Abstract
To regulate expression of a transferred gene in response to an exogenous compound, we have combined a high capacity adenoviral vector devoid of all viral coding sequences with a regulatory system that can be used to express a target gene in vivo in a selected site and at a desired time. This system uses a chimeric transactivator, GLp65, which consists of a mutated progesterone receptor–ligand binding domain fused to the GAL4 DNA binding domain and part of the activation domain of the human p65 protein, a component of the NF-κB complex. In the presence of the antiprogestin mifepristone, this chimeric regulator binds to a target gene containing the 17-mer GAL4 binding site, resulting in an efficient ligand-inducible transactivation of the target gene. We inserted the regulator GLp65 and a regulable human growth hormone target gene containing the 17-mer GAL4 binding site into the same adenoviral vector. To obtain tissue-specific expression of the target gene, we coupled the regulator to a liver-specific promoter. Infection of HepG2 cells and experimental mice with the adenovirus resulted in consistently high induction levels of human growth hormone in the presence of mifepristone whereas the transgene expression was undetectable in the absence of the ligand. Taken together, our regulable adenoviral vector represents an important tool for transgene regulation that can be used for potentially diverse applications, ranging from tissue-specific gene expression in transgenic animals to human gene therapy.
The ability to transfer foreign genes into an organism is a major goal in a wide variety of applications, ranging from tissue-cultured cells to transgenic animals and human gene therapy. Because endogenous genes are expressed at specific time points and at specific levels, constitutive expression of transferred genes is unsatisfactory. Different regulatory systems have been developed to approach this problem of target gene regulation. We have recently developed a regulable system (1–3) that can be used to express a target gene in vivo in a specific tissue, at a desired time, and under the control of an oral, nontoxic chemical. This system used a chimeric regulator, GLVP, consisting of a mutated human progesterone receptor–ligand binding domain (LBDΔ) fused to the yeast GAL4 DNA binding domain and the herpes simplex virus transcriptional activation domain VP16 (Fig. 1A). In the presence of the antiprogestin mifepristone, but not endogenous molecules present in mammalian tissues and organs, this chimeric regulator binds to a target gene containing the 17-mer GAL4 upstream activation sequence and results in efficient ligand-inducible transactivation of the target gene (2, 3). Most importantly, the gene regulator responded to mifepristone at a concentration that has no endogenous antiprogesterone or antiglucocorticoid activity.
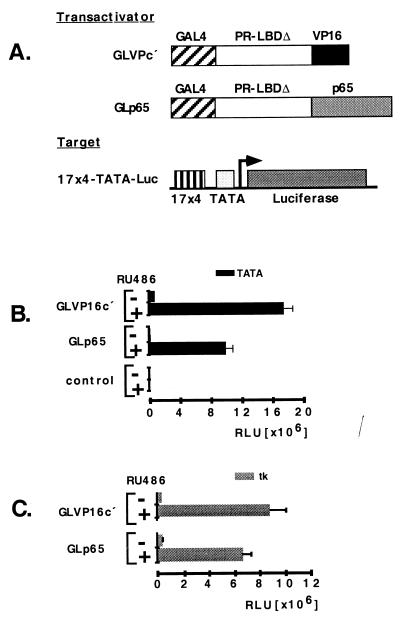
Figure 1.
(A) Regulatory system. The regulator GLVPc′ consists of a mutated human progesterone receptor–LBDΔ, a DNA binding domain of yeast GAL4 (GAL4–DNA binding domain), and an activation domain of herpes simplex virus (VP-16). Regulator GLp65 contains the activation domain of p65. The target consists of four GAL4 binding sites and a TATA-box linked to the luciferase reporter gene. Luc, luciferase; PR, progesterone receptor. (B) Mifepristone-dependent target gene induction by GLVP compared with GLp65. GLVPc′ or GLp65 (0.3 μg per well on a 6-well plate) were transiently transfected in Hela cells with the 17 × 4-TATA-luciferase as a reporter (0.3 μg per well on a 6-well plate) (B) by using 17 × 4-tk-luciferase as a reporter (C). The luciferase activity is shown as relative luciferase units (RLU). Control, transfection of the reporter and expression vector backbone; +, presence of mifepristone (Ru 486) [10−8]; −, absence of mifepristone (Ru 486). Error bars show standard deviation.
We decided to examine alternatives to the viral VP16 activation domain to preclude a possible immune response caused by the anticipated antigenicity of this domain. By replacing VP16 with a variety of human-derived activation domains, we show that a region of the human p65 (4), a member of the NFκB family, allows retention of the potent inducibility of GLVP.
To facilitate initial delivery of our inducible system in vitro and in vivo, we have developed an adenoviral vector-mediated gene transfer strategy. Previous results have shown that viral delivery has the following inherent problems: (i) expression of viral proteins in infected cells is believed to trigger a cellular immune response that precludes long-term expression of the transferred gene; and (ii) the insert capacity of adenoviral vectors previously has been limited to 8 kilobases of transgenic sequence. However, an adenoviral vector recently has been constructed (5, 6) that contains no viral coding sequences and possesses a very large insert capacity (up to 35 kilobases). To combine this improved adenoviral vector with our regulatory system, we have inserted into the vector a single regulatory cassette containing the regulator GLp65 and a regulable human growth hormone (hGH) target gene coupled to the 17-mer GAL4 binding site. To obtain tissue-specific expression of the target gene, we have coupled the regulator to the liver-specific transthyretin (TTR) promoter region (7, 8). Finally, to investigate the effect of an insulator on the regulable adenoviral target gene expression, the 5′element of the chicken β-globin domain (9) has been inserted between the target gene and the regulator. Using this adenoviral vector in combination with our inducible regulatory system, we successfully demonstrate potent inducible expression of hGH in both cultured (liver tumor-derived) HepG2 cells and in experimental mice.
MATERIALS AND METHODS
Construction of GLp65.
A HindIII-BamHI fragment of 680 bp was isolated from PAP CMV–GL914VPc′SV (3) and was cloned into the HindIII-BamHI site of a pUC18 plasmid. The resulting construct was named pUC-LBD914VPc′SV. The p65 activation domain (residues 286–550) was isolated from Gal4-p65 long (from Lienhard Schmitz, Deutsche Krebsforschungs Zentrum, Heidelberg) (4) by an EcoRI-BamHI digest. This fragment was ligated with a SalI linker TCGACGAGATATCAAGCAG to pUC-LBD914VPc′SV after VP16 was excised by SalI-BamHI, and the resulting plasmid was named pUC-LBD914p65. After digesting both this construct and PAP cytomegalovirus–GL914VPc′SV with HindIII-BamHI, the resulting fragments were ligated together to create the chimeric regulator GLp65.
Construction of Vector Containing Both Regulator and Target Gene.
Reporter plasmids p17 × 4-TATA-luciferase containing the adenovirus major late E1b TATA box and p17 × 4-tk-luciferase containing the thymidine kinase gene promoter have been described (10). To combine our regulator GLp65 with an hGH target gene on one plasmid, we created the following constructs. GLp65 first was isolated from PAP CMV–GLp65 by a complete KpnI and a partial BamHI digestion to generate a BamHI-KpnI fragment. This fragment then was ligated to PAP TTRBSV (3) to create PAP TTRB-GLp65SV. Second, TTRB GLVP SV was excised from PAP TAGH TTRB GLVP SV (3) by AscI-PacI digestion. TTRB-GLp65SV then was inserted in the AscI-PacI digested vector, resulting in PAP-GH-GLp65, which consists of the hGH genomic gene under the control of a TATA promoter and the GLp65 regulator driven by the liver-specific promoter TTRB (7, 8). PAP-GH-H-GLp65 was constructed in a similar manner, except that an additional insulator sequence from the 5′element of the chicken β-globin domain (obtained from G. Felsenfeld, National Institutes of Health) (9) was inserted by digesting PAP-GH-GLp65 with blunt-ended AscI and was ligated with a blunt-ended 2.4-kilobase BamHI-XhoI fragment from pBS-HS4, a 5′element of the chicken β-globin domain.
Adenoviral Constructs.
The plasmid pSTK119, which was used to construct the adenoviral vectors, has a 22.5-kilobase insert in the multiple cloning site of pBluescript KSII with, from the left to the right, the following essential features: the left terminus of adenovirus type 5 (nucleotides 1–440), a 16,054-bp EclXI/PmeI fragment of the human hypoxanthine-guanine phophoribosyltransferase gene (nucleotides 1,799–17,853 in gb: humhprtb), a 6,545-bp EcoRV fragment of the C346 cosmid (nucleotides 10,205–16,750 in gb: L31948), and the right terminus of adenovirus type 5 (nucleotides 35,818–35,935). To construct regulable adenoviral vectors, the regulatory expression cassette was isolated from PAP-GH-GLp65 by NotI digestion and was subcloned into the EclXI site of AdSTK119, resulting in GH-GLp65. The adenoviral vector GH-H-GLp65 was constructed in an analogous manner by using an insert isolated from PAP-GH-H-GLp65.
Cell Culture and Transient Transfection Assays.
HeLa (human epithelial cervix carcinoma) cells were grown in DMEM supplemented with 5% fetal bovine serum. Twenty-four hours before transfection, 3 × 105 cells were plated on 6-well collagen-coated dishes in DMEM with 5% dextran-coated charcoal stripped serum. Cells were transfected with the indicated amounts of DNA by using lipofectin (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Eighteen hours later, cells were washed with 1× Hanks’ balanced salt solution and DMEM, plus 5% stripped serum before an indicated amount of mifepristone (dissolved in 80% ethanol) was added. After 36 h, the cells were harvested, and cell extract was assayed for luciferase activity by using the luciferase assay system (Promega). Data is presented as the mean (±SD) of triplicate values.
Rescue of GH-GLp65 and GH-H-GLp65 Adenoviral Vectors.
Adenoviral constructs were cleaved by PmeI and were transfected into 293Cre4 cells. Subsequently, the cells were infected with loxP helper virus AdLC8cluc (11). To increase the titer, vector lysates were passed through 293-Cre4 cells several times, and the remaining helper viruses were separated by CsCl equilibrium density centrifugation. The detailed procedure for adenoviral rescue and virus characterization has been described (6). The concentration of the viral particles for GH-H-GLp65 was 4.1 × 1011/ml and for GH-GLp65 was 7.8 × 1011/ml. The particle/infectious units’ ratio was 20:1 with both vectors. The contamination of lox-P helper virus in the virus preparation was ≈0.01–0.05%. In addition, the viral preparation did not contain any replication competent adenoviruses.
Infection of HepG2 cells.
HepG2 (human epithelial hepatoblastoma) cells were maintained as described. Cells (2 × 105) were plated onto 6-well dishes in DMEM with 5% dextran-coated charcoal-stripped serum. Cells (2 × 105) were infected with 1 × 109 viral particles (5 × 107) infectious units, at a multiplicity of infection of 250. The viral particles were left on the cells for 3 h, then cells were washed with 1× Hanks’ balanced salt solution and DMEM containing 5% stripped serum, and the indicated amount of mifepristone was added. The levels of hGH in the medium were measured 24 h later by using a radioimmunoassay (Nichols Institute, San Juan Capistrano, CA) according to the manufacturer’s protocol.
Mouse Strains.
C57BL/6 mice were purchased from The Jackson Laboratory. All mice were 8–10 weeks old at the time of injection.
hGH Analysis in Adenoviral-Infected Mice.
C57BL/6 mice were infected by tail vein injection with 2 × 109 infectious units of either GH-GLp65 or GH-H-GLp65 diluted in PBS. Mice were given mifepristone (dissolved in sesame oil) or vehicle control at the specified dose and at indicated time points by i.p. or oral routes. At definite time points, mice were bled from the ophthalmic orbit by using a glass capillary or from the tail vein. Serum was obtained by blood incubation for 1 h at room temperature followed by centrifugation of the samples for 10 minutes at 10,000 × g. Serum hGH levels were detected by using a radioimmunoassay. When hGH levels exceeded the assay limit of 50 ng/ml, serum dilutions were performed in 1× PBS. The detection limit of the hGH assay is 0.5 ng/ml.
RESULTS
Regulator Modifications.
To optimize our regulatory system for gene therapy and to minimize possible toxic and immunogenic reactions, we replaced the viral VP16 activation domain with the human p65 activation domain (residues 286–550) and constructed the regulator GLp65 (Fig. 1A). To compare the ability of these regulators to induce a target gene in a mifepristone-dependent manner, we cotransfected regulator together with a reporter plasmid containing the luciferase gene driven by four copies of the consensus GAL4-binding site (17-mer) upstream of either a TATA or tk promoter into HeLa cells.
Fig. 1 B and C shows the potential of our different regulators to activate target gene expression in transient transfection. By using a TATA promoter, the basal activity of GLp65 is significantly lower than that of GLVPc′ in the absence of mifepristone. When mifepristone was added, both regulators showed ligand-dependent target gene expression. GLVPc′ induced slightly higher expression levels of the target gene as compared with GLp65. Because basal expression of GLp65 is usually lower, induction with this construct results in higher fold activation. Transfecting the expression plasmid backbone as a control resulted in no activation of the reporter plasmid. When using a tk promoter linked to the reporter plasmid (Fig. 1C), both regulators show similar low basal expression in the absence of mifepristone, as well as similar inducible target gene expression on mifepristone administration. These results demonstrate that the regulator GLp65, which contains the human p65 activation domain, has a similar potency in the induction of target gene expression in transient transfection when compared with GLVPc′. In addition, a lower basal expression level was observed in the absence of the ligand. Overall, the performance of our regulatory system seems to be promoter-dependent. In the case of the GLp65 regulator, optimal mifepristone-dependent transgene regulation appears to require a TATA promoter.
Construction of a Regulable Adenoviral Vector.
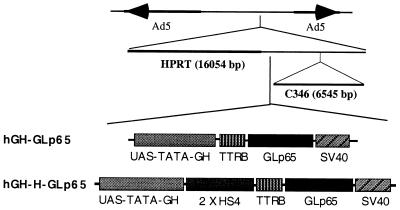
To facilitate initial delivery of our inducible system in vivo, we proposed the use of an adenoviral vector-mediated gene transfer strategy in which the virus has all viral coding sequences removed. Into this vector, we inserted a regulable expression cassette (Fig. 2). To achieve tissue-specific expression of the regulator and target protein, we first placed the regulator GLp65 encoding a GAL4 DNA-binding site, a progesterone receptor–LBD, and a p65 activation domain together with the SV40 polyA under control of the TTRB fragment, which contains a liver-specific promoter and enhancer. To combine the regulator with the target gene, we fused the coding sequence for hGH, under control of a GAL4 binding site and a TATA promoter, together with the GLp65 transcription unit. This adenoviral construct was named hGH-GLp65. Because the use of insulator sequences has been shown to improve gene expression in vivo on occasion, we used a 5′element of the chicken β-globin domain (2 × HS4) to investigate the insulator effect on adenoviral mediated gene transfer. To do this, we created a second adenoviral construct (hGH-H-GLp65) in which we inserted a chromosomal insulator between the hGH and the GLp65 cassette.
Figure 2.
Structure of hGH-GLp65 and hGH-H-GLp65. The constructs contain the left terminus of adenovirus type 5 (nucleotides 1–440), a 16,054-bp fragment of the human hypoxanthine-guanine phophoribosyltransferase gene, a regulatory cassette containing the regulator GLp65. UAS-TATA-GH, hGH under upstream activation sequence–TATA control; 2 × HS4, insulator, a 5′element of the chicken β-globin domain (from G. Felsenfeld); TTRB, liver-specific promoter enhancer (from R. Costa); GLp65, inducible gene switch p65 activation domain; SV40, poly(A), the 6,545-bp fragment out of the C346 cosmid and the right terminus of adenovirus type 5 (nucleotides 35,818–35,935). HGH-H-GLp65 contains an insulator sequence; hGH-GLp65 does not.
Inducible hGH Expression Using Adenoviral Constructs in Transient Transfection Assays.
After generating the adenoviral particles, we examined the ability of the viral vector to infect hepatocytes and regulate expression of hGH in cell culture. The infection was carried out for 3 h, then the medium was changed, and mifepristone was added as appropriate. hGH was measured in the medium after 48 h by a radioimmunoassay. As seen in Table 1, both adenoviral vectors regulate the expression of hGH in a mifepristone-dependent manner and express in the presence of mifepristone up to 20 μg of hGH/1 ml of medium. In the absence of mifepristone, hGH-H-GLp65 harboring the insulator shows no detectable expression of hGH whereas hGH-GLp65 seems to express hGH at a very low level in the absence of the ligand.
Table 1.
Adenovirus-mediated inducible hGH expression in hepatocytes
| Construct | Mifepristone | hGH, ng/ml |
|---|---|---|
| No virus | − | n.d. |
| No virus | + | n.d. |
| hGH-GLp65 | − | 5 |
| hGH-GLp65 | + | 19,000 |
| hGH-H-GLp65 | − | n.d. |
| hGH-H-GLp65 | + | 22,000 |
The viral construct without insulator (hGH-GLp65) was compared with the construct containing the insulator (hGH-H-GLp65). Cells 2 × 105 were infected with 1 × 109 viral particles. Three hours after infection, the media was changed, and antiprogestin mifepristone at a concentration of 10−8 was added. Twenty-four hours later, hGH was monitored by using a radioimmunoassay. The table displays the amounts of hGH in nanograms per milliliter cell media. n.d., no detectable levels of hGH.
Inducible hGH Expression Using Adenoviral Constructs in Vivo.
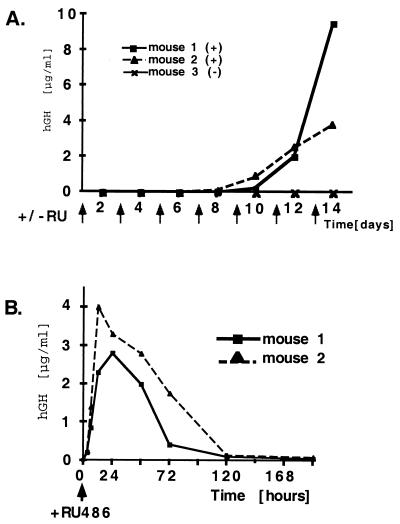
To assess the ability of the adenoviral constructs to effect regulable expression of hGH in vivo, we infected C57 black 6 mice with 1 × 109 infectious viral particles by tail vein injection. To investigate the time period between viral infection and hGH expression, mice received i.p. injections of mifepristone over a period of 2 weeks after a single tail vein injection of the virus. As shown in Fig. 3A, serum hGH is not detectable until day 8. At day 10 after viral infection, hGH is detectable, and the levels increase sharply. At day 14, up to 10 μg/ml hGH is detectable in the serum (50,000-fold induction). It is important to note that the transgene expression is undetectable in the absence of the ligand. These results indicate that optimal mifepristone inducible hGH expression is achieved 2 weeks after infection with the viral constructs.
Figure 3.
Induction of hGH on adenoviral transduction. (A) C57BL/6 mice (8–10 weeks old) were infected in the tail vein at day 0 with 1 × 109 infectious particle units of hGH-GLp65. Mifepristone (Ru 486) (250 μg/kg) was administered every second day after infection for a period of 2 weeks as indicated by arrows. Mice were bled at different time points, and serum hGH was analyzed by radioimmunoassay. Mice 1 and 2 received i.p. injections of mifepristone; mouse 3 (minus mifepristone) received sesame oil. hGH serum levels are shown micrograms per milliliter. (B) Kinetics of inducing hGH in mice 2 weeks after adenoviral infection. Mice infected for 2 weeks with the regulable adenoviral construct hGH-GLp65 were induced with 500 μg/kg mifepristone as indicated by an arrow. Blood was drawn from the mice 3, 6, 12, 24, 48, 72, 120, and 192 h after mifepristone (Ru 486) administration, and hGH was measured in the serum by a radioimmunoassay. hGH serum levels of individual mice are shown in micrograms per milliliter.
Kinetics of Induction of hGH Gene Expression.
To investigate the kinetics of the regulatory system, mice received a single mifepristone administration 2 weeks after the initial infection, and serum hGH levels were measured at different time intervals. Three hours after administration of the drug, hGH levels were detectable in the serum of the animals (Fig. 3B). A maximum level of hGH was observed 12 h after mifepristone administration. It decreased to low hGH serum levels at 120 h and was undetectable at 192 h. This decline of hGH expression correlates well with the metabolism of mifepristone in the mice. In contrast to the slow kinetics of hGH expression observed directly after the initial viral infection (Fig. 3A), the antiprogestin-mediated induction of hGH in these mice (Fig. 3B) is rapid and can be detected within hours.
Repetitive Induction of hGH Expression.
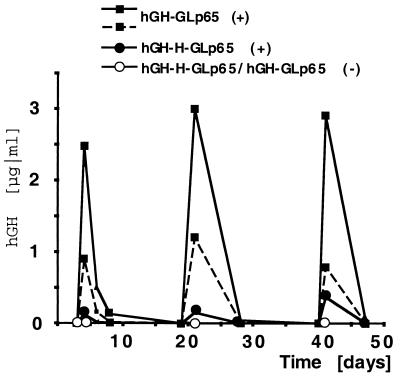
To examine whether hGH expression could be reinduced, an identical dose of mifepristone was administered at multiple time points to mice infected with regulable adenoviral vectors. Mice receiving multiple mifepristone administrations could be induced repeatedly over an extended period of time (Fig. 4). Twelve hours after a single administration of mifepristone (250 μg/kg), a strong induction of hGH (2.5 μg/ml) was detected, and, over time, these levels declined until hGH serum levels were no longer detectable. Similar expression levels of hGH could be obtained by repeated administration of the drug (250 μg/kg) whereas mice receiving only sesame oil had no detectable hGH serum levels. Another group of experimental animals could be reinduced up to five times over a period of 12 weeks (data not shown). This group of animals responded equally well to oral mifepristone administration with comparable hGH levels (data not shown). These results demonstrate that, by infecting mice with our regulable adenoviral vector, a transgene can be induced multiple times to the same extent on mifepristone administration in vivo.
Figure 4.
Repetitive induction of hGH in transduced mice. Mice infected with hGH-GLp65 or hGH-H-GLp65 adenoviral vectors were induced three times with 250 μg/kg mifepristone over a time period of 50 days. hGH was measured before, 12 h after, and 7 days after mifepristone administration. Graph shows independent mice that received mifepristone (+) or just carrier as a control (−). Serum levels of hGH are shown in micrograms per milliliter.
Insulator Effect on hGH Expression.
Fig. 4 also shows the in vivo effect of an insulator sequence when combined with an adenoviral vector. Both adenoviral vectors hGH-GLp65 (no insulator) and hGH-H-GLp65 (with insulator) are mifepristone inducible and show similar kinetics after viral infection. A possible difference between the two vectors is the expression level of the transgene. As the graph shows, hGH-GLp65 seems to have a higher expression level of hGH compared with the vector containing the insulator sequences (hGH-H-GLp65). This finding was observed consistently in all experiments presented. Mice infected with hGH-GLp65 consistently exhibited higher transgene expression levels than mice infected with the vector harboring the insulator. However, further and more detailed studies using additional animals are required to firmly support this notion. In contrast to this, the data we have obtained when transducing hepatic cell lines show similar expression levels with the two vectors. In addition, both adenoviral vectors show no detectable expression levels of hGH in the absence of mifepristone in vivo whereas in cell culture hGH-GLp65 shows low basal hGH expression. Thus, by using our regulable adenoviral vector, a difference between infection of cultured cells and experimental mice can be observed. The underlying mechanism of these differences has yet to be delineated.
Physiological Effect of hGH After Prolonged Expression.
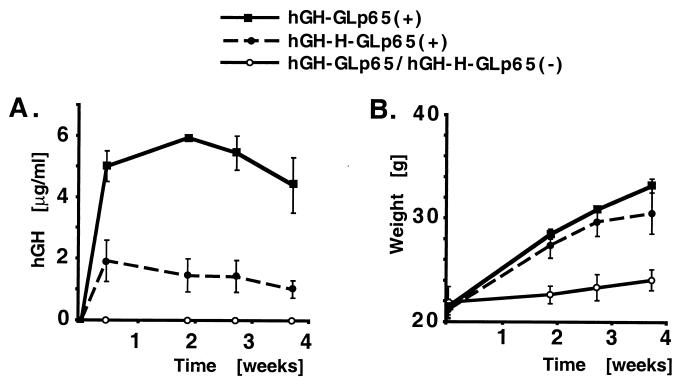
To achieve expression of hGH over a longer period of time, adenoviral infected mice received biodegradable pellets containing mifepristone introduced by s.c. implanting. Blood was drawn from these animals at indicated time points after implantation. Because it is known that constitutive expression of hGH in mice leads to growth stimulation (12), the weight of the animals also was monitored to show the physiological effect of the induced protein. Mice receiving the mifepristone pellet expressed hGH over a prolonged period of time whereas animals receiving only the carrier showed no detectable amounts of hGH (Fig. 5A). This data correlates with the weight gain seen for the mice receiving mifepristone (Fig. 5B). At day 3 after mifepristone administration, the mice showed hGH levels of up to 5 μg/ml. Over the next 10 days, expression levels rose to a concentration of 6 μg/ml. This hGH expression was monitored for up to 4 weeks. In response to the high levels of growth hormone expression, mice increased in weight by up to 60% within this time period. However, adenoviral-infected mice treated with carrier showed only a slight increase in weight. Over the time span of 4 weeks, hGH levels decreased very slightly in the animals. This was anticipated because hGH has been shown to be immunogenic in mice (13), and this marginal decrease in hGH could be caused by neutralizing antibodies raised against the protein. When hGH was induced multiple times over a short period of time, we were able to express the transgene repeatedly to the same extent for up to 2 months (Fig. 4). This experiment again shows that mice infected with adenoviral constructs harboring the insulator sequence have significantly lower hGH expression levels than when the infection was performed with the vector lacking this sequence.
Figure 5.
Long-term expression of hGH in transduced mice. Mice infected with hGH-GLp65 or hGH-H-GLp65 received, 4 weeks after infection, biodegradable pellets (360 μg/pellet, released in 60 days) by transplantation containing mifepristone (+) or carrier (−) only. Mice were weighed, and blood was drawn 3, 13, 20, and 27 days after drug administration. (A) hGH levels (micrograms per milliliter). The numbers of mice for each construct is three; bars show the standard error. (B) Weight of the mice (grams).
DISCUSSION
Recently, a variety of regulatory systems have been developed with the goal of regulating target gene expression (14–18). The desirable goals of such inducible systems are to achieve low basal expression with a high inducibility and rapid kinetics of induction on administration of a nontoxic and easily deliverable drug. The combination of our regulatory system with a high capacity adenoviral vector as described here made certain regulator modifications necessary. To facilitate future applications of our regulatory system in human gene therapy, it was necessary to replace the viral VP16 activation domain with other mammalian transcription factor activation domains because there is a higher probability that the VP16 protein could cause an immune response in human. In addition, high expression levels of the VP16 activation domain are known to have a squelching effect and can be toxic to cells (19, 20). After replacing VP16 with a variety of human derived activation domains, we chose p65, a partner of NF-κB in the human RelA heterodimeric transcription factor, because it is known to possess a strong potential to activate transcription (4). In comparison, the VP16 and the p65 derived regulators show similar inducibility on mifepristone induction. In fact, the magnitude of the GLp65 induction is superior to that of the GLVP regulator because of the low basal activity of the GLp65 regulator. Because of its nonviral p65 activation domain and its strong inducibility, the modified version (GLp65) of our inducible regulator has potential for future use in human gene therapy.
To complement the modification of our regulatory system and to enhance the efficiency of in vivo delivery, we decided to use a high-capacity adenoviral vector lacking all viral sequences (5, 6, 11), which could minimize toxicity and immunogenicity of the viral proteins known to cause short duration of target gene expression. Infecting mice with the regulable adenoviral vector, we show mifepristone-dependent induction of the transgene. The initial time delay of 8 days between viral infection and hGH inducibility on mifepristone administration was somewhat unexpected because other investigations using the new adenoviral vector have shown that, when under control of a constitutive promoter, target gene expression can be detected 3 days after infection (6). Two alternative reasons could explain the difference: (i) The liver-specific promoter used in our investigations to drive the GLp65 expression might need a defined concentration of transcription factors, and the assembly of specific transcriptional complexes might take some time, both of which could contribute to the delay of the regulator expression. (ii) To be able to induce target gene expression in a potent manner, the regulator concentrations need to exceed a specific threshold, which slowly builds up in the cells within the first few days after viral infection (Fig. 3A). Once the transduced gene is inducible in the animals, our regulatory system shows a fast response to the inducer such that maximal transgene expression can be attained 12 h after induction (Fig. 3B).
In our studies, we observed different expression levels of the transgene on administration of different amounts of mifepristone (data not shown). This is an important goal because, for gene therapy, the expression level of most transgenes appears to require a therapeutic “window” in which a successful gene transfer may be accomplished. The doses of mifepristone needed for induction in our regulatory system (0.1–0.5 mg/kg) is far below levels at which mifepristone is used as an antiprogestin (10 mg/kg) together with prostaglandin to terminate pregnancy. Administration of mifepristone at levels much higher than those necessary for transgene induction in our regulatory system have been administered safely to patients on a daily basis to treat different diseases (21, 22). Thus, it is likely that mifepristone, at this low concentration, can serve as a potent inducer for human gene therapy, even for a prolonged period of time.
Chromatin insulators are involved in position-independent expression of transgenes and have been shown to confer chromosomal integration site-independent transgene expression in transgenic mice (3). Using this insulator in combination with our regulable adenoviral vector, we obtained different effects, depending on whether the infection was carried out in transient transfection or in vivo. The mechanism of this differential effect caused by the insulator is still unclear and raises questions that have yet to be addressed.
The ability to transfer large DNA elements and the ability to regulate the transgene expression over a long period of time are important criteria for the success of human gene therapy in the future. Here, we combine a high capacity adenoviral vector deficient of all viral coding sequences with a single regulatory expression cassette to achieve persistent and inducible transgene expression in vivo. Induction was comparable when mifepristone was given by i.p. or oral routes. This combination represents an important advancement for transgene regulation that can be used for diverse applications, ranging from tissue-specific gene expression in transgenic animals to chronic human gene therapy.
Acknowledgments
We thank M.-J. Tsai for helpful discussion and Lucio Pastore for helping with the tail vein injections and for helpful comments. We thank Eduard Cho for technical support, Tyler Pierson for technical help and discussion, and Merck for providing 293Cre4 cells and AdLC8luc. We are also grateful to N. McKenna, Nail Baron, Kurt Schillinger, and Carena Chai for critical reading of the manuscript. This work was partially supported by a Fritz Thyssen Foundation and Deutsche Forschungsgemeinschaft postdoctoral fellowship from Germany (to M.M.B); by Grant 01KS9502 from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Germany) (to S.K.); and National Institutes of Health grants to S.Y.T. and B.W.O. The GLVP and GLp65 vectors have been licensed to Gene Medicine, Inc., and B.W.O. holds equity in that company.
ABBREVIATIONS
- hGH
human growth hormone
- TTR
transthyretin
- LBD
ligand binding domain
Footnotes
A Commentary on this article begins on page 324.
References
- 1.Wang Y, O’Malley B W, Jr, Tsai S Y, O’Malley B W. Proc Natl Acad Sci USA. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Xu J, Pierson T, O’Malley B W, Tsai S Y. Gene Ther. 1997;4:432–441. doi: 10.1038/sj.gt.3300402. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, DeMayo F J, Tsai S Y, O’Malley B W. Nat Biotechnol. 1997;15:239–243. doi: 10.1038/nbt0397-239. [DOI] [PubMed] [Google Scholar]
- 4.Schmitz M L, Baeuerle P A. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochanek S, Clemens P R, Mitani K, Chen H H, Chan S, Caskey C T. Proc Natl Acad Sci USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiedner G, Morral N, Parks R J, Wu Y, Koopmans S C, Langston C, Graham F L, Beaudet A L, Kochanek S. Nat Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 7.Yan C, Costa R H, Darnell J E, Jr, Chen J D, Van Dyke T A. EMBO J. 1990;9:869–878. doi: 10.1002/j.1460-2075.1990.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Wade M, Krall L, Grisham J, Xiong Y, Van Dyke T. Genes Dev. 1996;10:245–260. doi: 10.1101/gad.10.3.245. [DOI] [PubMed] [Google Scholar]
- 9.Chung J H, Whiteley M, Felsenfeld G. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 10.Smith C L, Onate S A, Tsai M J, O’Malley B W. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parks R J, Chen L, Anton M, Sankar U, Rudnicki M A, Graham F L. Proc Natl Acad Sci USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmiter R D, Norstedt G, Gelinas R E, Hammer R E, Brinster R L. Science. 1983;222:809–814. doi: 10.1126/science.6356363. [DOI] [PubMed] [Google Scholar]
- 13.Potter M A, Hymus S, Stockley T, Chang P L. Hum Gene Ther. 1998;9:1275–1282. doi: 10.1089/hum.1998.9.9-1275. [DOI] [PubMed] [Google Scholar]
- 14.Shockett P E, Schatz D G. Proc Natl Acad Sci USA. 1996;93:5173–5176. doi: 10.1073/pnas.93.11.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossen M, Bonin A L, Bujard H. Trends Biochem Sci. 1993;18:471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- 16.Baim S B, Labow M A, Levine A J, Shenk T. Proc Natl Acad Sci USA. 1991;88:5072–5076. doi: 10.1073/pnas.88.12.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 18.No D, Yao T P, Evans R M. Proc Natl Acad Sci USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ptashne M, Gann A A. Nature (London) 1990;346:329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- 20.Triezenberg S J, Kingsbury R C, McKnight S L. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 21.Grunberg S M, Weiss M H, Spitz I M, Ahmadi J, Sadun A, Russell C A, Lucci L, Stevenson L L. J Neurosurg. 1991;74:861–866. doi: 10.3171/jns.1991.74.6.0861. [DOI] [PubMed] [Google Scholar]
- 22.Brogden R N, Goa K L, Faulds D. Drugs. 1993;45:384–409. doi: 10.2165/00003495-199345030-00007. [DOI] [PubMed] [Google Scholar]