Abstract
Objective
To review the authors’ experience with ABO-matched, preserved, cadaveric, iliac artery grafts for treatment of transplant renal artery stenosis (TRAS).
Summary Background Data
TRAS is an important and treatable cause of hypertension and graft dysfunction in renal allograft recipients. Surgical treatment is reserved for lesions that are not amenable to percutaneous transluminal angioplasty (PTA) or for recurrence after PTA. Various surgical options for reconstruction of the transplant renal artery exist, although no single technique has been demonstrated to be superior. The authors have used preserved, blood type-matched, iliac artery grafts procured from cadaver organ donors to reconstruct transplant renal arteries in patients with specific lesions and following unsuccessful PTAs.
Methods
Between 1991 and 2001, 21 patients underwent reconstruction of allograft renal arteries using cadaveric iliac artery as conduit. Charts, operative notes, and imaging studies of all patients were reviewed. A successful intervention for TRAS was defined as technical success as well as a decrease in serum creatinine and/or blood pressure 6 weeks after the procedure. Development of a hemodynamically significant lesion following renal artery reconstruction was considered a recurrence.
Results
In patients treated with surgical reconstruction, hemodynamically significant TRAS occurred at or within 1 to 2 mm of the anastomosis in 13 patients, in the middle of the renal artery in 4, and secondary to a kink in 2 patients. Surgical treatment was undertaken in seven patients following unsuccessful PTA. Two patients had aneurysms of the iliac artery. Reconstruction using cadaveric iliac artery was successful in 19 of 21 (90%) patients, and only 1 these patients (4.8%) failed due to recurrence, with a median follow-up of 42 months. Graft loss secondary to TRAS occurred in two patients. The authors have not seen any long-term complications related to cadaveric iliac artery grafts, and the majority of the allografts continue to function well.
Conclusions
Surgical reconstruction of the transplant renal artery with blood type-matched iliac artery grafts should be considered a viable option for patients with specific anatomic lesions, those who have had an unsuccessful PTA, and those with recurrence following PTA.
Transplant renal artery stenosis (TRAS) is an increasingly recognized complication of kidney transplantation. It represents a potentially reversible cause of hypertension and allograft dysfunction. The incidence varies from 1% to 23% depending on the center, the definition of TRAS, and the intensity of screening done. 1 The detected incidence of TRAS increased from 2.4% to 12.4% at one center with the introduction of color Doppler ultrasonography. 2 The highest incidence (23%) was found in a series of patients undergoing angiography. 3 Several etiologic mechanisms have been proposed for TRAS, including suture technique, atherosclerotic arterial disease in the donor or recipient, and arterial trauma during organ procurement or transplant. Improper apposition of the donor and recipient vessels or excessive length of the renal artery can lead to torsion and kinking of the renal artery. 1,4 Others have speculated that TRAS is related to chronic rejection 2 or primary cytomegaloviral infection. 5,6
Percutaneous transluminal balloon angioplasty (PTA) is the preferred initial mode of therapy for both native renal artery stenosis and TRAS. PTA is minimally invasive and safe and a generally effective treatment for TRAS, with success rates reported between 20% and 88%. 7–10 The variable success rates for PTA may be related to the location and length of the stenotic lesion being treated. 4 In general, PTA works best for lesions that are short, linear, and relatively distal from the anastomosis. 1,11 When PTA is used for anastomotic or ostial lesions, the success rate is low and there is a higher incidence of complications. 7,12 Further, kinking of the transplant renal artery has been associated with a 60% failure rate of PTA. 9,11
Surgical techniques for repair of TRAS have included resection and revision of the anastomosis, renal artery patch angioplasty, localized endarterectomy, and renal artery bypass with either recipient saphenous vein or ipsilateral hypogastric artery. 1,4 In general, these are technically challenging operations due to the dense scar tissue around the allograft and the frequent adherence of the renal vein and ureter to the renal artery. No one technique of transplant renal artery reconstruction has been demonstrated to be superior to others. We report a novel strategy for surgical reconstruction of the transplant renal artery using preserved, blood type-matched, cadaveric donor iliac artery grafts. We reviewed the records of all patients at our center who had surgical reconstruction of their transplant renal artery with cadaveric iliac artery grafts following kidney transplant or simultaneous pancreas-kidney transplant (SPK) since 1991. The majority of reconstructions were done for TRAS, although two patients had aneurysmal disease of the iliac vessels. Surgical therapy was undertaken for anastomotic lesions, kinks in the transplant renal artery, technical failure of PTA, and recurrence after successful PTA. We found that treatment of TRAS with preserved, blood type-matched, cadaveric donor iliac artery grafts was successful, had minimal recurrence of stenosis, and resulted in good long-term graft survival.
METHODS
Patients
From January 1991 to June 2001, 79 patients underwent an intervention for TRAS at the University of Wisconsin, Madison. TRAS was defined as a hemodynamically significant lesion of the renal artery with associated signs and symptoms including hypertension, edema, and graft dysfunction. Twenty-one patients underwent surgical reconstruction with preserved, blood type-matched, cadaveric iliac artery. The remaining patients with TRAS underwent either PTA alone (48 patients) or various other techniques of surgical repair (11 patients). Charts, operative notes, and imaging studies were reviewed for details of their initial transplant, symptoms at presentation of TRAS, location of stenosis, pre- and postprocedure (4–6 weeks) blood pressure and serum creatinine, postoperative complications, recurrence of TRAS, and long-term graft survival. A successful procedure was defined by technical success and a decrease in serum creatinine and/or mean arterial blood pressure 6 weeks after the procedure. Recurrence was defined as the development of a hemodynamically significant lesion following transplant renal artery reconstruction with associated signs and symptoms of TRAS. Recurrence of the lesion was considered a failure. Mean arterial pressure was defined as diastolic blood pressure plus one third of the pulse pressure (systolic – diastolic blood pressure). Recurrent TRAS was suspected in patients who developed graft dysfunction, hypertension, or edema following intervention for TRAS. Patients with possible recurrence underwent ultrasound or magnetic resonance angiography, followed by arteriography as indicated.
Transplantation
Kidney allografts were placed into either iliac fossa according to standard surgical techniques. Most transplants were performed with end of the renal artery with or without a Carrel patch to the side of either common or external iliac artery and vein in the recipient. Two transplants were performed end to end with recipient hypogastric artery (patients 20 and 7). One patient received his transplant at another center and was referred for evaluation and treatment of TRAS (patient 7). Any ex vivo reconstructions of donor renal arteries at the time of transplant are described. The immunosuppressive protocols used at our institution have been published elsewhere. 13
Cadaveric Iliac Artery Grafts
Iliac vessels were procured from organ donors routinely and preserved in University of Wisconsin solution at 4°C for no more than 10 days before use. When a patient was identified as requiring transplant renal artery reconstruction, it was performed as soon as ABO-matched donor vessels became available. Bacterial cultures of the storage fluid were obtained before the procedure. One (5%) culture was positive for Lactobacillus, while the remainder of the cultures were negative. The patient who received this graft developed a wound infection.
Statistical Analysis
Data (age, time to presentation, blood pressure, and serum creatinine) are presented as mean ± standard error of the mean. Differences between mean arterial pressure and serum creatinine before and after renal artery reconstruction were analyzed by a two-tailed Student t test. Statistical significance was accepted within 95% confidence limits.
RESULTS
Patient Selection
Patients suspected to have TRAS based on signs and symptoms (graft dysfunction, hypertension, or edema) underwent duplex ultrasound and/or magnetic resonance angiography. In general, patients had an allograft biopsy demonstrating no rejection before extensive imaging and intervention for TRAS. If the ultrasound examination or magnetic resonance angiogram demonstrated TRAS, patients underwent angiography to define the location of the stenosis and for possible PTA. PTA was performed as the primary procedure if the lesion was considered amenable (i.e., a short, segmental stenosis, distal to the anastomosis) by the interventional radiologist. Anastomotic lesions and kinks were preferentially treated with surgical intervention during this period. Surgical therapy was undertaken when PTA was technically unsuccessful or when TRAS recurred following PTA.
Twenty-one patients underwent reconstruction of transplant renal arteries with ABO-compatible, cadaveric iliac artery grafts. Average age at transplant was 42.8 ± 2.4 years (range 34–64); 14 patients were men and 7 were women. Renal failure was secondary to diabetes mellitus (n = 7), hypertension (n = 6), glomerulonephritis (n = 5), polycystic kidney disease (n = 1), IgA nephropathy (n = 1), and lupus (n = 1). Eighteen received cadaveric kidney allografts (16 kidney alone and 2 SPK) and three live donor allografts. The mean time to presentation was 23.7 ± 9.2 months after transplantation (median 9.9 months; range 8 days to 16 years) (Table 1).
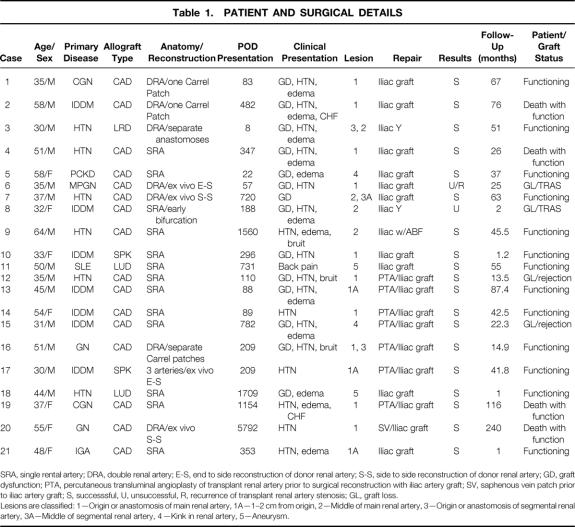
Table 1.PATIENT AND SURGICAL DETAILS
SRA, single rental artery; DRA, double renal artery; E-S, end to side reconstruction of donor renal artery; S-S, side to side reconstruction of donor renal artery; GD, graft dysfunction; PTA, percutaneous transluminal angioplasty of transplant renal artery prior to surgical reconstruction with iliac artery graft; SV, saphenous vein patch prior to iliac artery graft; S, successsful, U, unsuccessful, R, recurrence of transplant renal artery stenosis; GL, graft loss.
Lesions are classified: 1—Origin or anastomosis of main renal artery, 1A—1–2 cm from origin, 2—Middle of main renal artery, 3—Origin or anastomosis of segmental renal artery, 3A—Middle of segmental renal artery, 4—Kink in renal artery, 5—Aneurysm.
In seven patients surgical reconstruction was done following a PTA—in four cases after an unsuccessful PTA, and in three cases for recurrence of stenosis after PTA. One reconstruction with donor iliac artery was done after a recurrence of TRAS following surgical repair with a saphenous vein patch angioplasty. Twelve allografts had a single renal artery, seven had a double renal artery, and one had three renal arteries. Four allografts had an ex vivo reconstruction of donor renal arteries at the time of transplantation: two end-to-side and two side-to-side reconstructions were done as noted (see Table 1). All of the patients with TRAS had hemodynamically significant lesions of the transplant renal artery. Thirteen of the lesions were either at or near (within 1–2 cm) the anastomosis of the renal artery to the recipient iliac artery, four were in the middle of the transplant renal artery, and two were secondary to a kink in the renal artery. Two patients had iliac artery aneurysms, one of the hypogastric artery and one a poststenotic aneurysm of the common iliac artery.
Use of Preserved, Cadaveric Iliac Artery Grafts
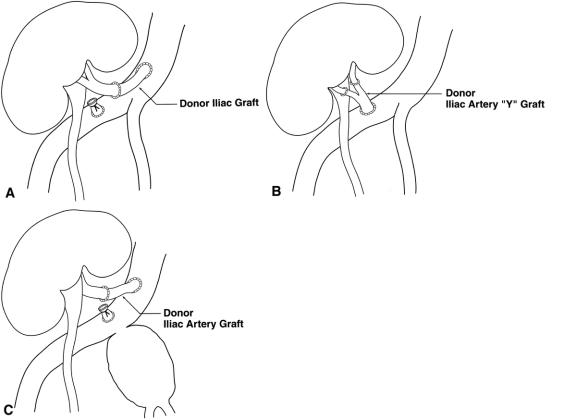
In general, the preserved, ABO-matched, donor iliac arteries were used as bypass conduits from the recipient iliac artery to the transplanted renal artery distal to the stenosis. The transplant renal artery was divided distal to the stenosis and the bypass graft was placed from the side of the recipient iliac artery to the end of the allograft renal artery (Fig. 1A). In two cases, a complete iliac Y-graft was used to manage double renal arteries (see Fig. 1B). Grafts were stored for up to 10 days before use in University of Wisconsin solution at 4°C. To perform iliac graft bypasses, careful dissection was carried out in the renal transplant hilum. In all cases the iliac and renal vessels were isolated via an intraperitoneal approach. Inflow was established by anastomosis of the donor iliac graft to the recipient iliac artery before ligating and dividing the renal artery or placing clamps on the renal artery in an attempt to reduce warm ischemia time. In patient 4, inflow was obtained from the contralateral iliac artery as this was the only suitable vessel available secondary to recipient atherosclerotic disease. In patient 9, severe aortoiliac obstructive disease as well as significant TRAS, necessitated placement of a PTFE aorto-bifemoral graft with reimplantation of the transplanted renal artery onto the graft using donor iliac artery.
Figure 1. Iliac artery grafts were used as conduit to reconstruct the transplant renal artery by obtaining inflow from the recipient iliac artery and performing an end-to-end anastomosis with the transplant renal artery distal to the stenosis (A). In patients with two transplant renal arteries, an iliac Y-graft was used to reconstruct both vessels (B). (C) Use of the iliac graft in patient 11, who had an internal iliac artery pseudoaneurysm; blood inflow had to be moved proximal to accommodate ligation of the aneurysm.
Two patients with iliac artery aneurysms were repaired using preserved iliac artery graft as conduit. In one instance (patient 11) a large pseudoaneurysm of the internal iliac artery was present, and to preserve renal transplant blood flow during vascular control of the aneurysm, an iliac artery graft was used to reposition inflow to a more proximal location on the common iliac artery such that clamps could then be placed distal to the allograft arterial inflow, permitting the aneurysm to be oversewn without renal warm ischemia (see Fig. 1C). This patient was previously reported due to his interesting presentation. 14 Another case of iliac artery aneurysm (patient 18) presented with hypertension and lower extremity edema. Magnetic resonance angiography revealed a stenosis of the recipient common iliac artery above the origin of the transplant renal artery and a poststenotic 2 × 3-cm aneurysm of the common iliac artery into which the transplant renal artery was inserted. A jump graft using preserved iliac artery as conduit was performed from the proximal common iliac artery to the end of the transplant renal artery. A PTFE graft was then used to bypass the excluded common iliac artery aneurysm.
Immediate Outcomes
Cadaveric iliac artery reconstruction resulted in a successful decrease in serum creatinine and/or mean arterial pressure in 90% (19/21) of the patients. Serum creatinine decreased from an average of 2.1 ± 0.2 preoperatively to 1.7 ± 0.2 postoperatively (P < .05, Student t test) (Fig. 2). Mean arterial pressure decreased from 119 ± 4 mm Hg to 107 ± 3 mm Hg (P < .05, Student t test) (see Fig. 2). Further, all patients who presented with edema (12 cases) had improvement in edema postoperatively. There was one (4.8%) recurrence of TRAS following reconstruction with donor iliac artery. There have been three postoperative complications: two wound infections and one fascial dehiscence. One of the wound infections occurred in the setting of a positive culture for Lactobacillus in the iliac artery graft storage fluid. This was treated with appropriate antibiotics without any further complications. There were no perioperative deaths.
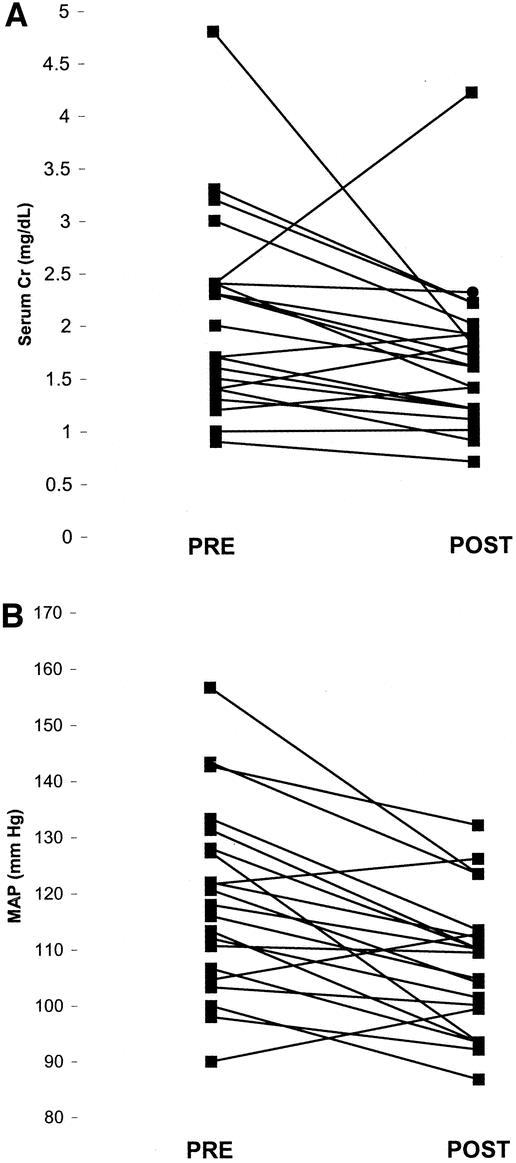
Figure 2. Serum creatinine and mean arterial pressure (MAP) were measured preoperatively and 6 weeks postoperatively following reconstruction of transplant renal arteries with cadaveric iliac artery grafts. The average serum creatinine preoperatively was 2.1 ± 0.2 mg/dL; it decreased to 1.7 ± 0.2 mg/dL (P < .05) postoperatively. The average MAP preoperatively was 119 ± 4 mm Hg; it decreased to 107 ± 3 mm Hg (P < 0.05) postoperatively.
Long-Term Follow-Up
Mean follow-up after renal artery reconstruction was 49 ± 12 months (median 42 months). Four patients have died with functioning grafts. Four grafts have failed: two secondary to rejection and two related to recurrent TRAS (patients 6 and 8). Patient 6 underwent transplant renal artery bypass with an iliac artery graft for an anastomotic lesion and subsequently developed a recurrent stenosis at the reconstructed site. He then underwent excision of the stenosis and reimplantation of the renal artery as a secondary procedure. He developed a second recurrence that ultimately resulted in graft loss. The second patient with graft loss due to TRAS (patient 8) is a 33-year-old, morbidly obese black woman who received a kidney transplant for end-stage renal disease secondary to insulin-dependent diabetes mellitus. Other premorbid conditions include a functional diagnosis of a hypercoagulable state that was made after she developed thrombosis of multiple upper extremity veins, superior vena cava, and left femoral vein. When she presented with graft dysfunction, hypertension, and edema 6 months after the transplant, she was found have stenosis of the transplant renal artery near an early bifurcation. She underwent transplant renal artery reconstruction with a donor iliac artery Y-graft. Her allograft dysfunction has progressed postoperatively over a 2-month period for unclear reasons, although a biopsy demonstrated moderate to severe ischemic damage of the allograft.
We have not seen any late vascular complications such as aneurysm formation or graft infection following the use of iliac artery grafts; however, there has been no systematic screening of these patients for iliac artery graft-related complications. Four of the 21 patients have had follow-up imaging (magnetic resonance angiography or duplex ultrasound) of the grafts related to concern over recurrence of TRAS. These four patients had normal transplant renal arteries without stenosis, and the iliac grafts were without aneurysm or stenotic lesions. We are actively pursuing follow-up imaging in other patients at this time.
DISCUSSION
Initial enthusiasm for PTA in the treatment of TRAS has been tempered by reports describing complications and recurrence of TRAS following percutaneous treatment. 11,15 Specifically, anastomotic lesions and kinks in the transplant renal artery may not respond well to angioplasty and may be best treated by surgical reconstruction. Furthermore, multiple surgical techniques have been reported, and no one technique has been defined as optimal. 1,4 We report a novel surgical technique to treat TRAS using blood type-matched, cadaveric iliac artery grafts. We have undertaken reconstruction of the transplant renal artery for specific indications including anastomotic lesions, kinks, unsuccessful PTA, recurrent lesions, and bypass in association with definitive surgical treatment of recipient iliac artery aneurysms following renal transplant. Surgical reconstruction with cadaveric iliac artery graft was successful in 19 (90%) cases, and only one recurrence has been noted to date. Long-term follow-up has revealed only two cases (9.5%) of graft loss related to TRAS and no significant vascular or immune complications related to the use of these grafts.
Numerous techniques have been reported for reconstruction of transplant renal arteries, including bypass or patch angioplasty with saphenous vein, bypass with in situ hypogastric artery, and excision/reimplantation of the renal artery. 16 Success rates have ranged from 66% to 100%, depending on the center. 17,18 Excision of the stenotic lesion with reimplantation of the renal artery has a reported success rate of 72% and an 11% incidence of recurrence. 7 However, excision and reimplantation may not be technically possible for many lesions. Most investigators report using saphenous vein as a conduit for bypass or reconstruction (vein patch) of the transplant renal artery. 17–21 In general, the use of saphenous vein grafts has resulted in a low but significant rate of recurrence, ranging from 0% to 12%. 17,18,21 However, vein harvest incisions in the lower extremity may have significant associated morbidity in immunosuppressed individuals. Furthermore, it is well known that autologous vein grafts used to treat native renovascular hypertension can dilate and develop aneurysmal degeneration over time. 22 There have even been case reports of late rupture of aortorenal saphenous vein grafts. 23,24 For these reasons, we were interested in finding a durable arterial tissue conduit, which avoids unnecessary morbidity in young immunosuppressed patients.
During cadaver donor multiorgan recoveries, the iliac vessels are routinely harvested in the event donor vessels need to be reconstructed with the primary transplant procedure. In fact, it is common practice to use the donor iliac artery Y-graft to reconstruct the pancreas allograft arterial anatomy before implantation. Furthermore, donor iliac vessels are frequently used in liver transplantation for both hepatic artery and portal vein reconstruction, and likewise for renal transplants when indicated. Large organ procurement organizations often have leftover vessels that can be retained for a period for subsequent secondary procedures for renal artery or hepatic artery stenosis. Use of cadaver donor iliac arterial conduit for hepatic allograft vascular reconstruction has been encouraging, with good initial technical success rates and long-term graft survival. 25 A brief report also described the use of donor vessels (iliac artery and vein or vena cava) in four patients to reconstruct the transplant renal artery, 26 although no operative details were given in this report.
With the use of cadaveric iliac artery grafts, we have not seen any late aneurysmal degeneration or rupture. The rate of recurrence in this small study using iliac arterial conduit was similar to that observed with saphenous vein. However, it is important to avoid cross-contamination with the blood of other patients and to avoid bacterial contamination of unused donor iliac grafts at the time of the initial transplant procedure in the primary recipient. To check for bacterial and fungal contamination we routinely culture storage fluid before use of the graft. In this series, we found only one case (5%) of bacterial contamination of the graft storage fluid, a lower incidence than has been reported in other studies for grafts stored in University of Wisconsin solution (20%). 25 An episode of cellulitis was, however, associated with this positive culture result, which was obtained after implantation of the graft. There has been no evidence to date in our patients for deep surgical infections involving the retroperitoneum, peritoneum, arterial wall, mycotic aneurysm, or endovascular infection.
At our center, we retain leftover cadaveric iliac vessels for up to 10 days, preserved in University of Wisconsin solution at 4°C. We have not had to delay surgical revascularization beyond 2 weeks because of lack of availability of donor vessels of the correct blood type. Most often, blood vessels are immediately available to allow surgical repair within several days. However, if there is an inadequate supply of vessels at any one transplant center, vessels could be shared between centers within a single organ procurement organization, or possibly between organ procurement organizations. Nationwide, there is clearly an ample supply of cadaveric iliac arterial grafts to provide conduit for all TRAS patients who might benefit.
We have successfully used preserved cadaveric iliac artery grafts to reconstruct transplant renal arteries for recurrent TRAS and following technically unsuccessful PTA. Reported success rates for PTA range from 70% to 90%, with a 20% to 40% incidence of recurrence. 7–11 Interventional radiologists have attempted to use expandable metallic endoprostheses for treatment of recurrent TRAS. 27 This is based on the successful use of stents as an adjunct to PTA in coronary, peripheral, and native renal vascular stenosis. 27 Use of intra-arterial stents for TRAS has had moderate clinical success in small numbers of patients. 27,28 The role of intra-arterial stents in the treatment of TRAS is not clear at this time and will require larger studies with longer follow-up. Our data suggest that surgical revascularization should be considered appropriate for recurrent TRAS following PTA.
We continue to use PTA as the initial therapy for TRAS in the majority of lesions, with the understanding that there is a significant incidence of recurrence following PTA. PTA is a relatively noninvasive procedure and can be done with minimal morbidity and discomfort to the patient. The majority of patients, when selected appropriately, will have a successful outcome following PTA. Patients who recur or who cannot undergo PTA for technical reasons should then be considered for operative repair. Primary surgical procedures are indicated for selected lesions such as anastomotic or ostial stenosis and kinks in the renal artery. In these circumstances we favor the use of ABO-matched, preserved cadaveric iliac artery graft as a useful arterial conduit to bypass or reconstruct the allograft renal artery.
Acknowledgments
The authors thank Drs. David Foley and Nancy Krieger for critical review of the manuscript. They also thank Joan Kozel for assistance with the figures and Barbara Voss, RN, for data collection.
Footnotes
Correspondence: Brian D. Shames, MD, H4/748 CSC, Department of Surgery, University of Wisconsin, 600 Highland Avenue, Madison, WI 53792.
E-mail: bd.shames@hosp.wisc.edu
Accepted for publication April 2, 2002.
References
- 1.Fervenza FC, Lafayette RA, Alfrey EJ, et al. Renal artery stenosis in kidney transplants. Am J Kidney Dis 1998; 31: 142–148. [DOI] [PubMed] [Google Scholar]
- 2.Wong W, Fynn SP, Higgins RM, et al. Transplant renal artery stenosis in 77 patients: Does it have an immunological cause? Transplantation 1996; 61: 215–219. [DOI] [PubMed] [Google Scholar]
- 3.Lacombe M. Arterial stenosis complicating renal allotransplantation in man: a study of 38 cases. Ann Surg 1975; 181: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray DWR. Graft renal artery stenosis in the transplanted kidney. Transplant Rev 1994; 8: 15–21. [Google Scholar]
- 5.Humar A, Uknis M, Papalois V, et al. Is there an association between cytomegalovirus and renal artery stenosis in kidney transplant recipients? [abstract]. Transplantation 2000; 69: S386. [Google Scholar]
- 6.Pouria S, State OI, Wong W, et al. CMV infection is associated with transplant renal artery stenosis. Q J Med 1998; 91: 185–189. [DOI] [PubMed] [Google Scholar]
- 7.Roberts JP, Ascher NL, Fryd DS, et al. Transplant renal artery stenosis. Transplantation 1989; 48: 580–583. [PubMed] [Google Scholar]
- 8.Greenstein SM, Verstandig A, McLean GK, et al. Percutaneous transluminal angioplasty. The procedure of choice in the hypertensive renal allograft recipient with renal artery stenosis. Transplantation 1987; 43: 29–32. [PubMed] [Google Scholar]
- 9.Fauchald P, Vatne K, Paulsen D, et al. Long-term clinical results of percutaneous transluminal angioplasty in transplant renal artery stenosis. Nephrol Dial Transplant 1992; 7: 256–259. [DOI] [PubMed] [Google Scholar]
- 10.Patel NH, Jindal RM, Wilkin T, et al. Renal artery stenosis in renal artery allografts: Retrospective study of predisposing factors and outcomes after percutaneous transluminal angioplasty. Radiology 2001; 219: 663–667. [DOI] [PubMed] [Google Scholar]
- 11.Benoit G, Moukarzel M, Hiesse C, et al. Transplant renal artery stenosis: experience and comparative results between surgery and angioplasty. Transplant Int 1990; 3: 137–140. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland RS, Spees EK, Jones JJ, et al. Renal artery stenosis after renal transplantation: The impact of the hypogastric artery anastomosis. J Urol 1993; 149: 980–985. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessandro AM, Pirsch JD, Knechtle SJ, et al. Living unrelated renal donation: the University of Wisconsin experience. Surgery 1998; 124: 604–610. [DOI] [PubMed] [Google Scholar]
- 14.Luzzio CC, Waclawik AJ, Gallagher CL, et al. Iliac artery pseudoaneurysm following renal transplantation presenting as lumbosacral plexopathy. Transplantation 1999; 67: 1077–1078. [DOI] [PubMed] [Google Scholar]
- 15.Viron B, Lacombe M, Raynaud AC, et al. Delayed extensive arterial dissection after percutaneous transluminal angioplasty for transplant renal artery stenosis. Nephron 1991; 58: 351–353. [DOI] [PubMed] [Google Scholar]
- 16.Rijksen JFWB, Koolen MI, Walaszewski JE, et al. Vascular complications in 400 consecutive renal transplants. J Cardiovas Surg 1982; 23: 91–98. [PubMed] [Google Scholar]
- 17.Benoit G, Hiesse C, Icard P, et al. Treatment of renal artery stenosis after renal transplantation. Transplant Proc 1987; 19: 3600–3601. [PubMed] [Google Scholar]
- 18.De Meyer M, Pirson Y, Dautrebande J, et al. Treatment of renal graft artery stenosis Comparison between surgical bypass and percutaneous transluminal angioplasty. Transplantation 1989; 47: 784–788. [DOI] [PubMed] [Google Scholar]
- 19.Grossman RA, Dafoe DC, Shoenfeld RB, et al. Percutaneous transluminal angioplasty treatment of renal transplant artery stenosis. Transplantation 1982; 34: 339–343. [DOI] [PubMed] [Google Scholar]
- 20.Dickerman RM, Peters PC, Hull AR, et al. Surgical correction of posttransplant renovascular hypertension. Ann Surg 1980; 192: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilney NL, Rocha A, Strom TB, et al. Renal artery stenosis in transplant patients. Ann Surg 1984; 199: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanley JC, Ernest CB, Fry NJ. Fate of 100 aortorenal vein grafts: Characteristics of late graft expansion, aneurysmal dilation and stenoses. Surgery 1973; 74: 931–944. [PubMed] [Google Scholar]
- 23.Travis JA, Hansen KJ, Miller PR, et al. Aneurysmal degeneration and late rupture of an aortorenal vein graft: Case report, review of the literature, and implications for conduit selection. J Vasc Surg 2000; 32: 612–615. [DOI] [PubMed] [Google Scholar]
- 24.Lavigne JP, Keppenne V, Limet R. Late rupture of a saphenous vein aortorenal graft. J Vasc Surg 1999; 29: 722–723. [DOI] [PubMed] [Google Scholar]
- 25.Martinez JA, Rigamonti W, Rahier J, et al. Preserved vascular homograft for revascularization of pediatric liver transplant: a clinical, histological, and bacteriological study. Transplantation 1999; 68: 672–675. [DOI] [PubMed] [Google Scholar]
- 26.Hohnke C, Adendroth D, Schleibner S, et al. Vascular complications in 1200 kidney transplantations. Transplant Proc 1987; 19: 3691–3692. [PubMed] [Google Scholar]
- 27.Fluck S, Preston R, McKane W, et al. Intra-arterial stenting for recurrent transplant renal artery stenosis. Transplant Proc 2001; 33: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 28.Sierre SD, Raynaud AC, Carreres T, et al. Treatment of recurrent transplant renal artery stenosis. J Vasc Interv Radiol 1998; 9: 639–644. [DOI] [PubMed] [Google Scholar]