Abstract
Objective
To assess the oncologic and cosmetic outcomes in women with breast carcinoma who were treated with breast-conserving therapy using oncoplastic techniques with concomitant symmetrization of the contralateral breast.
Summary Background Data
Although breast-conserving therapy is the standard form of treatment for invasive breast tumors up to 4 cm, in patients with large, ill-defined, or poorly situated tumors, cosmetic results can be poor and clear resection margins difficult to obtain. The integration of oncoplastic techniques with a concomitant contralateral symmetrization procedure is a novel surgical approach that allows wide excisions and prevents breast deformities.
Methods
This is a prospective study of 101 patients who were operated on for breast carcinoma between July 1985 and June 1999 at the Institut Curie. The procedure was proposed for patients in whom conservative treatment was possible on oncologic grounds but where a standard lumpectomy would have led to poor cosmesis. Standard institutional treatment protocols were followed. All patients received either pre- or postoperative radiotherapy. Seventeen patients received preoperative chemotherapy to downsize their tumors. Mean follow-up was 3.8 years. Results were analyzed statistically using Kaplan-Meier estimates.
Results
Mean weight of excised material on the tumor side was 222 g. The actuarial 5-year local recurrence rate was 9.4%, the overall survival rate was 95.7%, and the metastasis-free survival rate was 82.8%. Cosmesis was favorable in 82% of cases. Preoperative radiotherapy resulted in worse cosmesis than when given postoperatively.
Conclusions
The use of oncoplastic techniques and concomitant symmetrization of the contralateral breast allows extensive resections for conservative treatment of breast carcinoma and results in favorable oncologic and esthetic outcomes. This approach might be useful in extending the indications for conservative therapy.
Breast-conserving therapy (BCT), consisting of lumpectomy followed by radiotherapy, has become the standard form of treatment for invasive breast carcinomas up to 4 cm 1 and 5 cm 2,3 and is increasingly being used for ductal carcinoma in situ (DCIS) 4–6 and larger tumors. 7–9 However, with BCT for large tumors, there can be difficulty in obtaining clear excision margins, and the cosmetic outcome is often poor. 10,11 The tumor size in relation to the breast size is one of the most important factors when attempting to obtain a cosmetically favorable result. A conflict exists, therefore, between performing a resection wide enough to obtain optimal oncologic control and not removing so much breast tissue as to leave a deformed breast or a large discrepancy compared with the other side. One way of resolving this conflict is to use plastic surgery techniques such as remodeling mammaplasty to reshape the breast immediately following lumpectomy. This novel approach, referred to as “oncoplastic surgery” (W. Audretsch), has rapidly gained acceptance in Europe and is now widely practiced in some dedicated breast units. 12–18 When a remodeling mammaplasty is performed, because of the volume excised, contralateral symmetrization is often indicated to achieve symmetry. The aim of this prospective study was to assess the oncologic and cosmetic outcomes for patients who underwent BCT using oncoplastic techniques with a symmetrizing procedure on the opposite breast.
METHODS
One hundred and one consecutive women with breast carcinoma who underwent wide lumpectomy with remodeling mammaplasty and a symmetrizing contralateral mammaplasty at the Institut Curie between July 1985 and June 1999 were entered into a prospective study. Criteria for case selection included: patients with large tumors in whom a standard lumpectomy would have lead to breast deformity or gross asymmetry between the breasts, and the possibility of obtaining wide, clear margins of excision for the tumor with BCT. The contralateral symmetrizing procedure was performed concomitantly in 89 patients and at a later date in 12 patients. Patient and tumor characteristics, details of adjuvant therapy, surgical intervention, and complications of surgery were all entered into a computerized database. No patients were lost to follow-up. Patients were examined for postoperative complications, local or systemic recurrence of cancer, and cosmetic results. Radiotherapy to the breast and lymph nodes, chemotherapy, endocrine therapy, and axillary lymph node dissection were carried out without modification to our standard protocols. Seventeen patients who had a very large relative ratio between tumor and breast volumes received preoperative chemotherapy 19 to downsize the tumor. All patients were treated with postoperative radiotherapy, except for 13 patients who received it preoperatively because of a protocol at that time for large tumors. 20
Patient and Tumor Characteristics
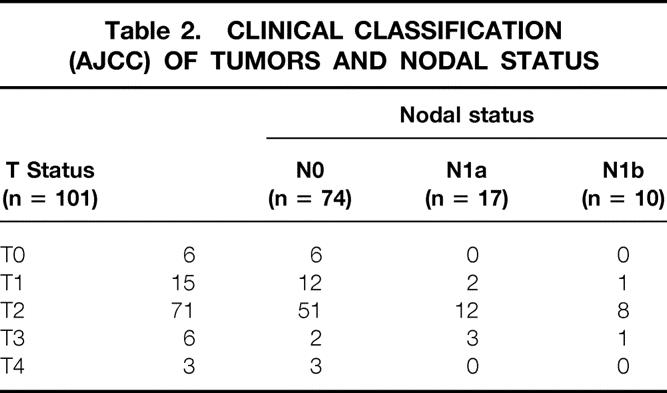
The average patient age was 53 years (range 31–91). Forty-seven patients (46.5%) were premenopausal. Fifty-four patients (53.5%) were postmenopausal; of these, 14 were receiving hormone replacement therapy at the time of initial diagnosis. The average size of the tumor determined clinically was 32 mm (range 10–70). Tumor histology is shown in Table 1. There were 93 patients with invasive carcinoma (ductal or lobular) and eight patients with DCIS or microinvasive carcinoma. The American Joint Committee on Cancer (AJCC) classification of the tumors is shown in Table 2. Three patients had skin involvement. Nine patients presented with no palpable mass and had microcalcifications on the mammogram. Ninety-one tumors were situated in the central area of the breast or in the inferior quadrants. Eight were in the superolateral and two were in the superomedial quadrants.
Table 1.HISTOLOGY OF TUMORS
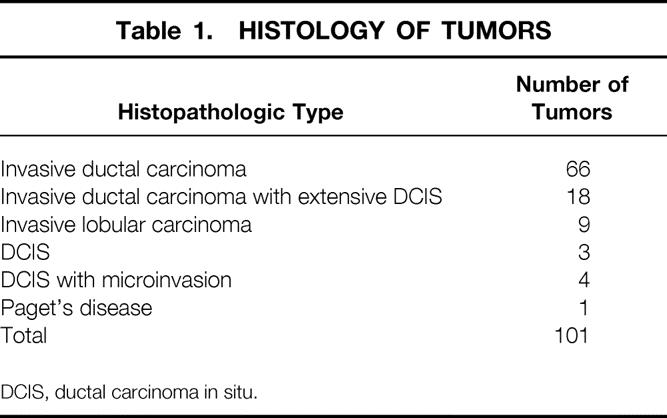
DCIS, ductal carcinoma in situ.
Table 2.CLINICAL CLASSIFICATION (AJCC) OF TUMORS AND NODAL STATUS
Surgical Procedure
For the first 15 patients operated on, there was a two-team approach (oncologic surgeon and plastic surgeon). All other patients were operated on by surgeons trained in both specialties. Preoperative markings were done with the patient in the upright position (Fig. 1). In 83% of cases, the mammaplasty involved a superior pedicle technique with an inverted T-scar, because the majority of tumors were situated in the central or inferior quadrants of the breast. The area surrounding the nipple–areolar complex (NAC) was de-epithelialized (Fig. 2). The next step involved the inframammary incision and wide undermining of the breast tissue off the pectoral fascia commencing inferiorly and proceeding superiorly beneath the tumor, the NAC (Figs. 2 and 3), and the medial and lateral aspects of the breast. The NAC was raised on a superiorly based flap (Fig. 4). The excision was then performed with the aim of incorporating the tumor excision with at least a 1-cm macroscopic margin of normal tissue, the skin overlying the tumor (if there was skin involvement or tethering by the tumor), and the tissue excised for the remodeling procedure as an en-bloc specimen (Fig. 5). The mobilization of the breast tissue allowed palpation of both deep and superficial surfaces of the tumor and aided in determining the lateral margins of excision around it. The remodeling procedure involved apposing the two medial and lateral glandular columns in the midline to fill in the defect and recentralization of the NAC to recreate a harmonious size and shape (Figs. 6 and 7). 21 When that side was completed, a contralateral mammaplasty using the identical technique was performed to achieve symmetry (Fig. 8). Other techniques were used for the rest of the cases and included the posterior pedicle technique (3%), 22 free nipple graft (6%), 23 or other procedures (8%). 24
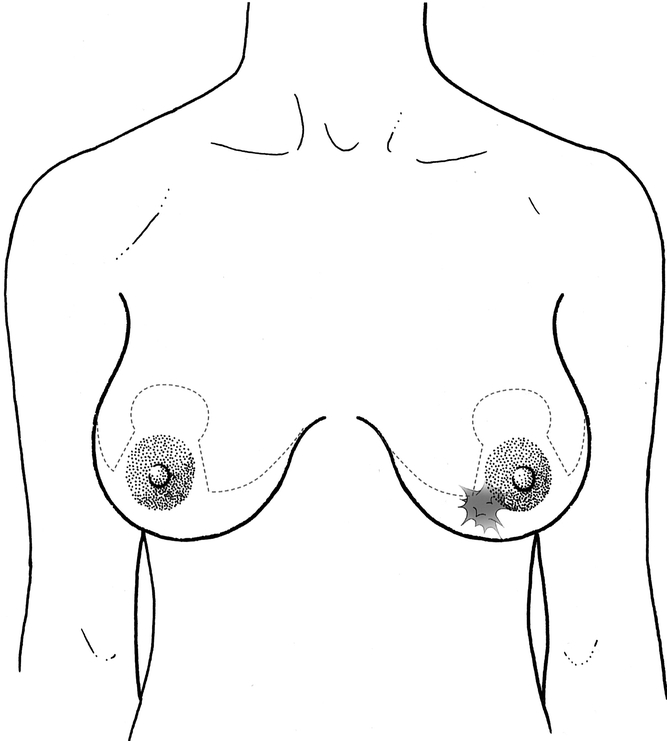
Figure 1. Preoperative skin markings done in the upright position, showing tumor location and dotted line for skin incision.
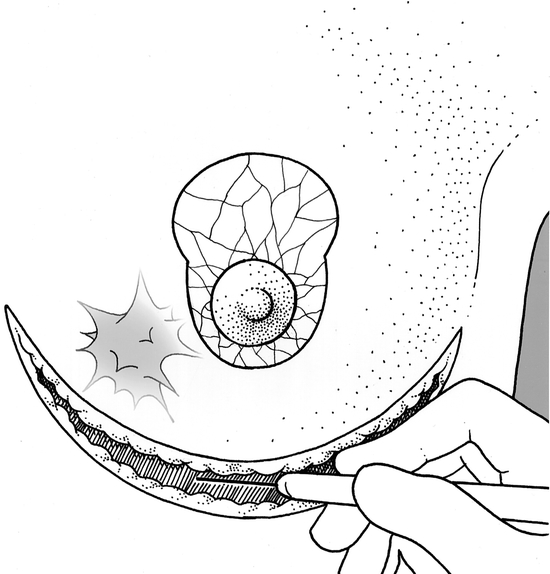
Figure 2. The area surrounding the nipple–areolar complex de- epithelialized and the inframammary skin incision.
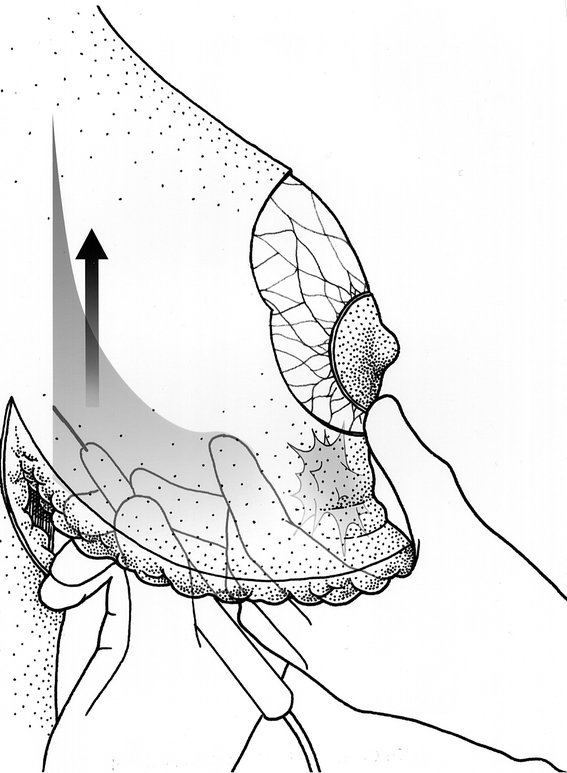
Figure 3. Undermining the breast off the pectoral fascia and palpation of the tumor.
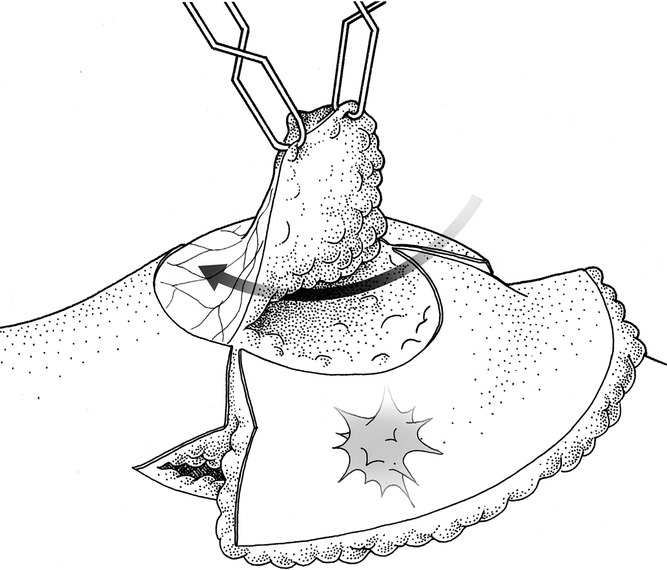
Figure 4. Developing the superiorly based flap for the nipple–areolar complex.
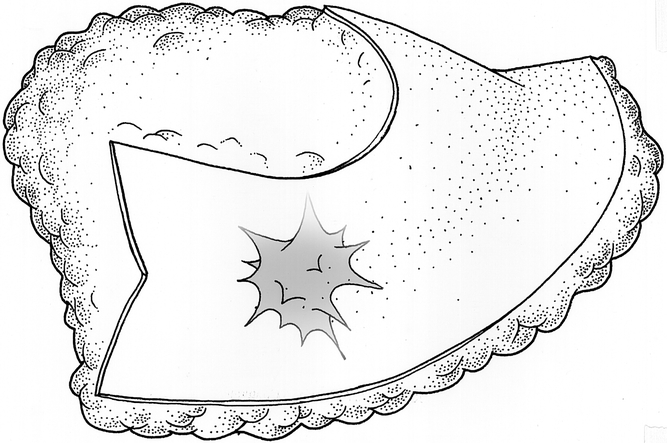
Figure 5. Excised tissue consisting of en-bloc specimen of tumor with wide margin of normal tissue and tissue excised for mammoplasty.
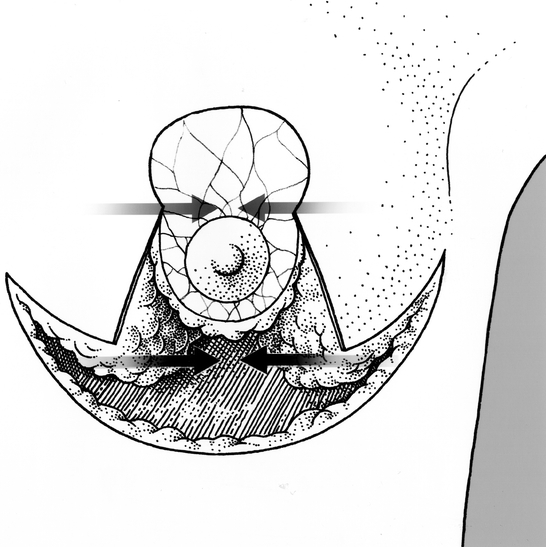
Figure 6. The residual defect. Arrows indicate apposition of medial and lateral pillars of gland.
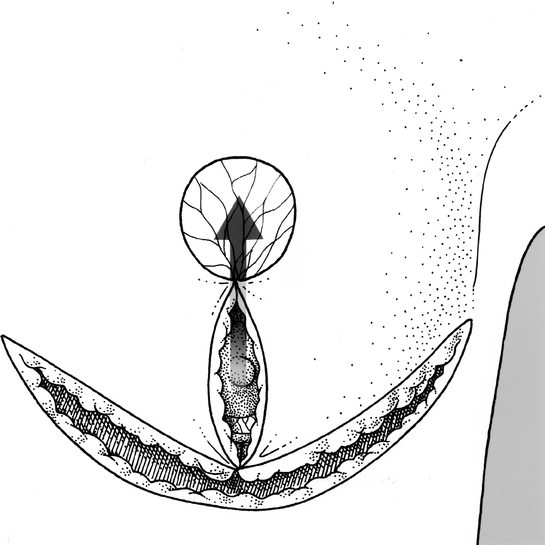
Figure 7. Reshaping the breast. Arrow indicates relocation of nipple–areolar complex to the de-epithelialized area.
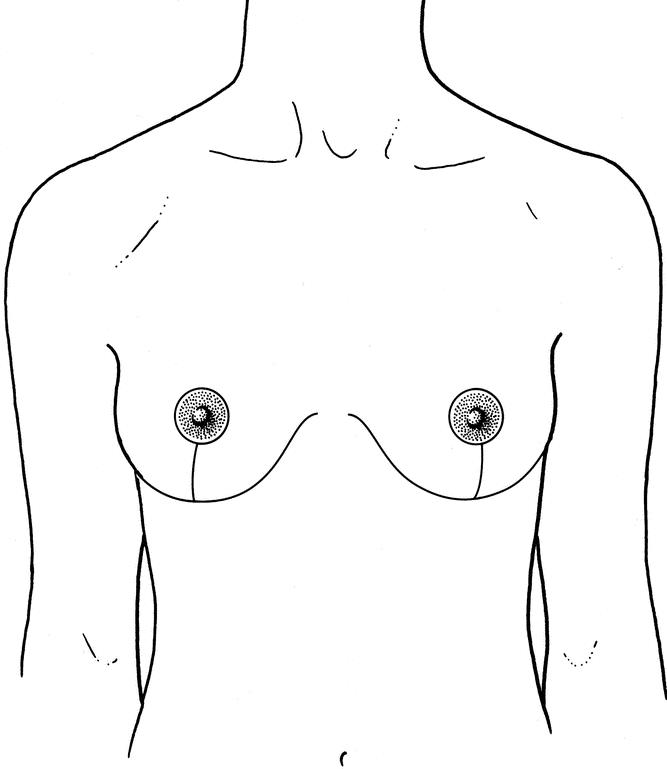
Figure 8. Resultant scars on both breasts after the procedure.
Axillary lymph node dissection (levels 1 and 2) was performed in 94 cases, using the horizontal submammary incision in 32 cases and a separate axillary incision in the rest. The lumpectomy specimen, complementary tissue excised for the remodeling procedure and axillary lymph node dissection, and the contralateral breast resection specimen were sent for histopathologic examination.
Statistical Analysis
Data were collected from the Institut Curie breast tumor database and clinical and pathologic case records of each patient. The Kaplan-Meier method was used to compute the probability of survival as a function of time. End points were survival (calculated from date of surgery to death or date of last follow-up), local recurrence, and metastasis-free interval. Five-year rates were expressed with their 95% confidence intervals. Late complications and cosmetic outcome were assessed using the Kaplan-Meier product limit survival curve. The effect of preoperative radiotherapy on cosmetic outcome over time was analyzed using the log-rank test. In these analyses the follow-up of each patient continued until the patient died or the date of the last follow-up contact was reached.
Follow-Up
All patients were reviewed by the surgeon, radiotherapist, and medical oncologist every 4 months for the first 3 years and then every 6 months thereafter. Bilateral mammograms were performed annually. A grading system was used for the cosmetic evaluation that has been described previously;14,25 briefly, a score of 5 to 1 (5 = excellent; 4 = good; 3 = fair; 2 = mediocre; 1 = poor) was given for five specific cosmetic parameters: volumetric symmetry of breasts, shape of breast mounds, symmetry of NAC placement, ipsilateral and contralateral scars, and postirradiation sequelae. Patients were reviewed by a panel of three people, including the surgeon and two nonmedical personnel, and given a score. Those with an average score of three or more were considered to have an acceptable result.
RESULTS
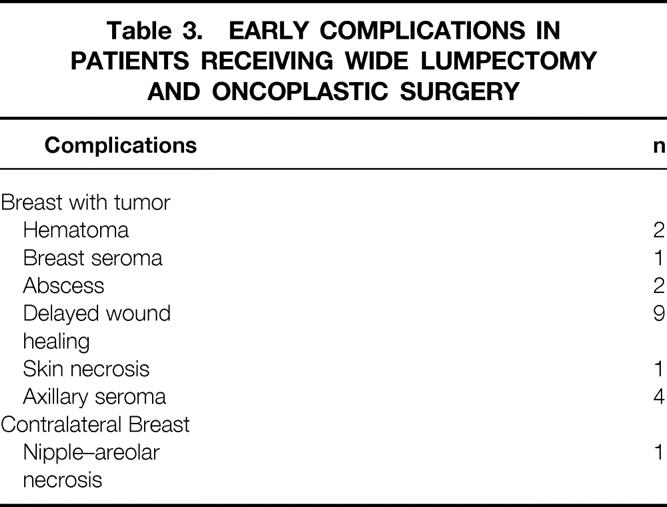
Mean operative time was 2 hours (range 86–162 minutes). Mean weight of breast tissue excised from the breast containing the tumor was 222 g (range 20–1,450). Mean weight of breast tissue from the contralateral normal breast was 264 g (range 20–1,900). Assessment of excision margins showed complete excision of the tumor in 90 patients; in 11 cases the margins were involved. The margins of the specimens were focally involved (<3 mm of the inked surface involved with tumor) in four cases and extensively involved (>3 mm of the inked surface involved with tumor) in three cases. In four cases the degree of margin involvement was not available. For patients with involved margins, six patients proceeded to a modified radical mastectomy and five patients had a boost to the tumor bed. In one case DCIS was found in the opposite breast; this patient received radiotherapy to both breasts. The average hospital stay was 6 days (range 2–12). There were early complications (<2 months after operation) in 20% of patients (Table 3). Of these, four patients required a reoperation. Four patients with delayed wound healing had a delay to their radiotherapy treatment, and in one of these patients chemotherapy was also delayed. Late complications (>2 months after operation) were mainly cosmetic and consisted of fat necrosis (three cases), breast fibrosis (three cases), and hypertrophic scarring (three cases). Two patients experienced breast pain and in one patient, wound healing took more than 2 months. There were more complications in patients who received preoperative rather than postoperative radiotherapy (10.8% vs. 27.0%, P = .18). Patients who received preoperative chemotherapy did not have more complications than the rest of the group.
Table 3.EARLY COMPLICATIONS IN PATIENTS RECEIVING WIDE LUMPECTOMY AND ONCOPLASTIC SURGERY
Tumor Recurrence and Patient Survival
Median follow-up was 46 months (range 7–168). Seven patients developed an ipsilateral breast recurrence. Three patients had a recurrence in the tumor bed and three patients in a different quadrant. One patient developed both a tumor bed recurrence and a recurrence in a different quadrant. Two patients developed a cancer in the contralateral breast. Thirteen patients developed metastases and eight died of the disease. The 5-year actuarial local recurrence rate was 9.4% (range 1.8–16.9%), and the 5-year actuarial overall survival and metastasis-free survival rates were 95.7% (range 91–100%) and 82.8% (range 72.5–93.2%), respectively.
Cosmetic Results
Six of the initial 101 patients who had a mastectomy for involved margins were excluded from the analysis. Of the remaining 95 patients, 88% had an acceptable result (excellent, good, or fair) at 2 years. This rate stabilized and was maintained in 82% of patients at 5 years. Clinical case examples are shown in Figures 9 and 10. In patients with poor results, the major cosmetic flaw was that the residual breast volume was too small in relation to patient morphology. Postoperative radiotherapy did not alter the cosmetic result, and good breast symmetry was maintained over time, but the cosmetic result was related to the timing of radiotherapy in the therapeutic sequence. Results were worse in the 13 patients who received preoperative rather than postoperative radiotherapy (42.9% vs. 12.7%, P < .002).
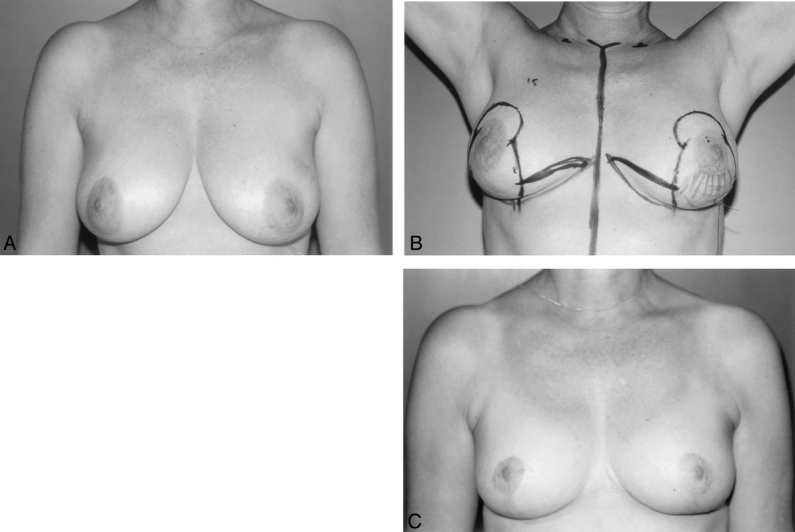
Figure 9. A 52-year-old woman with extensive microcalcifications in the lower pole of the left breast. (A) Core biopsy shows DCIS. A wire has been inserted under radiologic control to localize the tumor. (B) Preoperative skin markings. Hatched area indicates location of microcalcifications. Bold lines indicate lines of skin incision. (C) Two-year postoperative result after receiving postoperative radiotherapy to the left breast.
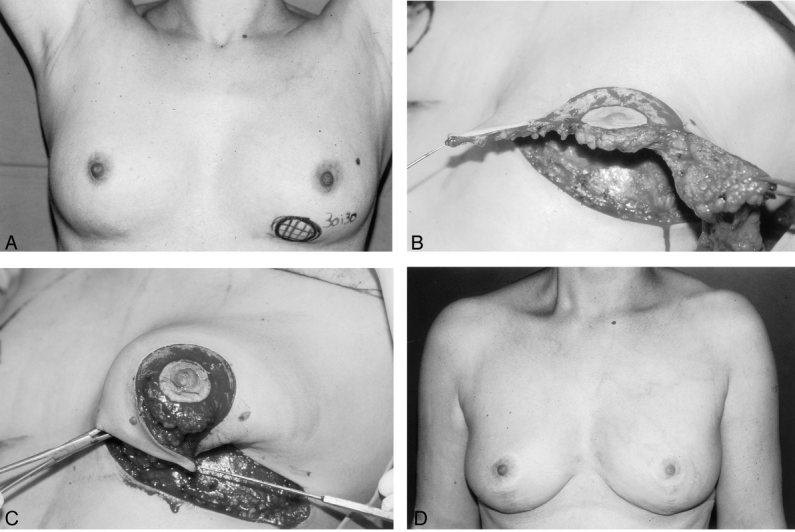
Figure 10. A 43-year-old woman with a 3 × 3-cm palpable invasive ductal carcinoma. (A) Mammogram showed extensive microcalcifications in the lower pole of the left breast. (B) After excision of the tumor and microcalcifications as an en-bloc specimen with tissue excised for the remodeling procedure. (C) The remodeling procedure: apposition of medial and lateral glandular pillars. (D) The result at 6 years after radiotherapy to the left breast.
DISCUSSION
The indications for BCT in breast cancer are expanding. The integration of plastic surgery techniques with BCT (oncoplastic surgery) in the treatment of breast cancer is a new approach that allows extensive resections. With a median weight of 222 g for our excision specimens, our study shows that it is feasible and can be done without modification to standard treatment protocols. The actuarial 5-year survival rate of 95.7%, metastasis-free survival rate of 82.8%, and local recurrence rate of 9.4% show that it is a safe approach. Furthermore, cosmetic results are favorable and are maintained in the long term.
Prospective randomized clinical trials have shown that BCT followed by radiotherapy gives equivalent survival rates for tumors up to 5 cm 2,3 compared with mastectomy. While there is a move toward treating larger tumors with BCT, one of the major limitations is the ability to perform a large enough resection without compromising the cosmetic result. The larger the tumor, the greater the risk of lumpectomy margins being involved with tumor. 26 There is a conflict, therefore, between obtaining an adequate excision margin around the tumor and not removing too much tissue, which might deform the breast. This conflict can be resolved in several ways. First, large or poorly situated tumors can be resected with the acceptance that there will be residual breast deformity, and the expectation that secondary reconstructive surgery can be offered at a later date. This raises false hopes, as these cases are difficult to treat, with the irradiated tissues responding poorly to surgery. Such reconstructions can involve complex flap procedures and often result in a disappointing and poor cosmetic result. 10,11,27 Second, BCT can be abandoned and the patient offered a mastectomy and a total breast reconstruction. Oncoplastic surgery offers a third modality of treatment. The breast can be reshaped immediately and the contralateral breast made to look like it. The patient leaves the operating room without asymmetry or deformity, and the whole procedure is done in a single sitting. There is the added psychological benefit to patients not having to undergo a second operation.
In the early stages of this study, oncoplastic mammaplasty was offered only to a selected number of patients with relatively large tumors in comparison to their breast volume; in this group, a standard lumpectomy was not feasible or would have resulted in a definite deformity. This included patients who had a poor or no response to preoperative chemotherapy 9 or radiotherapy 20 and patients for whom preoperative therapy was contraindicated because of their age or concurrent medical illness. We then extended the indications to patients who required a large excision margin for a poorly defined tumor or a large area of microcalcifications on the mammogram. There were 21 patients in our series with T0 and T1 tumors (eight patients had noninvasive or microinvasive carcinomas). Patients were also selected with regard to the location of the tumor in the breast. Ninety-one patients had a lower pole or central tumor location. These patients were selected because the cosmetic result following simple lumpectomy at these sites is poor and the breast is very difficult to reconstruct secondarily (Fig. 11). 10,14
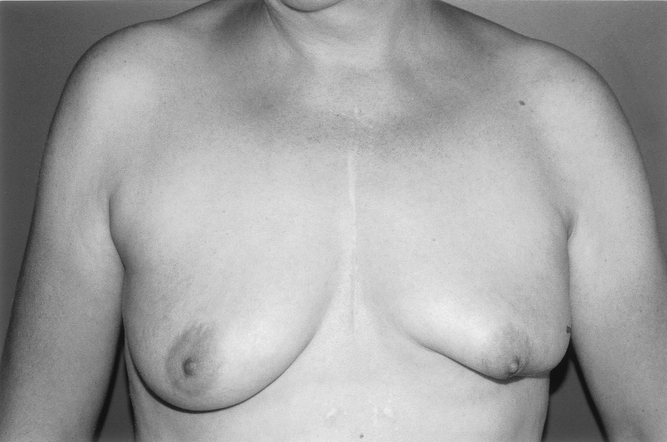
Figure 11. Typical breast deformity after breast-conserving surgery for a large tumor of the lower pole of the left breast.
Oncoplastic surgery is thus a useful tool in different situations. Although the indications for such an approach were rare in the initial years of this study, the results obtained encouraged us to integrate oncoplastic surgery into our surgical protocol every time a standard lumpectomy would not be suitable, where there was the need for a large resection, or where there was a high risk of deformity.
In terms of oncologic results, the 5-year local recurrence rate was 9.4%, which is comparable to results from previous studies, 2,3 even though we were treating relatively large tumors (median size 32 mm). The 5-year actuarial overall survival and metastasis-free survival rates were 95.7% and 82.8%, respectively, demonstrating the safety of these wide resections. However, although the first patients in this series were operated on more than 15 years ago, a longer follow-up of the whole population would be necessary to verify these results. To our knowledge there has only been one study, by Cothier-Savey et al, 15 reporting specifically on the oncologic results following oncoplastic surgery. Our results compare favorably with theirs, which were an actuarial 5-year local recurrence rate and overall survival rate of 8.5% and 86%, respectively, in a group of 70 patients who had oncoplastic surgery for breast carcinoma. Other authors have reported on the favorable cosmetic outcomes with oncoplastic surgery in the treatment of breast cancer but have not included the oncologic outcomes in their series. 13,17
While complete excision of the tumor with clear margins is mandatory, there is still debate about the optimal amount of normal tissue that should be removed with the tumor. The average tumor size in our group was 32 mm (range 10–70). We aimed for at least a 1-cm macroscopic margin around the tumor, but as it was removed as an en-bloc specimen with the tissue removed for the mammaplasty, there was frequently a much wider rim of normal tissue around the tumor. The use of oncoplastic techniques for our group of patients has shown that the cosmetic results are good, despite the relatively large excisions, and were well maintained over time in 82% of patients. The average weight of the resected en-bloc tumor specimen in our series was 220 g compared to the average weight of a lumpectomy specimen, which in our institute is 40 g. Some authors advocate very wide excision margins and even quadrantectomies for invasive ductal carcinoma. 28 Oncoplastic techniques may reduce the incidence of poor cosmesis with these very wide excisions.
Preoperative chemotherapy complicates the debate about margins of excision. For large tumors, it is our standard practice to give preoperative chemotherapy to downsize the tumor and resect the remaining palpable tumor after four to six cycles of chemotherapy. 9 However, pathologic examination of resected tissue in patients after chemotherapy has shown multiple foci of scattered residual tumor cells interspersed with marked fibrosis. 29 It may indeed be that it is necessary to remove a large margin of tissue around the residual tumor after initial chemotherapy. In our series, preoperative chemotherapy did not have an adverse effect on either the ability to obtain clear excision margins or the final cosmetic outcome, although there were only 17 patients in this group. Another option for downsizing large tumors is the use of preoperative radiotherapy, which was a protocol used extensively in our institution as well as others 30 before the era of preoperative chemotherapy. Although this is a valuable approach when followed by lumpectomy, we do not advocate preoperative radiotherapy before oncoplastic surgery and have now abandoned this practice because of the large number of early postoperative complications (27%) and poor cosmetic results (42.9%) compared with postoperative radiotherapy. Thus, this approach is fully compatible with pre- and postoperative chemotherapy and postoperative radiotherapy. The overall early complication rate of 20% seems relatively high but is explained by the prospective data collection of our study, which had us record every event (see Table 3) and included minor complications such as collections of seroma and minor delays in wound healing. Only four patients had a delay in postoperative treatment because of a complication.
In the long term, there have not been any difficulties in the follow-up of these patients. Clinical and radiologic follow-up has not been affected by the remodeling procedure, and mammographic changes are not a hindrance to radiographic evaluation.
Cosmesis following BCT and radiotherapy is worse in large-breasted versus small-breasted women. Gray et al, in their series of 257 patients undergoing BCT followed by radiotherapy, found that there was more asymmetry and retraction in the large-breasted (D-cup or larger) versus the small-breasted group, and that telangiectasia continued to worsen up to 5 years after surgery. 31 Moody et al, in their study of 559 women, reported that there were late radiation changes in 6% of small breasts versus 39% of large breasts. 32 Several reasons have been put forward to explain this. One of them is that there is a greater dose inhomogeneity in large breasts, possibly because of the greater dose separation in larger breasts or the poorer daily set-up reproduction in obese women. Another reason is that there is an increased fat content in large breasts, and the fatty tissue results in more fibrosis after radiotherapy than glandular tissue. 33 Oncoplastic surgery, which reduces the volume of the residual breast, might have a role in reducing these cosmetic problems for patients with large or fatty breasts. Another potential advantage to the reduction in the overall residual breast volume is that it might optimize radiotherapy treatment by reducing the inhomogeneous dosing that is found in larger breasts. 32
The incidence of a contralateral carcinoma is higher in women who already have a breast carcinoma. The contralateral mammaplasty gives an opportunity to palpate and visually examine tissue from the opposite breast, and this tissue can be analyzed histologically. Rietjens et al 34 reported a 4.5% incidence of occult carcinomas in the contralateral breast in a series of 440 patients undergoing contralateral reduction mammaplasty for breast reconstruction. In our series one patient had a contralateral cancer detected on histologic specimen analysis. Therefore, it does seem logical to offer women an immediate contralateral symmetrizing procedure at the same time as the remodeling mammaplasty. 35–37
While this is a useful method for obtaining acceptable oncologic control for breast cancer with satisfactory cosmetic results, it does require an operation to the contralateral breast; some women may not feel comfortable with this at the same time as their oncologic procedure. In our series of women, however, it was very well accepted, especially when done concomitantly. Another disadvantage is that the operating time is longer (average of 2 hours) and the procedure requires expertise in both plastic and oncologic surgery techniques. Sometimes surgeons from both specialties must be present if a dually qualified surgeon is not available, and this can be difficult to arrange logistically. In our department we have the advantage of surgeons who are trained in both oncologic and plastic surgery. Finally, there are limitations as to the type of patient to whom this procedure can be offered. Patients with very small breasts who would be left with very little tissue after a wide excision are not good candidates for such an approach.
CONCLUSIONS
The combination of plastic surgery techniques with oncologic surgery (oncoplastic surgery) gives the surgeon a new tool for the treatment of breast cancer. In selected cases, this approach has allowed us to perform wide resections and obtain good oncologic control with favorable cosmesis. Without compromising the multidisciplinary approach to breast carcinoma, oncoplastic surgery may have a role to play in extending the indications of BCT by allowing breast conservation and good cosmesis despite wide excisions.
Footnotes
Correspondence: Krishna B. Clough, MD, Department of General and Breast Surgery, Institut Curie, 26 rue d’Ulm, 75248 Paris, France.
E-mail: krishna.clough@curie.net
Accepted for publication December 5, 2001.
References
- 1.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 1995; 333: 1456–1461. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst 2000; 92: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 1995; 332: 907–911. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Dignam J, Wolmark N, et al. Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 1998; 16: 441–452. [DOI] [PubMed] [Google Scholar]
- 5.Julien JP, Bijker N, Fentiman IS, et al. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet 2000; 355: 528–533. [DOI] [PubMed] [Google Scholar]
- 6.Solin LJ, Kurtz J, Fourquet A, et al. Fifteen-year results of breast-conserving surgery and definitive breast irradiation for the treatment of ductal carcinoma in situ of the breast. J Clin Oncol 1996; 14: 754–763. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997; 15: 2483–2493. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GF, Birchansky CA, Komarnicky LT, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 1994; 73: 362–369. [DOI] [PubMed] [Google Scholar]
- 9.Scholl SM, Asselain B, Palangie T, et al. Neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer 1991; 27: 1668–1671. [DOI] [PubMed] [Google Scholar]
- 10.Clough KB, Cuminet J, Fitoussi A, et al. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg 1998; 41: 471–481. [DOI] [PubMed] [Google Scholar]
- 11.Berrino P, Campora E, Santi P. Postquadrantectomy breast deformities: classification and techniques of surgical correction. Plast Reconstr Surg 1987; 79: 567–572. [PubMed] [Google Scholar]
- 12.Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspectives in Plastic Surgery 1998; 11: 71–100. [Google Scholar]
- 13.Petit JY, Rietjens M, Garusi C, et al. Integration of plastic surgery in the course of breast-conserving surgery for cancer to improve cosmetic results and radicality of tumor excision. Recent Results Cancer Res 1998; 152: 202–211. [DOI] [PubMed] [Google Scholar]
- 14.Clough KB, Nos C, Salmon RJ, et al. Conservative treatment of breast cancers by mammaplasty and irradiation: a new approach to lower quadrant tumors. Plast Reconstr Surg 1995; 96: 363–370. [DOI] [PubMed] [Google Scholar]
- 15.Cothier-Savey I, Otmezguine Y, Calitchi E, et al. [Value of reduction mammoplasty in the conservative treatment of breast neoplasms. Apropos of 70 cases]. Ann Chir Plast Esthet 1996; 41: 346–353. [PubMed] [Google Scholar]
- 16.Huter J. [Tumor-adapted oncoplastic mastopexy and reduction-plasty]. Zentralbl Gynakol 1996; 118: 549–552. [PubMed] [Google Scholar]
- 17.Laxenaire A, Barreau-Pouhaer L, Arriagada R, Petit JY. [Role of immediate reduction mammaplasty and mammapexy in the conservative treatment of breast cancers]. Ann Chir Plast Esthet 1995; 40: 83–89. [PubMed] [Google Scholar]
- 18.Kohls A. [Oncoplastic variations in surgical treatment of pT2 breast carcinoma]. Zentralbl Chir 1998; 123: 113–115. [PubMed] [Google Scholar]
- 19.Scholl SM, Fourquet A, Asselain B, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 1994; 5: 645–652. [DOI] [PubMed] [Google Scholar]
- 20.Calle R, Pilleron JP, Schlienger P, et al. Conservative management of operable breast cancer: ten years’ experience at the Foundation Curie. Cancer 1978; 42: 2045–2053. [DOI] [PubMed] [Google Scholar]
- 21.Pitanguy I. Surgical treatment of breast hypertrophy. Br J Plast Surg 1967; 20: 78–85. [DOI] [PubMed] [Google Scholar]
- 22.Hester TR Jr, Bostwick J 3d, Miller L, Cunningham SJ. Breast reduction utilizing the maximally vascularized central breast pedicle. Plast Reconstr Surg 1985; 76: 890–900. [DOI] [PubMed] [Google Scholar]
- 23.Thorek M. Possibilities in the reconstruction of the human form 1922. Aesthetic Plast Surg 1989; 13: 55–58. [DOI] [PubMed] [Google Scholar]
- 24.Soussaline M. [Mammoplasty. Inverted V technic. Analysis of 225 cases]. Ann Chir Plast Esthet 1983; 28: 159–163. [PubMed] [Google Scholar]
- 25.Nos C, Fitoussi A, Bourgeois D, et al. Conservative treatment of lower pole breast cancers by bilateral mammoplasty and radiotherapy. Eur J Surg Oncol 1998; 24: 508–514. [DOI] [PubMed] [Google Scholar]
- 26.Silverstein MJ, Gierson ED, Colburn WJ, et al. Can intraductal breast carcinoma be excised completely by local excision? Clinical and pathologic predictors. Cancer 1994; 73: 2985–2989. [DOI] [PubMed] [Google Scholar]
- 27.Bostwick J 3d, Paletta C, Hartrampf CR. Conservative treatment for breast cancer. Complications requiring reconstructive surgery. Ann Surg 1986; 203: 481–490.3010888 [Google Scholar]
- 28.Veronesi U, Volterrani F, Luini A, et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur J Cancer 1990; 26: 671–673. [DOI] [PubMed] [Google Scholar]
- 29.Bonadonna G, Veronesi U, Brambilla C, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst 1990; 82: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 30.Calitchi E, Otmezguine Y, Feuilhade F, et al. External irradiation prior to conservative surgery for breast cancer treatment. Int J Radiat Oncol Biol Phys 1991; 21: 325–329. [DOI] [PubMed] [Google Scholar]
- 31.Gray JR, McCormick B, Cox L, Yahalom J. Primary breast irradiation in large-breasted or heavy women: analysis of cosmetic outcome. Int J Radiat Oncol Biol Phys 1991; 21: 347–354. [DOI] [PubMed] [Google Scholar]
- 32.Moody AM, Mayles WP, Bliss JM, et al. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol 1994; 33: 106–112. [DOI] [PubMed] [Google Scholar]
- 33.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst 1996; 88: 1659–1664. [DOI] [PubMed] [Google Scholar]
- 34.Rietjens M, Petit JY, Contesso G. The role of reduction mammaplasty in oncology. Eur J Plast Surg 1997; 20: 246–250. [Google Scholar]
- 35.Spear SL, Burke JB, Forman D, et al. Experience with reduction mammaplasty following breast conservation surgery and radiation therapy. Plast Reconstr Surg 1998; 102: 1913–1916. [DOI] [PubMed] [Google Scholar]
- 36.Smith ML, Evans GR, Gurlek A, et al. Reduction mammaplasty: its role in breast conservation surgery for early-stage breast cancer. Ann Plast Surg 1998; 41: 234–239. [PubMed] [Google Scholar]
- 37.Shestak KC, Johnson RR, Greco RJ, et al. Partial mastectomy and breast reduction as a valuable treatment option for patients with macromastia and carcinoma of the breast. Surg Gynecol Obstet 1993; 177: 54–56. [PubMed] [Google Scholar]


