codes (V580, V661, V671), procedure codes (9220, 9221, 9222, 9223, 9224, 9225, 9226, 9227, 9228, 9229), CPT codes (77261–77799), or revenue center codes (0330, 0333, 0339) indicating radiotherapy appeared in any claims. Patients were considered to have received adjuvant treatment if any of these codes were used at any time within the first 4 months after surgery.
We calculated the Charlson comorbidity index 23 by examining the inpatient and outpatient diagnostic and procedure codes recorded in the year before the diagnosis of lung cancer. 24,25 Patients were classified as having been treated at a teaching hospital if their record contained a charge for indirect medical education. Finally, the histopathologic factors analyzed in this study included tumor size (diameter), histologic grade (well, moderately, or poorly differentiated), T stage (recorded in the linked data as “in situ,” “local,” or “regional,” which were matched to T1, T2, or T3, respectively), and lymph node status. The primary outcome variable analyzed in this study was survival time after surgery.
Statistical Analysis
Tests of homogeneity using the log-rank chi-square test statistic were performed to determine whether any differences in survival existed according to year of resection or SEER registry. The Kaplan-Meier survival estimate was used to compare the survival rates of patients with post-resection pancreatic carcinoma for the various demographic, perioperative, and histopathologic factors with prognostic potential. 26 Statistical significance was assessed by the log-rank test. A multivariate Cox proportional hazards model was constructed based on the variables that have been previously shown to be negative prognostic factors in the surgical literature, as well as on those analyzed by the Kaplan-Meier method in this study. Multivariate analysis from the Cox proportional hazards model determined the independent prognostic factors associated with long-term survival. 27 All statistical methods were performed with Statistical Analysis Software (SAS), version 6.12 for Windows NT (SAS Institute Inc., Cary, NC, 1996).
RESULTS
Of the 401 patients who were diagnosed with nonmetastatic pancreatic cancer and underwent curative surgical resections, 5 were excluded because of insufficient data, yielding the study population of 396 patients. Survival was analyzed for the study population overall and for each of the three prognostic categories.
Patient Characteristics
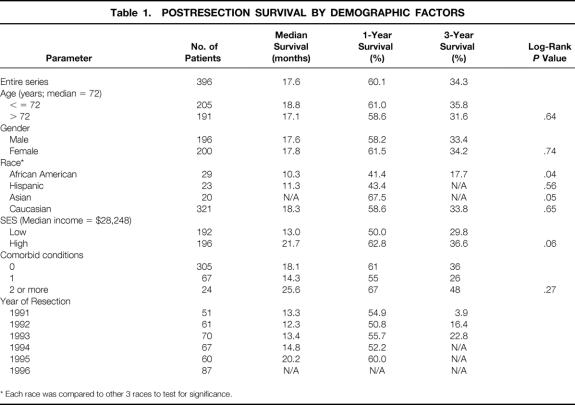
Table 1 shows the characteristics of the 396 patients meeting our eligibility criteria. The median follow-up time for the study population was 38.5 months (range 0.2–44.8), or 3.2 years. Although the last censored observation occurred at 90.4 months, the last recorded event occurred at only 44.8 months, or 3.7 years. Median survival for the overall study population was 17.6 months, with 1- and 3-year survival rates of 60.1% and 34.3%, respectively (Fig. 1).
Table 1.POSTRESECTION SURVIVAL BY DEMOGRAPHIC FACTORS
* Each race was compared to other 3 races to test for significance.
Figure 1. Overall survival (Kaplan-Meier) for study population of 396 patients who underwent curative surgical resection for nonmetastatic pancreatic adenocarcinoma.
The median age of the study population was 72 years. Two hundred five of the patients were 72 years of age or younger, and the other 191 patients were older than 72. There were 196 men and 200 women in the study. There were no significant differences in survival based on age or gender. Twenty-three percent of patients had coexisting comorbid conditions. Diabetes mellitus was the most common condition (13%), followed by chronic pulmonary disease (5.8%). Comorbidity was not significantly associated with survival.
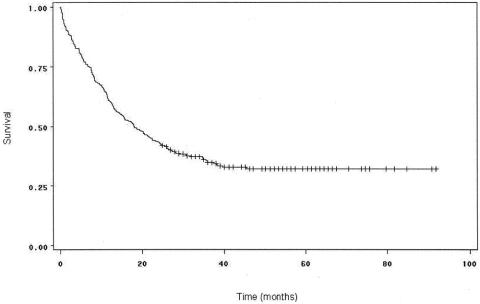
Twenty-nine of the patients were African American, 23 were Hispanic, 20 were Asian, and 321 were white. There was a significant difference in survival by Kaplan-Meier analysis between African Americans and non-African Americans (P = .04). However, this finding did not hold up on multivariate analysis. There was a strong trend toward improved survival for patients above the median income level compared to those below it on univariate analysis (P = .06) (Fig. 2), which later proved significant on multivariate analysis.
Figure 2. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients with high SES (n = 196) to patients with low SES (n = 192). Survival was not significantly better for patients with high SES by the Kaplan-Meier method (P = .06), but was by multivariate analysis (P = .02).
The Connecticut registry contributed the most patients to this study, followed by Detroit, Los Angeles, Iowa, San Jose/Monterey, and San Francisco. A test of heterogeneity across the different SEER regions yielded a P value of .05, indicating a significant difference in survival across the geographic catchment areas. Survival rates were found to be highest in the San Jose/Monterey region and lowest in the Los Angeles region.
Perioperative Factors
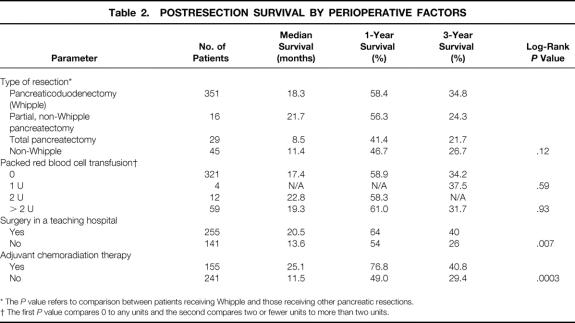
The postresection survival outcomes by perioperative factors are shown in Table 2. The median survival (18.3 months) and 1- and 3-year survival rates (58.4% and 34.8%, respectively) for patients undergoing pancreaticoduodenectomy were not significantly different from the survival outcomes of patients who underwent the partial, non-Whipple pancreatectomies (21.7 months median survival, 56.3% 1-year survival rate, and 24.3% 3-year survival rate) and total pancreatectomies (8.5 months median survival, 41.4% 1-year survival rate, and 21.7% 3-year survival rate). The difference in survival between post-Whipple and non-Whipple patients also was not significant (P = .12). Although all patients were included in the main analysis, we also repeated the analyses excluding the 30 (7.6%) patients who died in the 30-day perioperative period. These results were similar and are not reported.
Table 2.POSTRESECTION SURVIVAL BY PERIOPERATIVE FACTORS
* The P value refers to comparison between patients receiving Whipple and those receiving other pancreatic resections.
† The first P value compares 0 to any units and the second compares two or fewer units to more than two units.
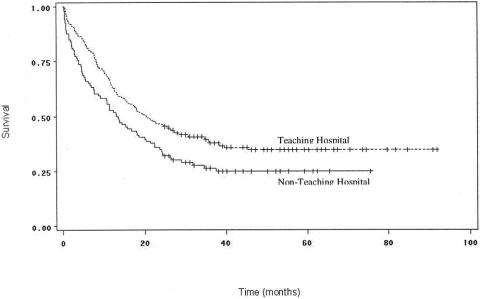
Sixty-four percent of patients underwent their surgery in a teaching hospital, and a total of 73% received some sort of perioperative care in such an institution. Treatment in a teaching center was associated with significantly better survival (P = .007;Fig. 3). Survival rates for patients who required zero units of packed red blood cell transfusions compared to any units (P = .59), as well as less than or equal to two units compared to three or more units (P = .93), were not significantly different on either univariate or multivariate analyses, however.
Figure 3. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients undergoing surgery in a teaching hospital to patients undergoing surgery in a nonteaching hospital. Survival was significantly better for patients who were treated in a teaching hospital (P = .007).
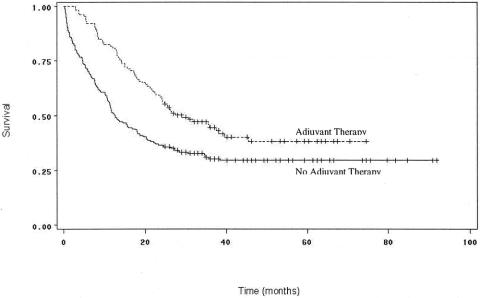
Adjuvant treatment with chemotherapy and/or radiation was found to be a highly significant prognostic determinant (Fig. 4). One hundred eighty-five patients received some sort of adjuvant therapy within 4 months of surgery: 49 were treated with radiation alone, and 11 received only chemotherapy. Of the other 125 patients who received both chemotherapy and radiation, median survival was 29 months, 1-year survival was 81%, and 3-year survival was 45%, significantly better than 12.5 months, 51%, and 30% for the 208 patients who did not receive adjuvant therapy (P = .0003). Patients who received adjuvant treatment were similar to those who did not except that they tended to be younger (71.3 vs. 73.3 years) and of higher SES (59% with above median income vs. 48% for patients who did not receive adjuvant therapy). Patients receiving adjuvant treatment were also more likely to have had nodal involvement (60% vs. 44%). There were no significant differences in sex, race, comorbidity, tumor size and histology, or rates of care in teaching centers between patients who did and did not receive adjuvant chemoradiotherapy. Administration of adjuvant treatment was homogeneously distributed across the different SEER registries, eliminating regional variations in practice patterns as a possible confounder.
Figure 4. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients who received adjuvant chemotherapy and/or radiation therapy (n = 185) to those who did not receive adjuvant therapy (n = 208). Survival was significantly better for patients who received adjuvant treatment (P = .0003).
Over time, the number of patients undergoing curative resection increased, from 51 patients in 1991 to 87 patients in 1996. Median survival also increased, from 13.3% in 1991 to 20.2% in 1995, the last year in which sufficient survival data were available. A test of heterogeneity across different years yielded a P value of .0001, indicating a significant difference in survival across the years of resection. A subanalysis comparing survival rates between 1991 and 1995, and between 1991 and 1996, showed significant differences in both comparisons (P = .0001). Use of adjuvant chemoradiotherapy did not change over time, but the proportion of patients who underwent surgery in a teaching center increased significantly from 55% in 1991 to 78% in 1996.
Histopathologic Factors
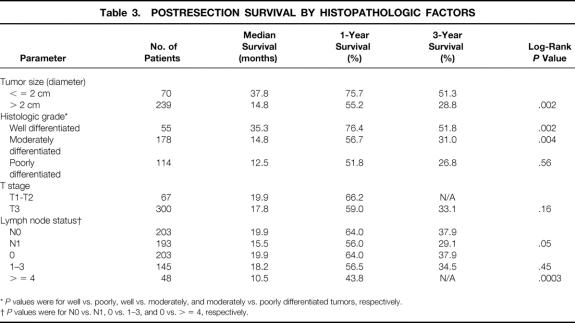
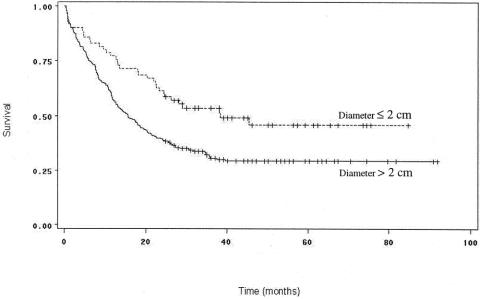
The postresection survival outcomes by histopathologic factors are shown in Table 3. Tumor size was an important predictor of survival by both univariate and multivariate analyses. The median tumor diameter in this cohort was 3 cm. Patients with tumors measuring less than 3 cm in diameter had significantly longer median and 3-year survival (23.9 months and 42%) than patients with tumors 3 cm or more in diameter (14.8 months and 29.6%;P = .03) (see Table 3, Fig. 5). Results were similar when the analyses were repeated using 2.5 cm and 2 cm as breakpoints, as have been used in other studies. 13,28–31 T stage was not independently prognostic, however.
Table 3.POSTRESECTION SURVIVAL BY HISTOPATHOLOGIC FACTORS
*P values were for well vs. poorly, well vs. moderately, and moderately vs. poorly differentiated tumors, respectively.
†P values were for N0 vs. N1, 0 vs. 1–3, and 0 vs. > = 4, respectively.
Figure 5. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients with tumors 2 cm in diameter or smaller (n = 70) to those with tumors larger than 2 cm (n = 239). Survival was significantly better for patients with tumor diameter 2 cm or less (P = .002).
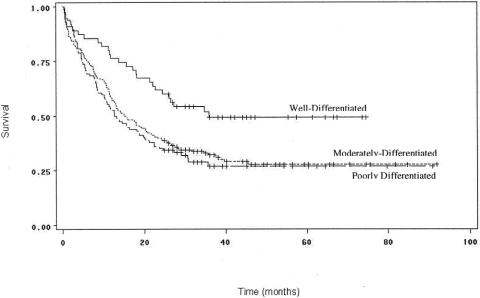
The histologic grade of the tumor was also a significant predictor of outcome by the Kaplan-Meier method (Fig. 6). Patients with well-differentiated tumors had significantly higher survival rates than those with moderately (P = .004) or poorly differentiated tumors (P = .002). These differences were confirmed by multivariate analysis. Rates of survival between patients with moderately and poorly differentiated tumors were not significantly different from each other (P = .56).
Figure 6. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients by histologic grade. Survival was significantly better for patients with well-differentiated tumors than those with moderately (P = .004) or poorly differentiated tumors (P = .002).
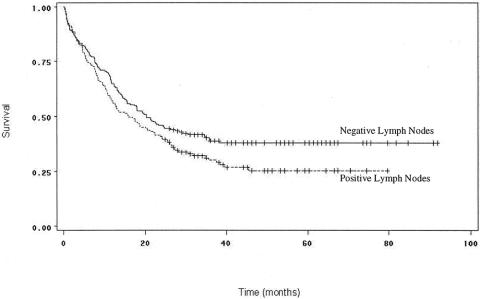
Lymph node status in the resected specimen was a significant prognostic variable (Fig. 7). Patients with negative lymph nodes had higher median survivals and 1-year and 3-year survival rates (19.9 months, 64%, and 37.9%, respectively) than patients with any resected lymph nodes positive for carcinoma (15.5 months, 56%, and 29.1%;P = .05). This finding was also supported by multivariate analysis. Further analysis of the lymph node status data (see Table 3) was conducted, separating one to three positive nodes and at least four nodes into different categories, as has been done previously. 13 While patients with one to three positive nodes did not have significantly different survival rates from patients with negative lymph nodes (P = .45), a highly significant difference was noted between patients with at least four positive nodes and those with negative nodes (P = .0003).
Figure 7. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients by lymph node status. Survival was significantly better for patients with negative lymph nodes (P = .05).
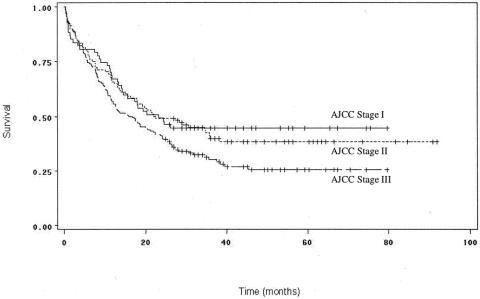
Using the TNM data obtained from tumor and lymph node status, the patients were categorized according to American Joint Committee on Cancer (AJCC) staging, which is increasingly becoming accepted as a prognostic standard for cancer management. 32–34 Figure 8 shows the survival curves for patients by AJCC stage. The survival outcomes for stage I (T1-T2, N0, M0) patients were not significantly different from the outcomes for stage II (T3, N0, M0) or stage III (T1-T3, N1, M0) patients (P = .75 and 0.05, respectively), but stage II patients exhibited significantly higher survival than stage III patients (P = .05).
Figure 8. Survival curves for patients undergoing curative resection for pancreatic adenocarcinoma, comparing patients by AJCC stage. Stage I patients did not have significantly different survival from stage II or stage III patients. Stage II patients had significantly higher survival than stage III patients (P = .05).
Multivariate Analysis
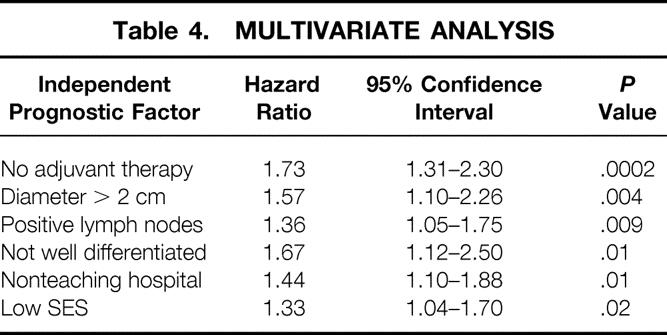
Multivariate survival analysis was performed to determine which potential prognostic factors were independent predictors of survival. These results are shown in Table 4. By multivariate analysis, six factors (one demographic, two perioperative, and three histopathologic) were found to be significant negative prognostic variables for pancreatic carcinoma survival following curative resection. Absence of adjuvant therapy was the strongest negative predictor of survival, measured by both the hazard ratio (HR; 1.73) and statistical significance (P = .0002). This was followed by tumor size more than 2 cm (HR = 1.57, P = .004), lymph node positivity (HR = 1.36, P = .009), not well-differentiated histology (HR = 1.67, P = .01), lack of care in a teaching center (HR = 1.44, P = .01), and low SES (HR = 1.33, P = .02).
Table 4.MULTIVARIATE ANALYSIS
DISCUSSION
Curative surgical resection of pancreatic carcinoma has been associated with improving outcomes in recent reports, with decreasing postoperative morbidity and mortality rates and increasing long-term survival over time. 11,17,35–38 This population-based study of multiregional outcomes, the first to use large administrative datasets to address this area of pancreatic cancer research, produced several interesting findings. Our cohort of 396 Medicare enrollees who underwent curative resection for pancreatic adenocarcinoma from 1991 through 1996 demonstrated median survivals and 1-year and 3-year survival rates of 17.6 months, 60.1%, and 34.3%, respectively, figures that compare favorably with those reported in the recent literature. 13,28–30,39,40
The most significant predictor of survival following pancreatic cancer resection in this study was the use of adjuvant combined chemoradiation therapy, a finding that is consistent with many published studies. 13,41–44 Almost 20 years ago, the Gastrointestinal Tumor Study Group showed in a randomized trial that adjuvant chemoradiotherapy could increase 2-year survival from 18% to 43%. 42,45 Although some small observational studies have been unable to detect improved survival, 28–30 more recent larger series conducted at Johns Hopkins (Baltimore, MD) 13,14 have substantiated the benefits of adjuvant chemoradiation. Two prospective randomized clinical trials are in progress, one in collaboration with the European Organization for Research and Treatment of Cancer (EORTC), the other in collaboration with the European Study Group for Pancreatic Cancer (ESPAC-1). 46–48 Although preliminary data from the EORTC showed no significant benefit of adjuvant radiotherapy and chemotherapy on 2-year survival, additional data from this ongoing trial, as well as those from the ESPAC-1 trial, should go a long way toward elucidating the role of adjuvant chemoradiation therapy in the treatment of pancreatic cancer patients.
Most previous studies have demonstrated that tumor-associated biologic characteristics are important in the prognosis of pancreatic cancer patients after resection. 13,28,29,39 For example, tumor size, histologic differentiation, and lymph node status have all been previously shown to be significant biologic determinants of survival. 11,13,28,30,41,49,50 The results of our study, in which all three factors were significantly associated with outcome in both univariate and multivariate analyses, lend support to these observations.
Our findings are also consistent with existing literature on perioperative factors. Geer and Brennan from Memorial Sloan-Kettering Cancer Center (New York, NY) found that the median survival of 18 patients who had tumors located in the body and tail of the pancreas that were resected with curative intent did not differ significantly from the 128 patients whose tumors were located in the head. 28 Our results, analyzed by the type of resection undertaken, were similar to this experience, as well as other published series. 29,39 No consensus has been reached regarding whether perioperative blood transfusion is a prognostic determinant. While some studies have confirmed that the presence of perioperative blood transfusions is an independent factor decreasing survival, 29,30,39,51 others have shown it is an important factor only when univariate but not multivariate analyses are used;13,52 still others have found that transfusions have no significant effect on survival. 28 Again, our data support the findings of the Memorial series, in which blood transfusion had no significant effect on survival.
There is evidence that the volume of procedures and experience of an institution can influence survival for high-risk cancer surgery like pancreatectomy. 5 Similarly, we found better outcomes when care was delivered in a teaching hospital, as teaching status is likely a proxy for high-volume specialized centers of excellence. Outcomes also seem to be improving over time. Several studies have reported that decade of resection, with stepwise improvement during each decade from the 1970s to the 1990s, is also a strong independent predictor of survival. 11,13,16 Although our study analyzed data during a relatively brief time interval from 1991 to 1996, we also observed an improving secular trend. As there was no change in the use of adjuvant therapy over this time period, but a steady increase in the proportion of patients receiving care in a teaching center, these improved outcomes may be at least partially due to progressive concentration of pancreatic surgery in specialized institutions.
This study is the first to note that being a pancreatic cancer patient of low SES may be as important a determinant of survival as poor biology or a bad operation. Both African American race and low SES were negative characteristics on univariate analysis. In the multivariate analysis, however, African American status was no longer found to be significant, while low SES was shown to be more predictive of negative outcomes, suggesting that in this case race was a proxy for low SES. Most disturbing, though, is our observation that low SES was associated not only with poor overall survival but also with a decreased likelihood of receiving adjuvant treatment. We had expected to see similar access to care among patient groups, since these individuals, by selection, had all already undergone pancreatic resection. However, it is plausible that other barriers exist, such as inadequate follow-up, lack of physician knowledge, or patient preferences. 53
There must be some caution in generalizing from the results of this study. Linked SEER–Medicare data capture only patients over 65 years of age, thereby excluding younger patients from the analysis. This amounts to approximately 27% of all patients diagnosed with pancreatic cancer being excluded, since 73% of these patients are 65 or older. 2 Similarly, the results may not be generalizable to the 19% of elderly patients who were not eligible for both parts of Medicare or who were enrolled in an HMO during the study period. 54 Additionally, administrative claims do not possess the richness of clinical information available from hospital-based chart reviews. 55–58 Therefore, we were unable to assess prognostic factors such as resection margins, ploidy of tumor DNA content, blood vessel and neural invasion, as well as case-specific measures such as operative time, blood loss, and surgeon’s experience. Furthermore, because of a requirement that all data be made anonymous, it was not possible to review pathologic specimens to ensure against misdiagnosis (e.g., other periampullary cancers, chronic pancreatitis) by the multiple pathologists involved. With improved imaging and pathologic techniques, however, we would expect misdiagnosis to be decreasing over time, leading to worsening overall survival statistics as the cohort would contain more true pancreatic cancers. Instead, we observed improving survival over time. Errors could also have been made in documenting diagnostic and other data in the SEER registry, although published validation studies have indicated a high level of accuracy for this process. 19 Moreover, it is unlikely that there would be systematic errors in these areas that would bias the conclusions of the analysis. Lastly, there is a potential for inaccurate coding in administrative claims. 55–58 For example, blood transfusions are voluntarily reported, leading to possible underreporting, which would tend to conservatively bias the result toward not being able to find a significant association with survival.
Despite these limitations, this study is the first population- and claims-based analysis of prognostic determinants for resectable pancreatic adenocarcinoma, and the largest multi-institutional U.S. experience reported to date. We found six important predictors of survival, the most powerful of which was the use of postoperative adjuvant therapy. An interesting finding that warrants further investigation is the effect of SES on both access to care and survival. These results indicate a potentially important deficiency in the quality of care received by poorer patients that directly affects their outcomes. Further research is needed to confirm these observations and to explore reasons for these disparities.
Footnotes
Dr. Lim is a National Institutes of Health Postdoctoral Fellow, supported in part by a grant from the National Institutes of Health (T32-CA09001-25).
Correspondence: Craig C. Earle, MD, MSc, FRCPC, Center for Outcomes and Policy Research, Dana-Farber Cancer Institute, 44 Binney St., Boston, MA 02115.
E-mail: craig_earle@dfci.harvard.edu
Accepted for publication February 25, 2002.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin 1999; 49: 8–31. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER cancer statistics review 1973–1996. NIH Publication No. 99–2789. Bethesda, Department of Health and Human Services, 1999.
- 3.Brennan MF, Kinsella TJ, Casper ES. Cancer of the pancreas. In De Vita VT, Helmann S, Rosenberg SA, eds. Cancer: principles & practice of oncology. Philadelphia: JB Lippincott; 1993: 849–882.
- 4.Reber HA, Gloor B. Radical pancreatectomy. Surg Oncol Clin North Am 1998; 7: 157–163. [PubMed] [Google Scholar]
- 5.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 6.Steele GD, Osteen RT, Winchester DP, et al. Clinical highlights from the National Cancer Data Base. CA Cancer J Clin 1994; 44: 72–80. [DOI] [PubMed] [Google Scholar]
- 7.Crile G. The advantages of bypass operations over radical pancreaticoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet 1970; 130: 1049–1053. [PubMed] [Google Scholar]
- 8.Shapiro TM. Adenocarcinoma of the pancreas: A statistical analysis of biliary bypass versus Whipple resection in good risk patients. Ann Surg 1975; 182: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudjonsson B. Carcinoma of the pancreas, critical analysis of costs, results of resection, and the need for standardized reporting. J Am Coll Surg 1995; 183: 483–503. [PubMed] [Google Scholar]
- 10.Gudjonsson B. Letter to the editor. J Am Coll Surg 1996; 183: 290–291. [PubMed] [Google Scholar]
- 11.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality and survival after the Whipple procedure. Ann Surg 1987; 206: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg 1995; 130: 295–300. [DOI] [PubMed] [Google Scholar]
- 13.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997; 225: 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trede M, Schwall G, Saeger HD. Survival after pancreaticoduodenectomy: 118 consecutive resections without an operative mortality. Ann Surg 1990; 211: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace PA, Pitt HA, Tompkins RK, et al. Decreased morbidity and mortality after pancreaticoduodenectomy. Am J Surg 1986; 151: 141–149. [DOI] [PubMed] [Google Scholar]
- 17.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993; 217: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas: Is it really improving? Ann Surg 1995; 221: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER program of the National Cancer Institute. Cancer 1995; 76: 2343–2350. [DOI] [PubMed] [Google Scholar]
- 20.Nattinger AB, McAuliffe TL, Schapira MM. Generalizability of the Surveillance, Epidemiology, and End Results Registry Population: Factors relevant to epidemiologic and health care research. J Clin Epidemiol 1997; 50: 939–945. [DOI] [PubMed] [Google Scholar]
- 21.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer-related health services research using a linked Medicare-tumor registry database. Med Care 1993; 31: 732–748. [PubMed] [Google Scholar]
- 22.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Pub Health 1992; 92: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1986; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde CN. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53: 1258–1267. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481. [Google Scholar]
- 27.Cox DR. Regression models and life tables. J R Stat Soc 1972; 34: 187–202. [Google Scholar]
- 28.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993; 165: 68–72. [DOI] [PubMed] [Google Scholar]
- 29.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg 1999; 65: 618–623. [PubMed] [Google Scholar]
- 30.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg 1991; 161: 120–124. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya R, Tomioka T, Izawa K, et al. Collective review of small carcinomas of the pancreas. Ann Surg 1986; 203: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TNM classification of malignant tumours. New York: John Wiley & Sons, Inc.; 1997.
- 33.Yarbro JW, Page DL, Fielding LP, et al. American Joint Committee on Cancer prognostic factors consensus conference. Cancer 1999; 86: 2436–2446. [DOI] [PubMed] [Google Scholar]
- 34.Fleming ID, Phillips JL, Menck HR, et al. The National Cancer Data Base report on recent hospital cancer program progress toward complete American Joint Committee on Cancer/TNM staging. Cancer 1997; 80: 2305–2310. [PubMed] [Google Scholar]
- 35.Trede M. The surgical treatment of pancreatic carcinoma. Surgery 1985; 97: 28–35. [PubMed] [Google Scholar]
- 36.Delcore R, Thomas JH, Hermreck AS. Pancreaticoduodenectomy for malignant pancreatic and periampullary neoplasms in elderly patients. Am J Surg 1991; 162: 532–535. [DOI] [PubMed] [Google Scholar]
- 37.Kellum JM, Clark J, Miller HH. Pancreatoduodenectomy for resectable malignant periampullary tumors. Surg Gynecol Obstet 1983; 157: 362–366. [PubMed] [Google Scholar]
- 38.Ozaki H. Retrospective study of resectable pancreatic cancer: results of cases collected from 12 institutions. Pancreas 1994; 9: 414–415. [Google Scholar]
- 39.Magistrelli P, Antinori A, Crucitti A, et al. [Surgical resection of pancreatic cancer]. Tumori 1999; 85: S22–S26. [PubMed] [Google Scholar]
- 40.Benassai G, Mastrorilli M, Quarto G, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol 2000; 73: 212–218. [DOI] [PubMed] [Google Scholar]
- 41.Kalser MH, Ellenberg SS. Adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Arch Surg 1985; 120: 899–903. [DOI] [PubMed] [Google Scholar]
- 42.Gastrointestinal Tumor Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 1987; 59: 2006–2010. [DOI] [PubMed] [Google Scholar]
- 43.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997; 15: 928–937. [DOI] [PubMed] [Google Scholar]
- 44.Miller AR, Robinson EK, Lee JE, et al. Neoadjuvant chemoradiation for adenocarcinoma of the pancreas. Surg Oncol Clin North Am 1998; 7: 183–197. [PubMed] [Google Scholar]
- 45.Gastrointestinal Tumor Study Group. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985; 120: 899–903. [DOI] [PubMed] [Google Scholar]
- 46.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999; 230: 776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neoptolemos JP, Kerr DJ, Beger H, et al. ESPAC-1 trial progress report: the European randomized adjuvant study comparing radiochemotherapy, 6 months chemotherapy and combination therapy versus observation in pancreatic cancer. Digestion 1997; 58: 570–577. [DOI] [PubMed] [Google Scholar]
- 48.Neoptolemos JP, Baker P, Beger H, et al. Progress report. A randomized multicenter European study comparing adjuvant radiotherapy, 6-mo chemotherapy, and combination therapy vs. no-adjuvant treatment in resectable pancreatic cancer (ESPAC-1). Int J Pancreatol 1997; 21: 97–104. [DOI] [PubMed] [Google Scholar]
- 49.Joensuu H, Alanen KA, Klemi P. Doubts on. “curative” resection of pancreatic cancer. Lancet 1989; 1: 953–954. [DOI] [PubMed] [Google Scholar]
- 50.Petrek JA, Sandberg WA, Bean PK, et al. Can survival in pancreatic adenocarcinoma be predicted by primary size or stage? Am Surg 1985; 51: 42–46. [PubMed] [Google Scholar]
- 51.Allema JH, Reinders ME, van Gulik TM, et al. Prognostic factors for survival after pancreaticoduodenectomy for patients with carcinoma of the pancreatic head region. Cancer 1995; 75: 2069–2076. [DOI] [PubMed] [Google Scholar]
- 52.Sperti C, Pasquali C, Piccoli A, et al. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg 1996; 83: 625–631. [DOI] [PubMed] [Google Scholar]
- 53.Mandelblatt JS, Yabroff KR, Kerner JF. Equitable access to cancer services: a review of barriers to quality care. Cancer 1999; 86: 2378–2390. [PubMed] [Google Scholar]
- 54.Riley GF, Potosky AL, Klabunde CN, et al. Stage at diagnosis and treatment patterns among older women with breast cancer: an HMO and fee-for-service comparison. JAMA 1999; 281: 720–726. [DOI] [PubMed] [Google Scholar]
- 55.Hsia DC, Krushat WM, Fagan AB, et al. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med 1988; 318: 352–355. [DOI] [PubMed] [Google Scholar]
- 56.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med 1997; 127: 666–674. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd SS, Rissing JP. Physician and coding errors in patient records. JAMA 1985; 254: 1330–1336. [PubMed] [Google Scholar]
- 58.McCarthy EP, Iezzoni LI, Davis RB, et al. Does clinical evidence support ICD-9-CM diagnosis coding of complications? Med Care 2000; 38: 868–876. [DOI] [PubMed] [Google Scholar]