Abstract
Objective
To review the first clinical cases of composite tissue allotransplantation (CTA) for reconstructive surgery and to discuss the outcome of and indications for these procedures in the context of chronic immunosuppression.
Summary Background Data
The first human hand transplant was performed in 1998. This procedure, as well as other composite tissue transplants, offers the potential for correcting untreatable large tissue defects. However, concerns remain regarding obligatory chronic immunosuppression and long-term functional results.
Methods
All the CTAs performed in humans that have been published or documented were reviewed. The preexisting clinical conditions and surgical procedures and the immunosuppressive therapy are described. The functional results and the complications or side effects of the treatment are detailed.
Results
Vascularized tendons (two cases), vascularized femoral diaphyses (three cases), knees (five cases), hands (four bilateral and seven unilateral cases), larynx (one case), and nonvascularized peripheral nerves (seven cases) have been transplanted in humans in the past decade. Rejection was prevented in most cases without difficulty. Early results are encouraging, particularly for hand and larynx transplants, but will need to be evaluated in the long term and in a larger number of patients.
Conclusions
CTA holds great potential for reconstructive surgery but is at present restricted by the risks of chronic immunosuppression and uncertain long-term results.
In September 1998, a 48-year-old man with right-hand amputation received a forearm transplant harvested from a brain-dead man. 1 This first human hand transplantation was performed in Lyon, France, by a team headed by Dubernard. Dispute over whether the procedure was justified has taken place in many forums and publications. 2–6 Despite the controversy, 10 more patients have received hand transplants, including bilateral hand allografts, and other anatomic parts such as larynx and knees have been transplanted. Composite tissue allotransplantation (CTA), referring to all nonorgan transplants, has entered the realm of clinical practice.
CTA is not a new technique but is a new practice that couples the principles of microsurgical reconstruction with those of human organ transplantation. In its purpose (correction of physical defects), objective (reconstruction with anatomically identical structures), and technique (the transfer of vascularized tissues), CTA represents the essence of reconstructive surgery. However, CTA introduces new dimensions (immunologic, ethical, and psychological) that modify the traditional rules of tissue reconstruction.
The first hand transplants have been regarded as experimental due to risks and uncertainty about their functional results. Nevertheless, there is general agreement in the surgical community about the significant potential of CTA. To address the role of CTA in future reconstructive surgery, the scientific data on this topic have been gathered and are discussed here.
RULES OF ALLOTRANSPLANTATION IN RECONSTRUCTIVE SURGERY
In its broadest sense, tissue transplantation is the basis of all modern plastic and reconstructive surgery. 7 Nasal reconstructions with antebrachial flaps described by Tagliacozzi in the 16th century are still regarded as the founding acts of modern reconstructive surgery, which is based on the use of the patient’s own (autologous) tissues transferred into the defect. This kind of autotransplantation differs from tissue allotransplantation, which is the transfer of tissues harvested from a different subject of the same species.
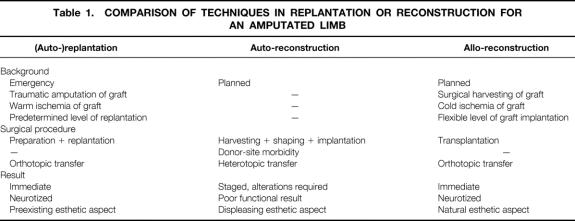
Thirty-five years ago, the advent of microsurgery opened the field of replantation of amputated tissues and reconstruction by free autologous tissue transfer. 8 New immunosuppressive agents have made tissue transfer between nonrelated subjects feasible. From a surgical point of view, harvesting tissues from a cadaveric donor has several advantages. First, tissue allotransplantation meets the preeminent objective of any tissue reconstruction, to replace “like with like.” For example, a thumb would be reconstructed with a thumb, not with a toe. Another major advantage of allografts is the avoidance of any donor site morbidity; this liberates the surgeon from the dilemma of healthy tissue destruction, a drawback of any reconstruction using autologous tissues. Thus, CTA works on two principles fundamental to any tissue reconstruction: improvement of the result and reduction of the morbidity related to donor site harvesting. In doing so, it allows the surgeon to achieve the optimal physical, functional, esthetic, and psychological result from reconstruction. Even compared to amputated tissue replantation, allotransplantation offers theoretical advantages that would give an improved functional and esthetic result (Table 1). However, these advantages of CTA must be balanced against the need for indefinite immunosuppressive treatment, which has potentially serious side effects.
Table 1.COMPARISON OF TECHNIQUES IN REPLANTATION OR RECONSTRUCTION FOR AN AMPUTATED LIMB
CTA adheres to the trends of solid organ transplantation: harvesting from cadaveric donors, immunologic incompatibility between donor and recipient, and life-long immunosuppression of the recipient. The attempt to obtain an efficient yet nontoxic immunosuppressive treatment has always been the limiting factor of organ transplantation. Introduced in 1982, cyclosporine improved the survival rate of grafts and patients by preventing graft rejection with reduced bone marrow toxicity. Other immunosuppressive agents introduced in recent years (tacrolimus, mycophenolate mofetil, monoclonal antibodies) have widened the therapies available to treat rejection and have limited the toxic effects specific to each drug with combination therapy. Composite tissue transplantation benefited from the progress of immunosuppression, but the heightened antigenicity of composite tissues has made the development of an effective yet nontoxic protocol difficult. 9 Unlike solid organ allografts, composite tissue allografts such as a hand are histologically heterogeneous and are composed of tissues that express varying degrees of antigenicity. Among these tissues are skin and muscle, which are highly antigenic. Other immunocompetent components such as bone marrow and lymph nodes, also the target of very severe rejection, may also participate in the immunologic reaction. 10 Many relevant studies have been conducted on animals and in 1997, a team from Louisville, Kentucky, demonstrated that an immunosuppressive tritherapy of tacrolimus, mycophenolate mofetil, and prednisone could prevent the rejection of an entire limb allograft without major toxicity in a preclinical model (adult swine) for up to 90 days. 11 This new scientific data fortified the knowledge gained from 30 years of human limb (auto)replantation, and in 1997 some specialists in tissue allotransplantation meeting in Louisville recommended the use of this immunosuppressive protocol to perform the first hand transplants in human. 12 Due to the complexity of the human immunologic system, graft rejection, immunosuppressive toxicity, and graft-versus-host disease (GVHD) were still risks. The human hand transplants that have been performed since 1998 have proved that the immunologic obstacle can be overcome and that the graft achieved bone consolidation and tissue healing similar to the patient’s own tissues. Nevertheless, the amputation of the first hand transplant in February 2001, after a long period of noncompliance to immunosuppressants, confirmed that any breach of the immunosuppressive treatment, even 2 years and 4 months after the intervention, led to graft rejection.
FIRST CLINICAL APPLICATIONS
In 1988 and 1989, a French team directed by Guimberteau performed two allotransplantations of the digital flexor tendon apparatus harvested from a nonrelated living donor (whose small finger had to be amputated) and from a cadaveric donor. The grafts were revascularized onto the recipient’s ulnar vessels. 13 Previously, nonvascularized allotransplants of fresh or frozen tendons had been performed, but the functional results of these allografts remained unsatisfactory due to the poor viability of the grafts and the disruption of the flexor system. The goal of the surgeons was to improve these results by transferring an entire flexor system (the tendon with its pulleys and sheaths) in which viability was preserved by immediate revascularization. Thus, it was a true CTA, and rejection had to be prevented by an immunosuppressive regimen, despite the low antigenicity of the tendon tissue. Cyclosporine was prescribed at a nontoxic dose of 7 mg/d for a 6-month period. The grafts were accepted without rejection by the two recipients; the anastomosis of vessels remained patent. One year postoperatively, the active flexion of these two fingers had improved from none preoperatively to 75° (patient 1) and 80° (patient 2) in the proximal interphalangeal joint, and 50° (patient 1) and 55° (patient 2) in the distal interphalangeal joint. The range of active extension slightly decreased by 5° (patient 1) to 25° (patient 2) in each interphalangeal joint.
In 1994, Hofmann and Kirschner, from Germany, started a program of vascularized bone allotransplantations for sequelae of chondrosarcoma or osteomyelitis of the lower limb. 14,15 Three patients received a femoral diaphysis and five patients an entire knee joint with its extensor system. The grafts were immediately revascularized by anastomosis of their pedicle to the femoral vessels 16 and were anchored to the femur and the tibia by an intramedullary nail. The immunosuppressive treatment began with the combination of cyclosporine, azathioprine, antithymocyte globulins, and methylprednisolone for the first 3 days and was then reduced to cyclosporine and azathioprine. After 6 months, azathioprine was withdrawn and cyclosporine alone was administered until complete bone consolidation of the two osteotomies. Clinical outcome published after a 2- to 5-year follow-up reported that four patients regained a favorable integration of their bone allograft and a satisfactory range of motion of their limb with a functional knee joint. 17,18 However, one femur allograft (out of three) and three knee allografts (out of five) became infected and were removed and replaced with a bone autograft or a knee prosthetic device. These complications were attributed to inadequate immunosuppressive treatment and insufficient monitoring of the rejection process.
In a field where the results have long been discouraging, recent nerve allografts have given hope to patients with extended peripheral nerve defects. 19 Between 1988 and 1998, Mackinnon (St. Louis, MO) performed peripheral nerve allotransplantations in seven patients ranging from 3 to 24 years old, following upper limb (four patients) or lower limb (three patients) trauma. 20 The grafts were harvested from limbs of cadaver donors and preserved in cold ischemia at 5°C for 7 days before implantation. The first five patients received cyclosporine, azathioprine, and prednisone; tacrolimus replaced cyclosporine for the last two patients. The immunosuppressive therapy was maintained until evidence of nerve regeneration (Tinel’s sign) traveled distal to the graft, with an average time of 18 months (range 12–26). One of the patients displayed rejection of the grafts 4 months postoperatively, which was attributed to the insufficient level of cyclosporine. The six other patients recovered distal sensibility and three regained motor function (upper limb grafts only), which remained stable subsequent to the withdrawal of immunosuppression. No side effects directly related to the immunosuppressive treatment were observed. Despite specific conditions that are not applicable to other tissues (the nonvascularization of grafts, prolonged preservation before transplantation, and withdrawal of the immunosuppressive treatment), these nerve allografts represent an advance that may benefit other CTAs such as the hand.
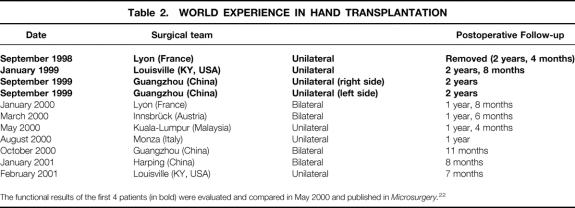
Since September 1998, 11 hand transplants have been performed around the world; of these, four were bilateral (Table 2). 1,21,22 The observations and results of the first four unilateral allografts were assessed during the second International Symposium on Composite Tissue Allotransplantation in Louisville, Kentucky, in May 2000. 23 At this time, the follow-up was 8 to 20 months. The comparison of these four cases illustrates the conditions under which the procedures were performed by three different teams and gave an initial glimpse into the functional results of these grafts. The grafts were harvested at a location above the elbow on the four donor subjects and stored at 4°C for 6 to 12 hours (cold ischemia time), preceded by perfusion of the brachial artery with University of Wisconsin preservation solution for organ transplants. The radius and ulna within the grafts were fixed to the recipient bones before vascular, tendon, and nerve repairs. The neurorrhaphy was kept as distal as possible (ranging from 21 to 1 cm from the wrist flexion cease) to reduce the distance of nerve regeneration. All four patients received an induction immunosuppressive treatment with tacrolimus, mycophenolate mofetil, and prednisone for the first 7 to 10 days. Two patients also received initial antithymocyte globulins and anti-CD25 monoclonal antibodies. This regimen was followed by a maintenance combination therapy of tacrolimus (adjusted to blood concentration of 5–10 ng/mL), mycophenolate mofetil (750–3000 mg/d), and prednisone (10–25 mg/d). The Lyon and Louisville patients experienced a few episodes of moderate rejection several weeks or months after the operation that were diagnosed by erythema on the hand allograft and confirmed with skin biopsies. Rejection was controlled in all cases with an increase in the prednisone dosage and topical steroids. Topical tacrolimus was also used by the French team. The Guangzhou patients did not experience any rejection during the first 8 months of follow-up. This may be due to the higher immunologic compatibility (3 HLA mismatches vs. 6 for the Lyon and Louisville patients), the initial irradiation or mechanical removal of the bone marrow contained in the graft radius and ulna, or the higher dosage of steroids used in the maintenance treatment.
Table 2.WORLD EXPERIENCE IN HAND TRANSPLANTATION
The functional results of the first 4 patients (in bold) were evaluated and compared in May 2000 and published in Microsurgery.22
The functional recovery of the first four hand transplants was evaluated by the Carroll test, which assesses the global functional capabilities of the upper limb in the everyday use. 24,25 On a scale of 0 to 99 points, the test result is considered poor at less than 50 points, fair between 51 and 74 points, good between 75 and 84 points, and excellent above 85 points. A prosthesis usually scores 25 or less, and the best replantations score within the 70 range. The functional capacity of the hand transplants was considered “fair” (Louisville: 52 points; Guangzhou 1: 65 points) and “good” (Guangzhou 2: 75 points). The Lyon patient did not perform the Carroll test but exhibited poor functional capacity, despite satisfactory initial functional recovery during the first 12 months. After 10 months, both patients from Lyon and Louisville were able to perform simple tasks of everyday life such as opening doors, holding and paging through a newspaper, filling a glass of water, or tying shoelaces. Good thumb–index and lateral pinch allowed them to perform prehensile activities under visual control to compensate for the lack of sensibility. 26 Nerve regeneration, a key determinate of functional recovery, was evaluated by the Tinel test and was considered remarkably rapid during the first few months for the four patients. 27 This observation was attributed to the favorable effect of tacrolimus on axonal regeneration previously observed in animals and humans. 20,28–30 However, nerve regeneration did not result in reinnervation of the intrinsic muscles or satisfactory distal sensitivity indicated by the Semmes-Weinstein test. While the Lyon patient ceased physical therapy, the surgical teams in Louisville and Guangzhou are still attempting to improve the functional results of the allografts with a therapy program extended for more than 2 years postoperatively. 31
The functional results achieved in the first hand transplants help to place in perspective the repercussions of long-term immunosuppressive treatment. During the first 8 to 20 months postoperatively in the first four hand transplant patients, the following complications were observed: insulin-dependent diabetes mellitus (Lyon), Cushing’s syndrome (Guangzhou 1), CMV colitis (Louisville), herpetic cutaneous infection (Lyon), and recurrent cutaneous mycoses (Louisville, Guangzhou 1). All these complications were treated successfully with decreased immunosuppression. The recipient of the first hand transplant in Lyon was not compliant with his immunosuppressive treatment and occupational therapy. The functional results did not meet his expectations despite some early return of function. Two years postoperatively, he discontinued his immunosuppressive medications and the graft was rejected in a few weeks and was removed. 32 He has since recovered from his previous conditions and is now free of medications. The other hand transplant recipients are following their immunosuppressive treatment and physical therapy, with viable grafts.
Another application of CTA in functional reparative surgery is that of the vascularized larynx allograft performed in January 1998 by Strome (Cleveland, OH). 33 A 40-year-old man, aphonous since age 20 after traumatic avulsion of his larynx, received an allograft of the complete pharyngolaryngeal system. The patient received an initial treatment of monoclonal antibodies OKT3, cyclosporine, mycophenolate mofetil, and methylprednisolone, followed by maintenance therapy of tacrolimus, mycophenolate mofetil, and prednisone. A rejection episode at 15 months postoperatively, diagnosed by aphony and laryngeal swelling, was confirmed by laryngeal biopsies and treated successfully with a transient increase of steroids. The patient exhibited high blood pressure for 6 months (treated with antihypertensive drugs) and three episodes of tracheobronchitis (treated with antibiotics). A pulmonary infection of Pneumocystis carinii occurred after the patient unexpectedly discontinued his preventive treatment; it was treated with antibiotics. Thyroid and parathyroid hormonal levels remained normal after the transplantation. The 40-month functional results were recently reported. 34 The patient obtained efficient deglutition after 3 months. At 16 months postoperatively, the various parameters of the voice (tone, quality, intensity, flow, and respiratory coordination) were normalized. The patient currently talks with a natural and intelligible voice. He can now feed himself without aspiration. Taste and odor sensations have improved. No stenosis hampers the airflow, and the tracheostomy will be closed.
FUTURE OF TISSUE ALLOTRANSPLANTATION
The 25 cases discussed here represent the first clinical applications of CTA. Early results of these operations proved that such transplantation is technically feasible, with graft survival depending on high-level and indefinite immunosuppression. New immunosuppressive agents can maintain survival of the different “components” of CTA, including skin, which is regarded by many as most antigenic. However, the potential side effects of chronic immunosuppression currently limit the widespread application of CTA in reconstructive surgery.
The first hand allografts were criticized by many for the risks of complications and uncertain functional recovery. Because having hands or a voice is not critical for life, they argue, it is unwarranted to subject disabled but “healthy” patients to the risks of significant complications. 35 Others who considered the quality of life as also important defended hand transplantations as a means to enhance the quality of life with acceptable risks. 36,37
Publication of the early results of the first CTAs has changed the nature of the debate. 38 At the second International Symposium on CTA in 2000, these early results were deemed by the symposium participants to be encouraging for the quality of recovered function and for the tolerance to the immunosuppressive treatment, which did not cause any major or irreversible complications. Amputation of one of the hand allografts was required in a patient who was not compliant with physical therapy, regular adjustment of medication, or close medical monitoring. While underscoring the critical importance of patient selection, this case also suggests that the transplant may be “reversible” for potentially unacceptable complications or even for dissatisfied patients. The limited side effects of the immunosuppressive treatment observed so far and the benefits of the transplantation (functional, but particularly psychological) manifesting in the restoration of body integrity helped to justify the procedures. Nevertheless, CTA is still regarded by some as a possible solution for exceptional indications at present. While awaiting long-term results, the National Committee of Ethics in France has agreed to four more hand transplants for bilateral amputees. 32
The principle of “like with like” reconstruction, specific to allotransplantation, gives hope to broadening the realm of reconstructive surgery to physical handicaps with no current solution. The use of vascularized tissues harvested from a different subject could theoretically be extended to all reconstructive procedures currently using autologous tissues to obtain better functional and esthetic results and to reduce the morbidity from tissue harvesting. Despite these advantages, the current application of CTA is limited by the following practical considerations.
Risks of Transplantation
The side effects of immunosuppression (metabolic disorders, malignancies, opportunistic infections) are the predominant limiting factor in tissue allotransplantation for reconstructive surgery. The risk of transmitting infection that might elude current techniques of detection should not be ignored. In addition, the indispensable matching of donor and recipient under cosmetic criteria (gender, ethnic background, morphology) is complicated by the current lack of organ and tissue donors in many countries. Current efforts in inducing tolerance to CTA in many laboratories hold promise for significant reduction of necessary immunosuppression and widespread clinical application. 39,40
Need to Determine Appropriate Indications
Success of these operations relies on the selection of acceptable indications, on an effective immunosuppressive protocol, and on the compliance with the treatment and therapy by the patient. General agreement in the medical community is needed regarding which physical defects justify reconstruction with an allotransplant. 41 Scalp avulsions, extended facial burns, large mandibular resections, and proximal limb amputations have already been suggested, and some have been carried out in animal experiments. 42 In contrast, allotransplantation is unsuitable in certain conditions. Breast reconstruction, for example, would be difficult to justify, given the excellent autologous alternative in most situations (and the difficulty of maintaining vascularity to a breast based on a reliable vascular pedicle).
Uncertainty of Long-Term Outcome
While the functional results of these allografts may improve with time and continued therapy, immunologic constraints might curtail these results in the long term. In organ transplantation, the functional capacities of solid organ allografts (mostly kidney and heart) decrease with time: 10 to 15 years postoperatively, an average of one third of the grafts are not functional. 43 This phenomenon is not clearly understood and is termed “chronic rejection.” Possible explanations include latent immunologic rejection, toxicity of the immunosuppressive treatment, ischemic injury, and accelerated aging of the grafts. It is unknown if composite tissue allografts will undergo chronic rejection with diminution of functional capacities. Furthermore, the functional results also depend on the integration of the graft in the body identity of the transplanted patient (i.e., the recognition and acceptance of the graft as “self”). Functional magnetic resonance imaging studies performed on the patient who received a double hand transplant in Lyon in January 2000 (Fig. 1) showed that the cerebral motor cortex can reorganize itself to recognize and activate the transplanted hands, even after the long period of deprivation due to the amputation. 44 While this cerebral plasticity is important in adapting to the everyday use of the hands, it does not predict the psychological acceptance of the grafts.
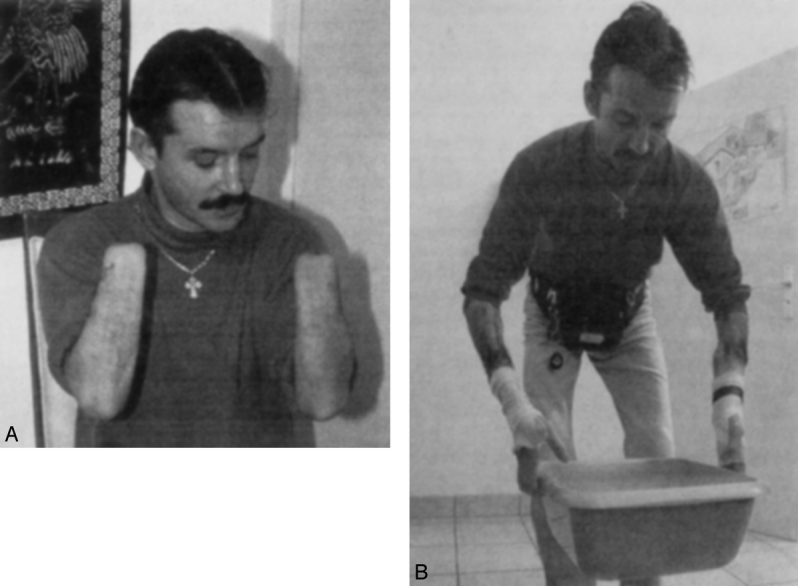
Figure 1. The 35-year-old patient before and after a double hand transplantation performed in Lyon, France, in January 2000.
In summary, CTA offers great potential for reconstructive surgery. The early results of the first clinical cases are encouraging but will need to be evaluated in the long term and in the context of chronic immunosuppression. CTA may not be the solution for all defects but may greatly improve the reconstructive options for a select group of patients. CTA is currently restricted to major disability conditions and must be performed with adherence to strict medical and ethical guidelines: professional competency, clear therapeutic objective, and informed consent of the patient.
Experimental research in transplantation is rapidly evolving. In the short term, new molecules will broaden the range of immunosuppressive agents, reduce the toxicity of current drugs, and perhaps prevent the lesions of chronic rejection. In the long term, tolerance induction through a preconditioning regimen of the recipient offers great promise by avoiding indefinite immunosuppression. 45 Such strategies could one day make CTA a safer and more widespread clinical procedure.
Footnotes
Dr. Petit is supported by a grant from the Académie Nationale de Médecine (France).
Correspondence: W. P. Andrew Lee, MD, Division of Plastic Surgery, Massachusetts General Hospital, WAC-453, 15 Parkman Street, Boston, MA 02114.
E-mail: lee.wpa@mgh.harvard.edu
Accepted for publication June 27, 2002.
References
- 1.Dubernard JM, Owen E, Herzberg G, et al. Human hand allograft: report on first 6 months. Lancet 1999; 353: 1315–1320. [DOI] [PubMed] [Google Scholar]
- 2.Foucher G. Prospects for hand transplantation. Lancet 1999; 353: 1286–1287. [DOI] [PubMed] [Google Scholar]
- 3.Tamai S. Reflections on human hand allografts. J Orthop Sci 1999; 4: 325–327. [DOI] [PubMed] [Google Scholar]
- 4.Lundborg G. Hand transplantation. Scand J Plast Reconstr Surg Hand Surg 1999; 33: 369–371. [DOI] [PubMed] [Google Scholar]
- 5.Lee WP, Mathes DW. Hand transplantation: pertinent data and future outlook. J Hand Surg [Am] 1999; 24: 906–913. [DOI] [PubMed] [Google Scholar]
- 6.Hettiaratchy S, Butler PE, Lee WP. Lessons from hand transplantations. Lancet 2001; 357: 494–495. [DOI] [PubMed] [Google Scholar]
- 7.Lee WP, Butler PE. Transplant biology and applications to plastic surgery. In: Aston SJ, Beasley RW, Thorne CHM, ed. Grabb and Smith’s Plastic Surgery. Philadelphia: Lippincott-Raven; 1997: 27–37.
- 8.Malt RA, McKhann CF. Replantation of severed arms. JAMA 1964; 189: 716–722. [DOI] [PubMed] [Google Scholar]
- 9.Gorantla VS, Barker JH, Jones JW Jr, et al. Immunosuppressive agents in transplantation: mechanisms of action and current anti-rejection strategies. Microsurgery 2000; 20: 420–429. [DOI] [PubMed] [Google Scholar]
- 10.Lee WP, Yaremchuk MJ, Pan YC, et al. Relative antigenicity of components of a vascularized limb allograft. Plast Reconstr Surg 1991; 87: 401–411. [DOI] [PubMed] [Google Scholar]
- 11.Jones JW Jr, Ustuner ET, Zdichavsky M, et al. Long-term survival of an extremity composite tissue allograft with FK506-mycophenolate mofetil therapy. Surgery 1999; 126: 384–388. [PubMed] [Google Scholar]
- 12.Llull R. An open proposal for clinical composite tissue allotransplantation. Transplant Proc 1998; 30: 2692–2703. [DOI] [PubMed] [Google Scholar]
- 13.Guimberteau JC, Baudet J, Panconi B, et al. Human allotransplant of a digital flexion system vascularized on the ulnar pedicle: a preliminary report and 1-year follow-up of two cases. Plast Reconstr Surg 1992; 89: 1135–1147. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann GO, Kirschner MH, Wagner FD, et al. Allogeneic vascularized grafting of a human knee joint with postoperative immunosuppression. Arch Orthop Trauma Surg 1997; 116: 125–128. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann GO, Kirschner MH, Wagner FD, et al. Allogeneic vascularized transplantation of human femoral diaphyses and total knee joints–first clinical experiences. Transplant Proc 1998; 30: 2754–2761. [DOI] [PubMed] [Google Scholar]
- 16.Kirschner MH, Menck J, Hofmann GO. Anatomic bases of a vascularized allogenic knee joint transplantation: arterial blood supply of the human knee joint. Surg Radiol Anat 1996; 18: 263–269. [DOI] [PubMed] [Google Scholar]
- 17.Kirschner MH, Brauns L, Gonschorek O, et al. Vascularised knee joint transplantation in man: the first two years’ experience. Eur J Surg 2000; 166: 320–327. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann GO, Kirschner MH. Clinical experience in allogeneic vascularized bone and joint allografting. Microsurgery 2000; 20: 375–383. [DOI] [PubMed] [Google Scholar]
- 19.Bain JR. Peripheral nerve and neuromuscular allotransplantation: current status. Microsurgery 2000; 20: 384–388. [DOI] [PubMed] [Google Scholar]
- 20.Mackinnon SE, Doolabh VB, Novak CB, et al. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg 2001; 107: 1419–1429. [DOI] [PubMed] [Google Scholar]
- 21.Jones JW, Gruber SA, Barker JH, et al. Successful hand transplantation. One-year follow-up. Louisville Hand Transplant Team. N Engl J Med 2000; 343: 468–473. [DOI] [PubMed] [Google Scholar]
- 22.Francois CG, Breidenbach WC, Maldonado C, et al. Hand transplantation: comparisons and observations of the first four clinical cases. Microsurgery 2000; 20: 360–371. [DOI] [PubMed] [Google Scholar]
- 23.Barker JH, Breidenbach WC, Hewitt CW. Proceedings of the second International Symposium on Composite Tissue Allotransplantation. Microsurgery 2000; 20. [DOI] [PubMed]
- 24.Carroll D. A quantitative test of upper extremity function. J Chronic Dis 1965; 18: 479–491. [DOI] [PubMed] [Google Scholar]
- 25.Russell RC, O’Brien BM, Morrison WA, et al. The late functional results of upper limb revascularization and replantation. J Hand Surg [Am] 1984; 9: 623–633. [DOI] [PubMed] [Google Scholar]
- 26.Lee WP, Breindenbach WC, Hodges A, et al. Lessons from hand transplantation. In: Hand Surgery Quarterly; American Association for Hand Surgery, summer 2001.
- 27.Owen ER, Dubernard JM, Lanzetta M, et al. Peripheral nerve regeneration in human hand transplantation. Transplant Proc 2001; 33: 1720–1721. [DOI] [PubMed] [Google Scholar]
- 28.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci 1995; 15: 7509–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg 1999; 103: 1928–1936. [DOI] [PubMed] [Google Scholar]
- 30.Fansa H, Keilhoff G, Altmann S, et al. The effect of the immunosuppressant FK 506 on peripheral nerve regeneration following nerve grafting. J Hand Surg [Br] 1999; 24: 38–42. [DOI] [PubMed] [Google Scholar]
- 31.Hodges A, Chesher S, Feranda S. Hand transplantation: rehabilitation: case report. Microsurgery 2000; 20: 389–392. [DOI] [PubMed] [Google Scholar]
- 32.Dubernard JM, Owen ER, Lanzetta M, et al. What is happening with hand transplants? Lancet 2001; 357: 1711–1712. [DOI] [PubMed] [Google Scholar]
- 33.Strome M. Human laryngeal transplantation: considerations and implications. Microsurgery 2000; 20: 372–374. [DOI] [PubMed] [Google Scholar]
- 34.Strome M, Stein J, Esclamado R, et al. Laryngeal transplantation and 40-month follow-up. N Engl J Med 2001; 344: 1676–1679. [DOI] [PubMed] [Google Scholar]
- 35.Hand, Forearm Transplantation Background Paper. American Society of Surgery of the Hand. ASSH, ed. 2000; http://www.hand-surg.org/members/transplant.asp.
- 36.Siegler M. Ethical issues in innovative surgery: should we attempt a cadaveric hand transplantation in a human subject? Transplant Proc 1998; 30: 2779–2782. [DOI] [PubMed] [Google Scholar]
- 37.Simmons PD. Ethical considerations in composite tissue allotransplantation. Microsurgery 2000; 20: 458–465. [DOI] [PubMed] [Google Scholar]
- 38.Manske PR. Hand transplantation. J Hand Surg [Am] 2001; 26: 193–195. [DOI] [PubMed] [Google Scholar]
- 39.Mathes DW, Randolph MA, Lee WP. Strategies for tolerance induction to composite tissue allografts. Microsurgery 2000; 20: 448–452. [DOI] [PubMed] [Google Scholar]
- 40.Petit F, Minns AB, Hettiaratchy SP, et al. New trends and future direction of research in composite tissue allotransplantation. Transplant Proc (in press).
- 41.Edgell SE, McCabe SJ, Breidenbach WC, et al. Different reference frames can lead to different hand transplantation decisions by patients and physicians. J Hand Surg [Am] 2001; 26: 196–200. [DOI] [PubMed] [Google Scholar]
- 42.Hohnke C, Russavage JM, Subbotin V, et al. Vascularized composite tissue mandibular transplantation in dogs. Transplant Proc 1997; 29: 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auchincloss HJ, Sykes M, Sachs DH. Transplantation immunology. In: Paul WE, ed. Fundamental Immunology. Philadelphia: Lippincott-Raven, 1999: 1175–1235.
- 44.Giraux P, Sirigu A, Schneider F, et al. Cortical reorganization in motor cortex after graft of both hands. Nat Neurosci 2001; 4: 691–692. [DOI] [PubMed] [Google Scholar]
- 45.Mathes DW, Lee WP. Composite tissue transplantation: more science and patience needed. Plast Reconstr Surg 2001; 107: 1066–1070. [DOI] [PubMed] [Google Scholar]