Abstract
Most chemotherapeutic drugs kill cancer cells by indirectly activating checkpoint-mediated apoptosis after creating nonselective damage to DNA or microtubules, which accounts for their toxicity toward normal cells. We seek to target cancer cells by directly activating checkpoint regulators without creating such damage. Here, we show that β-lapachone selectively induces apoptosis in cancer cells without causing the death of nontransformed cells in culture. This unusual selectivity against cancer cells is preceded by activation of S-phase checkpoint and selective induction of E2F1, a regulator of checkpoint-mediated apoptosis. This study suggests direct checkpoint activation as a strategy against cancer.
Checkpoints are built into each phase of the cell proliferation cycle to safeguard genomic integrity (1–3). Checkpoint regulation to control DNA damage consists of sensing damage, transduction of information, and ultimately the execution of DNA damage responses by effectors (3). Cells seem to undergo apoptosis when the DNA damage is irreparable, or when conditions are adverse for their growth (3, 4). Checkpoints are depressed in cancer cells, resulting in accumulation of genetic damage (1–5).
Checkpoints have been explored as targets for cancer therapy. One strategy has been to enhance cellular sensitivity to chemotherapy by abrogating G2 checkpoint function (6). Another strategy has been to inhibit cell proliferation by using inhibitors of cyclin-dependent kinases (CDKs; ref. 7). These CDKs are essential components of the cell proliferation machinery both in normal and cancer cells. Therefore, they are primarily antiproliferative and have limited selectivity. Most chemotherapeutic drugs indirectly activate checkpoint-mediated apoptosis (4, 5) by first creating DNA or microtubule damage in cancer as well as in normal cells. Such nonspecific damage largely accounts for the severe toxicity and the limited selectivity against cancer cells.
β-Lapachone (3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b] pyran-5,6-dione) is an investigational anticancer agent that induces cell death in human cancer cells with a wide spectrum of activity (8, 9). It does not cause damage to DNA (10). We have reported its potent antitumor activity in xenografted human cancer models (8). The mechanism of cell death triggered by β-lapachone is unknown. It inhibits the catalytic activity of topoisomerase I (10). However, the concentration of β-lapachone required to inhibit topoisomerase I is above concentrations that induce apoptosis. NAD (P) H: quinone oxidoreductase (NQO1) has been proposed to be a target of β-lapachone (9). However, β-lapachone induced apoptosis in HL-60 and MDA-MB-468 cells that are deficient in NQO1 (9). Furthermore, NCM 460 cells express a level of NQO1 equal to that of SW 480 cells (unpublished data), yet the former cell line is resistant to β-lapachone.
Here, we report that β-lapachone selectively induces apoptosis in transformed cells but not in proliferating normal cells, which is an unusual property that is not shared by conventional chemotherapeutic agents. It activates checkpoints in the absence of DNA damage. This selective induction of apoptosis is preceded by the rapid and sustained increase of E2F1 level and activity in cancer cells. These results suggest direct checkpoint activators as selective agents against transformed cells.
Materials and Methods
Colony Formation Assay.
Exponentially growing cells were seeded at 1,000 cells per well in six-well plates and allowed to attach for 48 h. β-Lapachone was dissolved at a concentration of 20 mM in DMSO and were added directly to cells in <2 μl of concentrated solution (corresponding to a final DMSO concentration of <0.1%). Control plates received the same volume of DMSO alone. After 1–4 h, cells were rinsed, and drug-free medium was added. Cultures were observed daily for 10–20 days and then were fixed and stained with modified Wright-Giemsa stain (Sigma). Colonies of 30 cells were scored as survivors (8). Cells were maintained at 37°C in 5% CO2 in complete humidity. Human breast cancer cell lines MCF-7 and MCF 10A were cultured in MEM-α (GIBCO) supplemented with 10% (vol/vol) FCS/2 mM l-glutamine/1 mg/ml insulin. Normal colonic epithelial cell line NCM 460 was cultured in M3:10 culture media (Incell, San Antonio, TX). Human colon adenocarcinoma cell lines SW 480 and DLD1 were cultured in DMEM supplemented with 10% (vol/vol) FCS and 2 mM l-glutamine (GIBCO).
Cell Death Assay.
Cell death was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay or by trypan blue exclusion, as indicated. Briefly, cells were plated in a 96-well plate at 10,000 cells per well, cultured for 48 h in complete growth medium, then treated with β-lapachone for 4 h and cultured with drug-free medium for 24 h. MTT solution was added to the culture medium, and after 2 h, optical density was read with an ELISA reader. For the trypan blue exclusion assay, cells were cultured and treated in the same way. They were harvested, and trypan blue dye solution was added to the cell suspension. Total cell counts and viable cell numbers were determined with a hemacytometer (8). Apoptosis was determined by three independent assays. One assay determined the sub-G1 fraction of propidium iodide-stained nuclei, as described (8).
Western Blot Analysis.
Nuclear extract or whole-cell lysates were prepared and resolved by SDS/PAGE as described (8). The enhanced chemiluminescence (ECL) assay system was used to determine specific protein levels in these cell extracts. The filter was incubated with a second antibody that was conjugated with horseradish peroxidase. Finally, the filter was developed with detection reagents (RPN 2109; Amersham Pharmacia) and exposed to a hyperfilm-ECL (RPN 2103). To measure cytochrome c released from mitochondria whole-cell lysate, S-100 fractions were prepared from exponentially growing cells. The ECL assay system was used to detect the cytochrome c released from mitochondria (S-100 fraction) and poly(ADP-ribose) polymerase immunoblot analyses (8).
Cell Cycle Analysis and CDK Assay.
Cell cycle analysis was carried out by flow cytometry after staining cells with propidium iodide; nuclear extract preparation and kinase assays were performed as described (11). For each assay, 50 μg of cell extract was immunoprecipitated with 1 μg of purified polyclonal antibody against human cyclin A or CDK2. Histone H1 was used as the substrate for kinase assays.
Electrophoretic Mobility-Shift Assay.
DNA-binding activity of E2F1 was determined by using a 32P-labeled, 100-bp double-stranded DNA fragment containing E2F consensus motifs (12). It was chemically synthesized, and the recessed 3′ ends were labeled with [α-32P]dATP and Klenow fragments. Nuclear extracts were incubated at 30°C for 20 min with the 32P-labeled substrate DNA (20,000 cpm per reaction) in 20 μl of binding buffer (10 mM Tris⋅HCl, pH7.5/3 mM CaCl2/50 mM NaCl/0.1 M sucrose). Glycerol was added to a concentration of 5%. The reaction mixture was electrophoresed through a nondenaturing 4% polyacrylamide gel. The gel and buffer system contained 0.167× Tris-borate.
Results
Selective Killing of Transformed Cells by β-Lapachone.
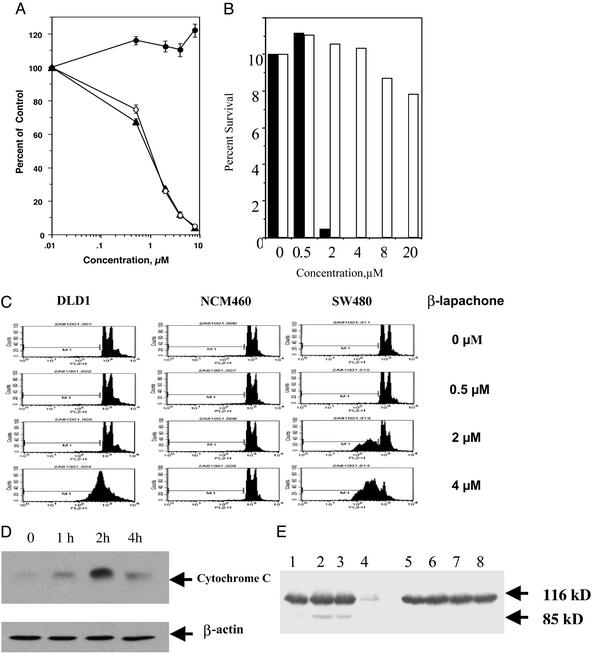
Unlike conventional chemotherapeutic agents, β-lapachone selectively induced cell death in human cancer cells, but not in normal cells under the similar treatment conditions (Fig. 1). It induced massive apoptosis in human myeloma cells that are resistant to dexamethasone and in myeloma cells from a patient who had failed conventional therapies. In contrast, proliferating peripheral blood mononuclear cells (PBMC) stimulated by phytohemagglutinin (PHA) were not affected by β-lapachone (Fig. 1A), suggesting that it is not a generalized antiproliferative or apoptotic agent.
Figure 1.
β-Lapachone selectively induced apoptosis of human cancer cells and not normal cells. (A) Multiple myeloma (MM) cells and proliferating peripheral blood mononuclear cells (PBMC) were treated with β-lapachone (0, 0.5, 2, 4, or 8 μM) for 24 h. Cell death was measured by the MTT assay. Results are the average of triplicates from one of the three independent experiments. MM.As (▴) is a cell line from a myeloma patient; MM.1R (○) is a myeloma cell line that is resistant to dexamethasone; PBMCs (●) were stimulated with PHA at 2 μg/ml for 72-h incubation before β-lapachone treatment. Control cells were treated with an equal volume of DMSO. (B) Human breast cancer cells (MCF-7) and nontransformed breast epithelial cells (MCF-10A) were treated with β-lapachone at the concentrations indicated for 4 h and incubated in drug-free media for 10–14 days. ▪, MCF-7; □, MCF-10A. Cell survival was determined by colony formation assay (8). Results are from one of two independent experiments. (C) Human colonic cancer cells (DLD1, SW480) and normal human colonic epithelial cells (NCM460) were treated with β-lapachone for 4 h. Cells were incubated in drug-free media for an additional 20 h and were harvested for flow cytometry analysis. Apoptosis was determined by the sub-G1 fraction (8). (D) Human colonic cancer cells (DLD1) were treated with β-lapachone at 4 μM for different times as indicated. Cytoplasmic cytochrome c was determined by immunoblotting with anti-cytochrome c. β-Actin was used as a loading control. (E) Human colon cancer (SW 480, lanes 1–4) and normal colonic epithelial cells (NCM 460, lanes 5–8) were treated with β-lapachone at 0 μM (lanes 1 and 5), 0.5 μM (lanes 2 and 6), 2 μM (lanes 3 and 7), or 4 μM (lanes 4 and 8) for 4 h, followed by incubation in drug-free media for an additional 4 h before cells were harvested for analysis of poly(ADP-ribose) polymerase by Western blot.
To investigate whether this selectivity holds true for carcinoma cells, we tested the effect of β-lapachone on proliferating nontransformed epithelial cells, in comparison with human cancer cells with a similar doubling time. β-Lapachone abolished colony formation by MCF-7 cells, a human breast carcinoma cell line. In contrast, the number of colonies of MCF-10A, a nontransformed human breast epithelial cell line, was not significantly reduced (Fig. 1B). β-Lapachone induced apoptosis (Fig. 1C) in human colonic adenocarcinoma cells with genomic instability (SW 480) and those with microsatellite instability (DLD1; ref. 13). Under the same treatment conditions, no apoptosis was detected in spontaneously immortalized normal human colonic epithelial cells (NCM 460 cells; Fig. 1C).
β-Lapachone rapidly induced the release of mitochondrial cytochrome c, which signals the onset of apoptosis, within 1 h after treatment and peaked at 2 h in human colon cancer cells (SW 480; Fig. 1D). Poly(ADP-ribose) polymerase was selectively cleaved in colon cancer cells (SW 480) but not in normal colonic epithelial cells (NCM 460; Fig. 1E). These results show that β-lapachone is a highly selective apoptosis inducer in cancer cells, suggesting sensitivity of cancer cells to checkpoint activators.
β-Lapachone Activates S-Phase Checkpoint.
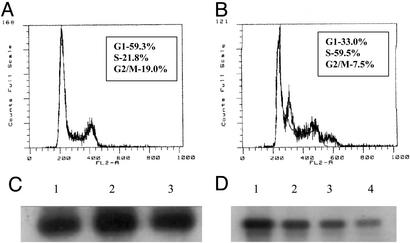
β-Lapachone predominantly induces S-phase checkpoint delay in cancer cells (Fig. 2 A and B). It is not a direct CDK inhibitor (Fig. 2C). However, cyclin A/CDK2 kinase activity was down-regulated in cells within 8 h after treatment with β-lapachone (Fig. 2D), which is consistent with a consequence of S-phase checkpoint activation.
Figure 2.
Activation of S-phase checkpoint by β-lapachone (A and B) is accompanied by indirect inhibition of cyclin A/CDK2 (C and D). DU145 prostate cancer cells were treated with vehicle control (A) or β-lapachone at 4 μM (B) for 4 h and incubated in drug-free medium for an additional 4 h. Cells were harvested for flow cytometry analysis (8). (C) Cyclin A/CDK2 was immunoprecipated from untreated cells, and the direct effect of β-lapachone on the kinase activity was determined with histone H1 as the substrate (8, 24). Lane 1, control; lane 2, 2 μM; lane 3, 4 μM. (D) DU145 prostate cancer cells were treated with or without β-lapachone for 4 h and incubated in drug-free medium for an additional 4 h. Nuclear extracts were prepared. Cylin A CDK2 were immunoprecipated, and the kinase activity was determined as described. Lane 1, control; lane 2, 0.5 μM; lane 3, 2 μM; lane 4, 4 μM.
β-Lapachone Rapidly and Specifically Induces E2F1 in Cancer Cells.
Because β-lapachone does not cause DNA damage (10), we determined its effect on checkpoint regulators. E2F has been implicated in regulating the G1/S transition and the S-phase checkpoint and also functions as a critical linkage between checkpoint activation and apoptosis (14–16). Although activation of E2F is required for cells to enter S phase, inactivation of E2F is necessary for cells to exit S phase. Sustained E2F activity results in apoptosis, as mediated by p53-dependent or -independent pathways (14–16).
To investigate the mechanism of checkpoint activation and apoptosis by β-lapachone, we determined its effect on E2F1. The protein level of E2F1, as determined by Western blotting, was markedly elevated in human pancreatic cancer cells (Fig. 3A) within 30 min of exposure at 0.5–4 μM. This rapid increase in E2F1 lasted >4 h, through completion of mitochondrial cytochrome c release (Fig. 1D). As shown in Fig. 3B, E2F1 was induced within 20 min by β-lapachone in human colon cancer cells, preceding cytochrome c release from mitochondria, suggesting the involvement of E2F1 in the early stage of apoptosis induced by β-lapachone.
Figure 3.
β-Lapachone selectively induced elevation of E2F1 protein in human pancreatic cancer cells (A) and colon cancer cells (B), but not in normal colonic epithelial cells (C). (A) Human pancreatic cancer cells (Paca-2) were exposed to β-lapachone at 0 μM (lane 1), 0.5 μM (lane 2), 2 μM (lane 3), and 4 μM (lane 4) for 0.5 h and were harvested for determination of E2F1 level by Western blot. Monoclonal antibody against E2F1 was obtained from Santa Cruz Biotechnology. (B) SW480 and NCM460 were treated with β-lapachone at 2 μM and were harvested after 20 min (lane 2), 1 h (lane 3), 2 h (lane 4), or 4 h (lane 5). Control cells were treated with an equal volume of DMSO (lane 1). Whole-cell extracts were prepared, and the E2F1 level was determined by Western blotting with a monoclonal antibody against E2F1. (C) Effects of β-lapachone on E2F2, -3, -4, and -5 were similarly determined from the same set of cell lysates of B with monoclonal antibodies from Santa Cruz Biotechnology. β-Actin was used as a loading control. (D) SW 480 cells (lanes 1–5) and NCM 460 cells (lanes 6–8) were treated with β-lapachone at 4 μM for 0.5 h (lanes 2 and 7), 1 h (lanes 3 and 8), 2 h (lane 4), or 4 h (lane 5). Control cells were treated with an equal volume of DMSO (lanes 1 and 6). Nuclear extracts were prepared, and E2F1 activity was determined by the electromobility shift assay by using a 32P-labeled, 100-bp, double-stranded DNA fragment containing three E2F consensus sequences (18). Arrow denotes the location of different forms of E2F protein–DNA complexes. Results represent one of three independent experiments. (E) Expression of pRB and phosphorylation status in cell lines used. Lane 1, DLD1; lane 2, SW480; lane 3, NCM 460; lane 4, MCF7; lane 5, MCF10A. Whole-cell lysates were prepared, and Western blotting was used to analyze pRB by using a monoclonal antibody from Santa Cruz Biotechnology.
The E2F1 level is under tight control in normal cells because of its important functions in cell proliferation and apoptosis (14–17). Because β-lapachone caused apoptosis only in cancer cells, we measured its effect on E2F1 in normal cells. β-Lapachone did not significantly induce E2F1 level in normal colonic epithelial cells (NCM 460; Fig. 3B) or in nontransformed human breast epithelial cells (MCF 10A). This selectivity of β-lapachone on E2F1 correlates with the selective induction of apoptosis in cancer cells. β-Lapachone is different from CD437, which induces E2F1 in normal cells (18), an effect possibly related to the DNA adduct formation in cells treated with CD 437 (19). Consistent with this possibility, E2F1 is elevated by radiation-induced DNA damage (12). It seems that E2F1 induction by DNA damage follows a much slower kinetics and proceeds at a lower magnitude than β-lapachone (12, 19, 20).
E2F is a family of transcriptional factors, consisting of six members (14–16). To investigate the specificity of β-lapachone on E2F1, we determined changes in the levels of other members of the E2F family. Transient elevation of E2F2 and E2F3 were observed 1–2 h after β-lapachone treatment (Fig. 3C). There was no elevation in E2F4 and E2F5, consistent with the fact that these two members function differently than E2F1, E2F2, and E2F 3 (14–16).
E2F1 functions as a transcription factor by binding to its specific motif (14–16). To determine whether E2F1 activity is elevated after β-lapachone treatment, we measured its DNA-binding activity by the electromobility shift assay (12). As shown in Fig. 3D, within 30 min of treatment with β-lapachone, E2F1 activity was significantly elevated within nuclei of SW 480 cells and lasted >4 h, through completion of mitochondrial cytochrome c release (Fig. 1D). Importantly, E2F1 activity was not significantly elevated in NCM 460 cells (Fig. 3D), which is consistent with the lack of elevation of E2F1 protein level (Fig. 3B). Similarly, E2F1 activity was significantly induced by β-lapachone in MCF 7 cells, but not in MCF 10A cells (unpublished data).
The Induction of E2F1 by β-Lapachone Is Not Related to Rb Status.
E2F1 activity is controlled by the tumor suppressor Rb pathway (14–16). Chen et al. (21) have shown that transformed cells with defective Rb or increased E2F1 are more sensitive to small peptide inhibitors of cyclin A/CDK, an effect which is presumably mediated through E2F1. To determine whether the differential induction of E2F1 activity by β-lapachone in normal and cancer cells is related to Rb status, we determined the Rb level and phosphorylation in cancer and nontransformed cells before treatment with β-lapachone. Although Rb is in a hyperphosphorylated state in MCF 7 (Fig. 3E, lane 4) compared with hypophosphorylated Rb in MCF 10A (lane 5), there was no difference in Rb phosphorylation between SW 480 (lane 2) and NCM 460 cells (lane 3). These results suggest that the selectivity of β-lapachone on E2F1 is independent of Rb status, which may be caused by the magnitude and rapidity of E2F1 induction by β-lapachone.
Discussion
The selectivity of β-lapachone against cancer cells supports the possibility of treating cancers by selectively inducing cancer cell death with checkpoint activators, i.e., activated checkpoint therapy (ACT). ACT differs from both secondary checkpoint activation and E2F1 elevation triggered by DNA damage from chemotherapy and radiation therapy (12, 20). Rather, ACT directly activates the regulatory pathways that link checkpoint activation with apoptosis. Depressed checkpoint function is a hallmark of malignancy (1–5). However, cancer cells retain effector pathways that connect checkpoint activators with apoptosis. The E2F pathway is one of the preserved checkpoint regulators in cancer cells that serve as important connections between checkpoints and apoptosis (14–16, 21).
It seems that the selectivity of β-lapachone against cancer cells occurs at the step of E2F1 induction (Figs. 2 and 3) and not the downstream apoptotic pathways. E2F1 has been shown to induce p53-dependent and -independent apoptosis, including p73 and Apaf1 pathways (14–16, 22). Correspondingly, β-lapachone induces apoptosis in cancer cells harboring p53 mutations, e.g., SW 480 cells (Fig. 1), and DU 145 cells in vivo (8), suggesting that β-lapachone is not subject to chemoresistance from p53 mutations in cancer cells.
The precise mechanism of E2F1 induction by β-lapachone remains to be defined. Given the selectivity and rapid kinetics, the mechanism is likely to be critical for efficient control of E2F1 levels in cancer cells. β-Lapachone inhibits cyclin A/CDK2 activity in cells (Fig. 2D). However, it was not a direct inhibitor of cyclin A/CDK2 (Fig. 2C), and the inhibition occurs after the increase of E2F1. Understanding the mechanism of E2F1 induction may uncover additional potential therapeutic targets in cancer cells.
It is particularly interesting that cells use the E2F switch for regulating both proliferation and apoptosis, representing another example that shows that replication of DNA is closely linked to apoptosis to safeguard the genomic integrity and to prevent excessive cell proliferation. Consistent with this model, inactivation of E2F1 in c-myc transgenic mice enhances tumorigenesis, suggesting tumor-suppressor function of E2F1 (23). It seems that there are two thresholds that determine E2F1 function (16). Once the E2F1 level reaches the first threshold, it promotes cells to pass the restriction point, therefore committing cells to DNA replication. However, after this important commitment, E2F1 switches to promote apoptosis if its level reaches the next threshold in the presence of irreparable DNA stress. We propose that β-lapachone rapidly and directly induces E2F1, thereby converting E2F1 from a proliferation regulator to a regulator of checkpoint-mediated apoptosis. (Fig. 4)
Figure 4.
Proposed apoptotic mechanism of β-lapachone.
The high degree of selectivity of β-lapachone is an unusual property that is not shared by conventional anticancer agents. Importantly, β-lapachone did not interfere with the proliferation of normal lymphocytes, demonstrating that it is not a generalized antiproliferative agent. These results suggest direct checkpoint activation as an avenue for developing selective anticancer agents.
Acknowledgments
We thank Dr. David E. Fisher for critical reading of the manuscript. This work was supported by a grant from Cyclis Pharmaceutical Inc. (Norwood, MA) and by the GI Cancer Research Fund from Beth Israel Deaconess Medical Center (to C.J.L.).
Abbreviations
- CDK
cyclin-dependent kinase
- β-lapachone
3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b] pyran-5,6-dione
References
- 1.Hartwell L H, Weinert T A. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 2.Pardee A B. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B B, Elledge S J. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 4.Rich T, Allen R L, Wyllie A H. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Lane D, Levine A J. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 6.Lau C C, Pardee A B. Proc Natl Acad Sci USA. 1982;79:2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sausville E A. Trends Mol Med. 2002;8, Suppl. 4:S32–S37. doi: 10.1016/s1471-4914(02)02308-0. [DOI] [PubMed] [Google Scholar]
- 8.Li C J, Li Y Z, Pinto A V, Pardee A B. Proc Natl Acad Sci USA. 1999;96:13369–13374. doi: 10.1073/pnas.96.23.13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pink J J, Planchon S M, Tagliarino C, Varnes M E, Siegel D, Boothman D A. J Biol Chem. 2000;275:5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 10.Pardee A B, Li Y Z, Li C J. Curr Cancer Drug Targets. 2002;2:227–242. doi: 10.2174/1568009023333854. [DOI] [PubMed] [Google Scholar]
- 11.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 12.Blattner C, Sparks A, Lane D. Mol Cell Biol. 1999;19:3704–3713. doi: 10.1128/mcb.19.5.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengauer C, Kinzler K W, Vogelstein B. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 14.Sears R C, Nevins J R. J Biol Chem. 2002;277:11617–11620. doi: 10.1074/jbc.R100063200. [DOI] [PubMed] [Google Scholar]
- 15.Nahle Z, Polakoff J, Davuluri R V, McCurrach M E, Jacobson M D, Narita M, Zhang M Q, Lazebnik Y, Bar-Sagi D, Lowe S W. Nat Cell Biol. 2002;4:859. doi: 10.1038/ncb868. [DOI] [PubMed] [Google Scholar]
- 16.Trimarchi J M, Lees J A. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- 17.Lomazzi M, Moroni M C, Jensen M R, Frittoli E, Helin K. Nat Genet. 2002;31:190–194. doi: 10.1038/ng891. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Rishi A K, Dawson M I, Tschang R, Farhana L, Boyanapalli M, Reichert U, Shroot B, Van Buren E C, Fontana J A. Cancer Res. 2000;60:2025–2032. [PubMed] [Google Scholar]
- 19.Zhao X, Demary K, Wong L, Vaziri C, McKenzie A B, Eberlein T J, Spanjaard R A. Cell Death Differ. 2001;8:878–886. doi: 10.1038/sj.cdd.4400894. [DOI] [PubMed] [Google Scholar]
- 20.Meng R D, Phillips P, El-Deiry W S. Int J Oncol. 1999;14:5–14. [PubMed] [Google Scholar]
- 21.Chen Y N, Sharma S K, Ramsey T M, Jiang L, Martin M S, Baker K, Adams P D, Bair K W, Kaelin W G., Jr Proc Natl Acad Sci USA. 1999;96:4325–4329. doi: 10.1073/pnas.96.8.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa Y, Nishimura N, Furukawa Y, Satoh M, Endo H, Iwase S, Yamada H, Matsuda M, Kano Y, Nakamura M. J Biol Chem. 2002;277:39760–39768. doi: 10.1074/jbc.M200805200. [DOI] [PubMed] [Google Scholar]
- 23.Rounbehler R J, Rogers P M, Conti C J, Johnson D G. Cancer Res. 2002;62:3276–3281. [PubMed] [Google Scholar]