Abstract
Objective
To evaluate early and late morbidity of laparoscopic adjustable gastric banding for morbid obesity and to assess the efficacy of this procedure by analyzing its results.
Summary Background Data
Laparoscopic adjustable gastric banding is considered the least invasive surgical option for morbid obesity. It is effective, with an average loss of 50% of excessive weight after 2 years of follow-up. It is potentially reversible and safe; major morbidity is low and there is no mortality.
Methods
Between April 1997 and June 2001, 500 patients underwent laparoscopic surgery for morbid obesity with application of an adjustable gastric band. There were 438 women and 62 men (sex ratio = 0.14) with a mean age of 40.4 years. Preoperative mean body weight was 120.7 kg and mean body mass index (BMI) was 44.3 kg. m−2.
Results
Mean operative time was 105 minutes, 84 minutes during the last 300 operations. Mean hospital stay was 4.5 days. There were no deaths. There were 12 conversions (2.4%), 2 during the last 300 operations. Fifty-two patients (10.4%) had complications requiring an abdominal reoperation. Forty-nine underwent a reoperation for minor complications: slippage (n = 43, incisional hernias (n = 3), and reconnection of the catheter (n = 3). Three patients underwent a reoperation for major complications: gastroesophageal perforation (n = 2) and gastric necrosis (n = 1). Seven patients had pulmonary complications and 36 patients experienced minor problems related to the access port. At 1-, 2-, and 3-year follow-up, mean BMI decreased from 44.3 kg. m−2 to 34.2, 32.8, and 31.9, respectively, and mean excess weight loss reached 42.8%, 52%, and 54.8%.
Conclusions
Laparoscopic adjustable gastric banding is a beneficial operation in terms of excessive weight loss, with an acceptably low complication rate. It can noticeably improve the quality of life in obese patients. Half of the excess body weight can be effortlessly lost within 2 years.
Morbid obesity carries major health hazards and reduces the quality of life. It is also life-threatening, since pulmonary, vascular, endocrine, and skeletal complications reduce life expectancy. 1 Demographic studies show a recent increase in the prevalence of morbid obesity, especially among youth, essentially related to changes in nutritional behavior. It will be a major public health challenge during the coming years. 2
Dietary methods for weight control have inconsistent success and a high rate of weight regain. 3 Therefore, surgical options are increasingly considered in the treatment of morbid obesity. Two major categories of surgical procedures haven been considered: the first was based on generating malabsorption (e.g., an intestinal bypass) and the second on stomach volume restriction, by constructing a small proximal pouch that prevents massive food intake and limits the perception of hunger. The latter category was more frequently used in morbid obesity. The calibrated vertical banded gastroplasty, first described by Mason et al., 4 represented an acceptable compromise between long-lasting efficacy and low morbidity. 5 On the same basis, the adjustable gastric band 6 produced similar results in terms of weight loss, with a lower risk of life-threatening complications because there was no opening or suturing of the digestive tract. The laparoscopic approach brought major advantages in terms of safety and comfort.
Success criteria are mainly health improvement and disappearance of cofactors of morbidity, during at least a 5-year follow-up. Excess weight loss is a reliable monitoring tool. In no circumstances were cosmetic concerns taken into account when operative indications were considered.
The aim of this study was to evaluate early and late morbidity and to assess the efficacy of this procedure by analyzing its results.
METHODS
Patient Characteristics
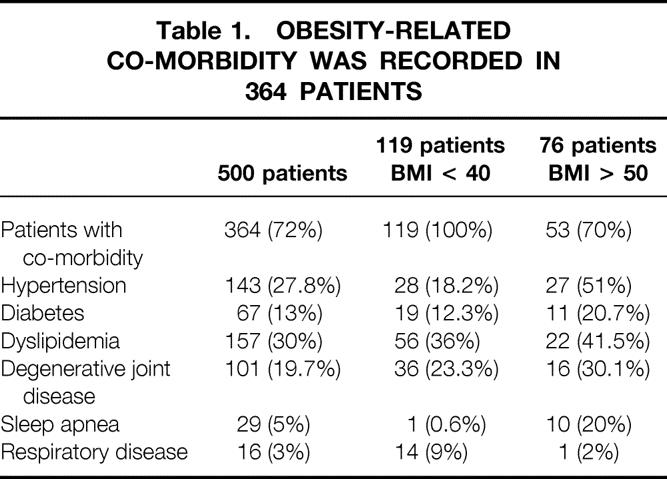
Between April 1997 and June 2001, 500 consecutive patients were operated on for morbid obesity in the Surgical Unit at the European Georges-Pompidou Hospital of the University of Paris 5. All patients were carefully selected and strictly met the criteria for bariatric surgery as defined by the NIH Consensus Development Panel report. 7 Ideal body weight was determined according to the Metropolitan Life Insurance Company’s 1983 height/weight tables. Excess weight was defined as the difference between the patient’s weight and the theoretical medium-frame ideal body weight. There were 438 women and 62 men (sex ratio = 0.14) with a mean age of 40.4 years (range 16.3–66.3). Preoperative mean body weight was 120.7 kg (range 85–195) and mean body mass index (BMI) was 44.3 (range 35–65.8). Seventy-six patients were superobese (BMI > 50; range 50–65.8), with a mean weight of 146 kg (range 113–205) and mean age of 41.2 years (range 20.9–66.3). Twelve patients had previously undergone cholecystectomy, three of them by a laparoscopic approach. A total of 513 comorbidity conditions related to overweight were recorded in 364 patients (72%) with a mean number of comorbidities per patient of 1.4 among them and 1.26 in the whole series. These included hypertension, diabetes, dyslipidemia, degenerative joint disease, sleep apnea, and respiratory disease. Detailed comorbidity data are listed in Table 1. We observed 154 comorbidity conditions in the 119 patients with BMI less than 40 (1.3 per patient) and 87 conditions in 53 of the 76 superobese patients (1.64 per patient).
Table 1.OBESITY-RELATED CO-MORBIDITY WAS RECORDED IN 364 PATIENTS
Preoperative Management
All patients underwent a preoperative evaluation including history, physical examination, and nutritional and psychiatric evaluation. Therapeutic care was multidisciplinary (cardiologist, nutritionist, dietitian, endocrinologist, psychologist, pneumologist, and anesthesiologist), according to many recommendations. 6,8,9 No patient was operated on based on the surgeon’s decision alone. Laboratory evaluation included complete blood count, serum biochemical analysis, including blood glucose, cholesterol, and fatty acid measurements, and thyroid function tests. All patients underwent preoperative abdominal ultrasound. In case of left hepatic lobe hypertrophy (>15 cm), laparoscopic port insertion varied accordingly. When gallstones were detected, simultaneous cholecystectomy was planned. Gallstones were found in 38 patients (7.6%). Specific explorations such as upper gastrointestinal endoscopy, barium swallow, or spirometry were requested only in case of relevant history.
During the first visit, detailed information was provided in oral and written form on all surgical and nonsurgical therapeutic options, their potential risks, benefits, and side effects, and the possibility of conversion to open procedure. Dietary restrictions related to gastric banding were discussed in detail with the patients, first during a collective meeting and a second time during the preoperative dietitian interview. A 1-month delay was mandatory before any decision was taken. During this period, the patient was encouraged to meet other patients who had gastric banding at least 1 year previously. Only afterward was a detailed informed consent form signed; with this form, the patient accepts the procedure-related constraints, particularly the frequent postoperative follow-up visits.
Prophylaxis against venous thromboembolism consisted of preoperative low-molecular-weight subcutaneous heparin for 10 days and perioperative elastic stockings.
Events related to changes in quality of life, comorbidities, and patient satisfaction were assessed during a minimum 24-month follow-up. Changes in quality of life was assessed by the Bariatric Analysis and Reporting Outcome System (BAROS). 10 This system, based on a point scale, evaluates three main criteria: weight loss, medical well-being, and quality of life after treatment. Quality of life evaluated five parameters: self-esteem, physical activity, social life, work conditions, and sexual satisfaction. The final score took into account postoperative complications and reoperations. Among 185 patients with follow-up of more than 24 months, 140 were available for analysis.
Operative Technique
The procedure consisted of laparoscopic placement of a gastric band (Lap-Band System, BioEnterics, Carpinteria, CA) creating a proximal 15-mL pouch at the cardia. The band, which had an expandable balloon, was connected to a port securely sutured subcutaneously on the left rectus abdominis muscle. 6,8 Later, under radiologic guidance, the port was injected with fluid so that the balloon was inflated, calibrating the inner diameter of the ring. This allowed personalized controlled emptying of the pouch.
The patient was positioned in an elevated recumbent position. The surgeon operated standing between the legs of the patient and the first assistant on his or her right. The video monitor was located beyond the patient’s right shoulder. Pneumoperitoneum was created using a Palmer-Veress needle. The 10-mm optical trocar was inserted first, 10 cm below the xiphoid notch. Then, three 10-mm cannulas were placed under the rib margin (Fig. 1). The fourth cannula on the left had a larger diameter (18 mm) to allow the introduction of the band. All cannulas were shifted to the left when preoperative ultrasound revealed an enlarged left liver lobe (>15 cm high). A 10-mm liver retractor was inserted through a paraxiphoid cannula and the left lobe was elevated to expose the cardiac area and the diaphragmatic crus.
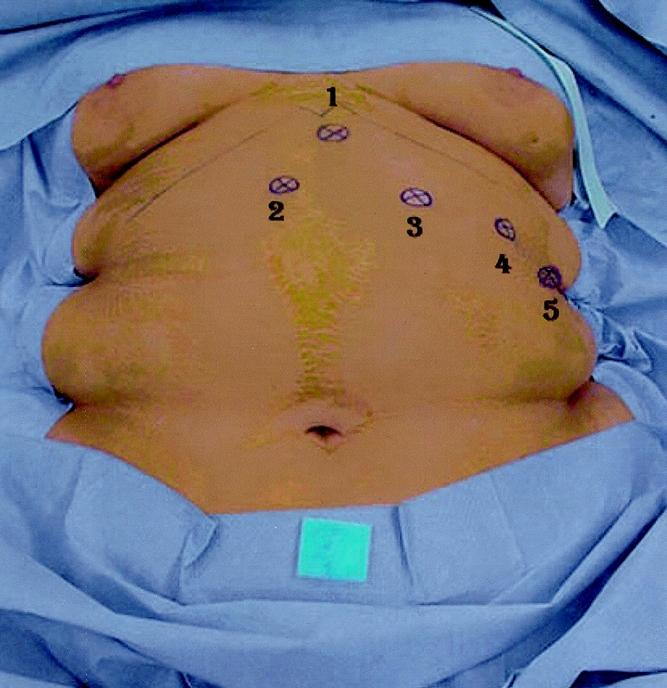
Figure 1. Position of the cannulas.
Gastric dissection started at the angle of the cardia by division of the phrenogastric ligament. Two different techniques were applied afterward. The earlier, used in the first 378 patients, consisted of inserting an orogastric balloon tube. The intraluminal balloon (BioEnterics) was calibrated at 15 cc3 and pulled against the cardia, demarcating the appropriate volume of the pouch. The dissection started at the balloon equator, at the level of the first right gastric vessels, burrowing a tunnel in contact with the posterior gastric wall through a nonextensible space behind the mesoesophagus, above the lesser peritoneal sac. Since October 2000, we revised the procedure into what we call pars flaccida approach (Figs. 2–4). Dissection on the left side was identical. Over the lesser omentum, we opened the peritoneal sheet close to the edge of the right crus, then gradually created a retrogastric tunnel reaching the left crus and the phrenogastric ligament. This avoided the use of the balloon, which was sometimes difficult to identify and occasionally lacerated the esophagus. The band was secured by an anterior gastrogastric valve using four nonabsorbable seromuscular stitches. This covered the anterior part of the band completely. During both procedures, when laceration of the posterior gastric wall was suspected, a methylene blue dye test was carried out.

Figure 2. First step of the pars flaccida approach: opening of the lesser omentum. 1, Pars flaccida of the lesser omentum; 2, liver; 3, lesser curvature of the stomach; 4, right crus.

Figure 3. Second step of the pars flaccida approach: dissection of the right crus. 1, Pars flaccida of the lesser omentum; 2, liver; 3, lesser curvature of the stomach; 4, right crus.

Figure 4. Third step of the pars flaccida approach: retrogastric channel. 1, Pars flaccida of the lesser omentum; 2, liver; 3, lesser curvature of the stomach; 4, right crus.
Three weeks later, the inner diameter of the band was calibrated for the first time with water injection. Additional calibrations were later considered based on clinical evaluation of symptoms and weight loss during follow-up.
Early Postoperative Period
On day 1, Gastrografin swallow was systematically performed to rule out undetected gastric or esophageal injuries, before oral intake. Only fluids were allowed on day 1 and then semifluids until the first calibration appointment. The patient was discharged on day 3 and reviewed shortly afterward on day 10. Calibration was done 1 month postoperatively. Thereafter, the patient was reviewed every 3 months during the first postoperative year and then twice a year. All patients were reviewed by the surgeon to evaluate the need for band recalibration or to detect complication and, when necessary, by the dietitian and the psychologist.
RESULTS
Follow-Up
Mean follow-up was 13.0 months (range 1–49.5); it was more than 2 years in 185 patients. None of the 500 patients was lost to follow-up. Table 2 shows the distribution of patients by length of follow-up.
Table 2.CLASSIFICATION OF THE PATIENTS BY LENGTH FOLLOW-UP
Operative Data
Mean overall operative time was 105 minutes (range 20–380); it was 84 minutes for the last 300 operations. Band placement was impossible in seven patients (1.4%) because of gastric perforation (n = 2), large hiatal hernia (n = 2), large gastric diverticula at the angle of the cardia (n = 1), and enlarged left liver lobe with steatosis (n = 2). Conversion to laparotomy was necessary in 12 patients (2.4%), 2 during the last 300 procedures. Forty-three patients with gallstones underwent concomitant cholecystectomy. In this group the mean operative time was 150 minutes (range 90–220). No operative or postoperative death occurred. There were no transfusions.
Operative Morbidity
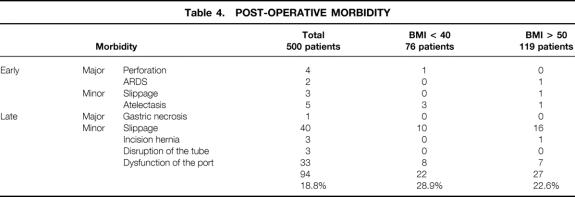
The overall morbidity rate was 18.8%. Ninety-four patients experienced complications, 14 in the early postoperative course and 80 during follow-up. Twenty-two patients (28.9%) with a BMI less than 40 and 27 patients (22.6%) with a BMI less than 50 had complications. Gastric perforation occurred in four patients (0.8%). Two were promptly recognized perioperatively: one was successfully treated by laparoscopic suturing and the other needed conversion to open surgery. The other two perforations were detected the next day by Gastrografin swallow and were successfully managed under laparotomy by suturing and band removal.
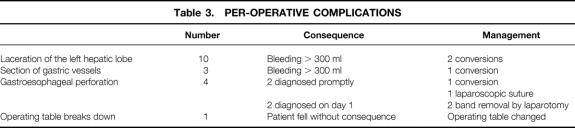
Among the first patients in our series, posterior slippage was detected by Gastrografin meal in three patients and required laparoscopic repositioning. Table 3 shows the perioperative complications and their management, and Table 4 lists postoperative complications.
Table 3.PER-OPERATIVE COMPLICATIONS
Table 4.POST-OPERATIVE MORBIDITY
Mean hospital stay was 4.5 days (range 3–42). Thirty-seven patients stayed in the intensive care unit for 3.8 days (range 1–35), eight patients because of postoperative complications: acute respiratory distress syndrome or atelectasis (n = 4), bleeding from liver laceration (n = 1), gastric perforation (n = 3), or early postoperative monitoring of sleep apnea (n = 29).
There were seven pulmonary complications. Five patients developed atelectasia treated by bronchoscopic aspiration and two patients had respiratory distress following conversion to laparotomy, leading to admission to the intensive care unit. Data on postoperative recovery are summarized in Table 5.
Table 5.POST-OPERATIVE RECOVERY

Reoperations
Among the above-mentioned complications, 52 patients required reoperation under general anesthesia. There were three major complications: one case of gastric necrosis necessitating gastrectomy because of significant band slippage and strangulation, and two perforations requiring esophageal suturing. Other complications requiring reoperation were as follows: 43 slippages, 3 incisional hernias secondary to conversion, and 3 port infections requiring catheter disconnection.
The mean delay for late slippage was 13.4 months (range 4–24). It was related to stomach intussusception through the band with subsequent dilatation of the pouch, resulting in acute food intolerance (n = 10) or pain and insufficient weight loss (n = 33) due to chronic dilatation. The prolapse was usually anterior and the band appeared in a horizontal position on a plain x-ray. The total number of slippages was 55: 12 of them were well tolerated, and in only 3 patients did the band need deflation. In 43 patients the band was removed laparoscopically because weight loss was considered satisfactory. In 10 other patients, another bariatric procedure was performed (a new gastric band was placed in five and a laparoscopic Mason procedure was performed in five). No slippage was observed in patients who underwent surgery after October 2000, when we began using the pars flaccida approach. Minor complications related to the access port occurred in 36 patients due to displacement (n = 22), local infection (n = 8), leakage (n = 3), and disconnection (n = 3). Five patients who had port infection underwent subsequent laparoscopy for catheter location and insertion of a new access port. Twenty-eight patients were successfully managed under local anesthesia.
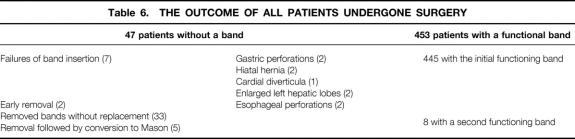
There was no clinical evidence of thromboembolic complications or gastric erosion related to the band. At the completion of our study, 501 bands were inserted in 491 patients, 448 patients had a functional band, 5 had a laparoscopic Mason procedure, and 47 did not have a band. Details are summarized in Table 6.
Table 6.THE OUTCOME OF ALL PATIENTS UNDERGONE SURGERY
Weight Loss and Band Management
Of the 491 patients with an inserted band, 37 were never calibrated because their intake was limited enough to achieve adequate weight loss. The other 454 patients were calibrated within 3 months postoperatively. Of these, 290 (48%) needed only one calibration adjustment, 110 had two calibration adjustments, 40 had three adjustments, and 14 patients had four or more calibrations during the 24-month follow-up. On three occasions, band slippage necessitated deflation without reoperation. During the year 2000, there were 342 adjustments of the balloon (24 deflations and 318 inflations).
The excessive weight loss was 42.8%, 52%, and 54.8% at 1, 2, and 3 years (Fig. 5). The mean BMI decreased from 44.3 to 34.2, 32.8, and 31.9 at, respectively, 1, 2, and 3 years (Fig. 6).
Figure 5. Excess weight loss from 0–48 months.
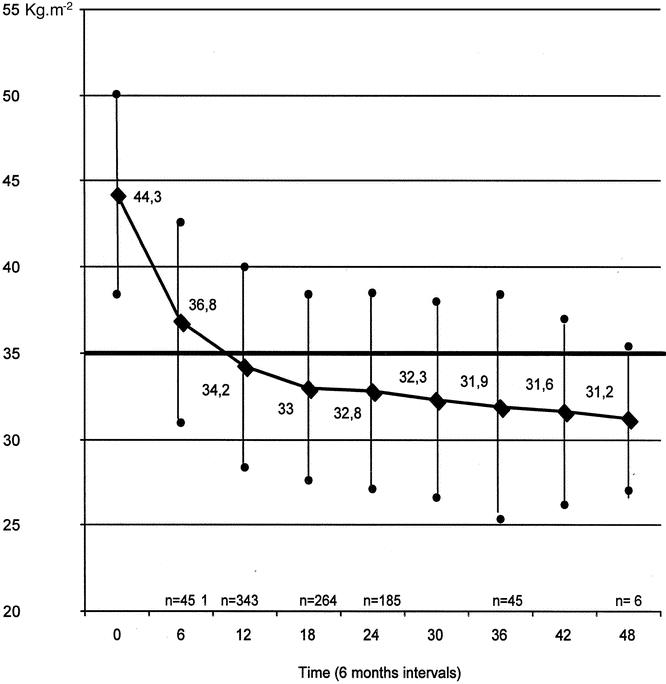
Figure 6. Change in BMI from 0–48 months.
There were 55 superobese patients (BMI > 50). These patients lost a great deal of weight but their BMI reduction was less significant (mean 37.2 after 2 years). This result was insufficient to protect them from obesity-related vital complications. Among the 343 patients with at least 1 year of follow-up, 16 (9%) were considered a failure with an excess weight loss of less than 20%. Among these, 11 were subsequently revealed to be bad candidates because their psychiatric profile showed either compulsive bulimic behavior or “sweets-eaters.”
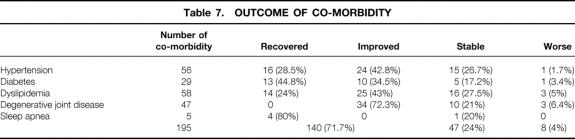
Evolution of Comorbidity
Table 7 demonstrates the outcome of comorbidity in patients with a follow-up greater than 2 years. With a total of 195 comorbidity occurrences in 185 patients, 140 (71.7%) improved or recovered, 47 (24%) remained unchanged, and 8 (4%) worsened. The 140 improved co-morbidity occurrences were observed in 133 patients; the 55 unchanged or worsened occurrences were seen in 55 patients. There was a significant improvement in the medical condition of these patients (P < .05).
Table 7.OUTCOME OF CO-MORBIDITY
Quality of Life
The mean BAROS scores were 3.64 at 6 months, 3.79 at 12 months, 4.38 at 18 months, and 5.20 at 24 months. The failure rate for quality of life improvement was 6%, fair results were noted in 18%, good results in 49%, and excellent results in 27%. Quality of life significantly improved with the excessive weight loss as early as 6 months in 76% of our patients.
DISCUSSION
As long as follow-up of any large cohort of patients with laparoscopic gastric banding remains under 4 years, the selection criteria for surgery should not be modified. The major indication for surgery remains limited to medical reasons; cosmetic considerations should never be taken into account, even though the procedure was judged to be easy and safe. It should be used only to treat morbid obesity with reduced life expectancy. 7
Alternative bariatric operations of the restrictive type are those that induce malabsorption via a jejunoileal, biliopancreatic, or gastric bypass. Gastric bypasses that allow patients to “lose weight while eating” show the best results, and excess weight loss was considerable: 55% and 70% at 1 and 5 years, respectively, 11 but at the cost of a fragile anastomosis and potentially serious nutritional deficiencies. Roux-en-Y gastric bypass has recently been adapted to laparoscopy with an acceptably low rate of complications (3.3% major and 27% minor) and an excellent weight loss of 77% at 30 months, but it remains a challenging procedure. 12 In a series of 275 patients, a total of 12 gastrointestinal leaks occurred (4.4%), 3 of them with postoperative peritonitis, and 1 patient died of secondary jejunojejunal obstruction. We consider these procedures difficult, lengthy (two to three times longer than our procedure), and more dangerous.
Of the restrictive procedures, which induce slow gastric emptying by creating a small pouch, Mason’s gastroplasty remains the gold standard. The vertical banded gastroplasty allows a 40% to 50% excess weight loss at 1 year 5 with a maximum efficacy at 12 to 18 months, declining somewhat thereafter but still efficient at 10 years.
Our gastric banding operation offers results comparable to the stapling technique. As a reversible and adaptable procedure, it promises to be a good alternative as long as its morbidity rate remains low, at least similar to the Mason’s operation. Prolonged follow-up should determine its efficacy in long-term weight control. None of our 500 patients died and 20% of them experienced complications, mostly minor. These results are comparable to other series. 9,13,14 The last 300 patients of our series had a lower morbidity rate than the first 200, demonstrating technical progress and follow-up enhancement, particularly reducing slippage and port dysfunction rates.
In earlier series, the band was inserted through the lesser sac of the peritoneum. 6 Consecutive modifications were subsequently introduced that aimed at reducing the pouch size (from 25–15 cc), 8 fixing the band behind the stomach in case it entered the lesser sac, 15 and covering it completely by anterior gastrogastric suturing. Subsequently, most authors recommend placing the band above the lesser sac outlined by the short gastric vessels, through the fibrous space under the mesoesophagus. 14,15 The slippage rate was 8.5% in our study, which was high compared to other series (3–9%9,13,14). To avoid band slippage we developed the pars flaccida approach. Since then (October 2000), no slippage was observed.
Late band slippage remained the most common complication for earlier patients. It led to gastric pouch dilatation. Its onset was delayed: 13 months in our study, 6 to 12 months by others. 9,14,15 It was probably related to too-loose stitching of the band on the anterior wall of the stomach and a premature intake of solid food. Dilatation of the gastric pouch usually produces an interruption in the weight loss curve and is readily diagnosed by Gastrografin or barium swallow. It necessitates prompt deflation of the band. Should food intolerance persist, laparoscopic band removal may be carried out, especially if excess weight loss was satisfactory; otherwise the band should be promptly repositioned or replaced. 13,14
In 11 patients with unreliable alimentary behavior, weight loss was inadequate: they admitted to violating their dietary restrictions with excessive intake of sweets (“sweets-eaters”) or by immoderate bulimic behavior. If this had been known preoperatively, these patients (nine women and two men) would have been classified as poor candidates for gastric restrictive surgery. At present, despite meticulous routine preoperative psychological evaluation, there is no foolproof method to detect these patients who are at risk of failure. They may be better candidates for gastric bypass.
Because of the dietary behavior of the French people, who have a reputation for elaborate cooking and a taste for good meals, most of our patients accept dietary re-education based on restricting quantity while preserving taste. A recent report on gastric banding by DeMaria et al showed discouraging results in a limited series of 37 obese American patients. 16 We believe that dietary re-education of patients is essential. The main reason for weight loss failure has so far been noncompliance with the dietitian’s advice. Patients do not need to be on a strict low-calorie diet but must avoid hypercaloric fluids and semifluids in spite of a well-functioning restriction band. We therefore attempt to preselect our patients carefully and turn down “sweets-eaters.” DeMaria et al also reported a high rate of esophageal dilatation (71%); this was considerably lower in our series (4/491). It is usually suspected when the patient’s weight starts to increase again, due to the loss of the restrictive function of the gastric pouch because of the new pouch of the dilated esophagus.
We showed in our study that laparoscopic gastric banding is efficient, with a reduction of the mean BMI from 44.3 to 34.2, 32.8, and 31.9 at 1, 2, and 3 years, respectively. Mean excessive weight loss was increased to 42.8%, 52%, and 54.8%, respectively. Improvement of quality of life was strongly demonstrated on personal, psychological, sexual, and social grounds. Near 80% of our patients lost 60% of their excess weight, improving their general condition and reducing cofactors of morbidity. 17 We showed a significant regression of comorbidity related to diabetes, 18 hypertension, 19 dyslipidemia, 20 coronary pathology, 21 and painful degenerative joint disease. This plays an important part in the improvement of health status and quality of life as well as in the reduction of medications.
CONCLUSIONS
In our experience, the Lap-Band System is well adapted to the French population. The operative risk is acceptable (we had no death) and the incidence of severe morbidity is low. The goal is to reduce the patient’s weight to decrease the high death rate due to obesity. This procedure should not be recommended for esthetic motivations, but only on medical grounds. The laparoscopic approach allows early mobilization, a short hospital stay, early return to work, and fewer wound complications. It produces at least 50% excess weight loss after 2 years in 80% of patients and improves the patient’s quality of life significantly. The device is partially reversible, allowing prompt return to the previous condition after weight loss and dietary re-education. The long-term risk of band slippage and the stabilization of weight loss need longer follow-up study. However, the risk of slippage was considerably decreased since we began using the pars flaccida approach.
Multidisciplinary care is essential in obese patients as it is a complex pathology. The surgeon, the nutritionist, and the psychologist must monitor the patient together during the entire weight loss program.
Footnotes
Correspondence: Franck Zinzindohoue, MD, Department of Digestive & General Surgery, Hopital Europeen G. Pompidou, 20-40 rue Leblanc, 75908 Paris, France.
E-mail: franck.zinzindohoue@hop.egp.ap-hop-paris.fr
Accepted for publication July 22, 2002.
References
- 1.Sjöström LV. Mortality of severely obese subjects. Am J Clin Nutr 1992; 55: 516–523. [DOI] [PubMed] [Google Scholar]
- 2.Oppert JM, Rolland-Cachera MF. Prévalence, évolution dans le temps et conséquences économiques de l’obésité. Med Sci 1998; 14: 939–943. [Google Scholar]
- 3.Goodrick GK, Foreyt JP. Why treatments for obesity don’t last. JAMA 1991; 91: 1243–1247. [PubMed] [Google Scholar]
- 4.Mason EE, Tang S, Renquist KE, et al. A decade of change in obesity surgery. Obesity Surg 1997; 7: 189–197. [DOI] [PubMed] [Google Scholar]
- 5.Nightengale ML, Sarr MG, Kelly KA, et al. Prospective evaluation of vertical banded gastroplasty as the primary operation for morbid obesity. Mayo Clin Proc 1991; 66: 773–782. [DOI] [PubMed] [Google Scholar]
- 6.Belachew M, Legrand M, Vincent V, et al. L’approche coelioscopique dans le traitement chirurgical de l’obésité morbide. Technique et résultats. Ann Chir 1997; 51: 165–172. [PubMed] [Google Scholar]
- 7.National Institutes of Health Consensus Conference. Gastrointestinal surgery for severe obesity. Am J Clin Nutr 1992; 55: 487S–619S. [DOI] [PubMed] [Google Scholar]
- 8.Cadière GB, Bruyns J, Himpens J, et al. Laparoscopic gastroplasty for morbid obesity. Br J Surg 1994; 81: 1524. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien PE, Brown WA, Smith A, et al. Prospective study of a laparoscopically placed, adjustable gastric band in the treatment of morbid obesity. Br J Surg 1999; 86: 113–118. [DOI] [PubMed] [Google Scholar]
- 10.Oria EH, Moorehead KM. Baratric analysis and reporting outcome system (BAROS). Obes Surg 1998; 8: 487–497. [DOI] [PubMed] [Google Scholar]
- 11.Pories WJ. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995; 222: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg 2000; 232: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dargent J. Coeliochirurgie de l’obésité morbide: la gastroplastie par anneau modulable, 320 observations. Ann Chir 1999; 53: 467–471. [PubMed] [Google Scholar]
- 14.Zimmermann JM, Mashoyan PH, Michel G, et al. Laparoscopic adjustable silicone gastric banding: une étude préliminaire personnelle concernant 900 cas opérés entre Juillet 1995 et Décembre 1998. Jour de Coelio-chir 1999; 29: 25–31. [Google Scholar]
- 15.Favretti F, Cadiere GB, Segato G, et al. Laparoscopic adjustable silicone gastric banding: How to avoid complications. Obes Surg 1997; 7: 352–358. [DOI] [PubMed] [Google Scholar]
- 16.DeMaria EJ, Sugerman HJ, Meador JG, et al. High failure rate after laparoscopic adjustable silicone gastric banding for treatment of morbid obesity. Ann Surg 2001; 233: 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brolin RE, Kenler A, Gorman RC, et al. The dilemma of outcome assessment after operations for morbid obesity. Surgery 1989; 105: 337–345. [PubMed] [Google Scholar]
- 18.Krall JG, Heymsfield SB. Morbid Obesity. Gastroenterol Clin North Am 1987; 16: 255–275. [PubMed] [Google Scholar]
- 19.Bell RM, Bivins BA, Griffen W. The effect of surgically induced weight loss on hypertension clinical trends. J Chronic Dis Ther Res 1982; 6: 126–132. [Google Scholar]
- 20.Gonen B, Halverson JD, Schonfeld G. Lipoprotein levels in morbidity obese patients with massive surgically-induced weight loss. Metabolism 1983; 32: 492–496. [DOI] [PubMed] [Google Scholar]
- 21.Hall JC, Watts J, O’Brien PE. Gastric surgery for morbid obesity. Ann Surg 1990; 211: 419–42 [DOI] [PMC free article] [PubMed] [Google Scholar]