Abstract
Huntington's disease (HD) is a neurodegenerative disease caused by polyglutamine (polyQ) expansion in the protein huntingtin (htt). Pathogenesis in HD seems to involve the formation of neuronal intranuclear inclusions and the abnormal regulation of transcription and signal transduction. To identify previously uncharacterized htt-interacting proteins in a simple model system, we used a yeast two-hybrid screen with a Caenorhabditis elegans activation domain library. We found a predicted SH3 domain protein (K08E3.3b) that interacts with N-terminal htt in two-hybrid tests. A human homolog of K08E3.3b is the Cdc42-interacting protein 4 (CIP4), a protein involved in Cdc42 and Wiskott–Aldrich syndrome protein-dependent signal transduction. CIP4 interacted in vitro with full-length htt from lymphoblastoid cells. Neuronal CIP4 immunoreactivity increased with neuropathological severity in the neostriatum of HD patients and partially colocalized to ubiquitin-positive aggregates. Marked CIP4 overexpression also was observed in Western blot from human HD brain striatum. The overexpression of CIP4 induced the death of striatal neurons. Our data suggest that CIP4 accumulation and cellular toxicity may have a role in HD pathogenesis.
Huntington's disease (HD) is a dominant neurodegenerative disorder that is caused by polyglutamine (polyQ) expansion tract in huntingtin (htt), a ubiquitously expressed protein of unknown function (1, 2). HD results in selective neuronal loss, especially in the striatum and cerebral cortex (3), and the polyQ size in HD patients inversely correlates with the age of onset and severity of symptoms (4).
The pathogenesis in HD and other polyQ diseases remains unclear (5). The accumulation of ubiquitinated polyQ-containing protein aggregates in neuronal inclusions (NIIs) and cell death are pathological hallmarks of HD, but the role of NIIs as a potential cause of cell death is controversial. In mice expressing htt exon 1, the appearance of ubiquitinated NIIs before the onset of neurologic symptoms has suggested that NIIs may be toxic to neurons (6). However, the translocation of soluble mutated htt in the nucleus might be required to produce neuronal death (7). Transgenic mice that express full-length htt (8, 9) and analysis of human HD brain tissue (10) also have suggested that NIIs may not be essential to neuronal death. Neuritic aggregates also may be involved and, compared with NIIs, have been suggested to show a strong correlation with the pathology in mice models (11). Besides aggregation, the appearance of misfolded truncated htt species (12) may alter the interaction of htt with several proteins essential to neuronal cell activity. Noticeably, htt interactors include several transcriptional regulators that may not function properly in HD (13). Mechanisms for abnormal transcription may include the sequestration of transcriptional regulators such as the cAMP-responsive-element-binding protein (CREB)-binding protein (CBP) (14, 15) and abnormal histone acetylation (16). Abnormal transcription in HD has been documented by several reports on gene expression changes in HD models (17).
The pathogenesis of HD also may involve abnormal signal transduction and transport. Noticeably, htt interacts directly or indirectly with signaling pathways regulated by Rho GTPases. The htt-associated protein HAP1 binds to Duo, a Trio-like protein that contains a Rac1 guanine nucleotide exchange factor (GEF) domain, raising the possibility that polyQ-expanded htt may affect a ras-related signaling pathway (18). Normal htt is associated with the Cdc42 interactor Grb2, the Ras GTPase-activating protein (RasGAP), and epidermal growth factor (EGF) receptor (19). Mutant htt disrupts cellular signaling mediated by the EGF receptor in PC12 cells (20). PolyQ expansion impairs the ability of htt to bind the mixed-lineage kinase 2 (MLK2), a protein that contains a Cdc42/Rac interactive binding motif, likely contributing a toxic activation of MLK2-mediated signal transduction (21). PolyQ expansion also may impair the ability of htt to bind PSD-95, a protein that functions as a scaffold to assemble signaling proteins such as SynGAP, a phenomenon that may inhibit glutamate-mediated excitotoxicity (22).
In the present study, we have identified a Caenorhabditis elegans protein (K08E3.3b) that interacts with N-terminal htt in two-hybrid tests. A human homolog of K08E3.3b is the Cdc42-interacting protein 4 (CIP4; ref. 23), a Wiskott–Aldrich syndrome protein (WASP) interactor and Cdc42 effector protein involved in cytoskeletal organization (24). Our data suggest that CIP4 accumulation and toxicity in striatal neurons may play a role in HD pathogenesis.
Materials and Methods
Yeast Two-Hybrid Screens.
We subcloned DNA fragments encoding either normal (18 Glns) or mutated (81–128 Glns) N-terminal htt species, the first 50 aa of normal ataxin-3 (23 Glns), and lamin C into the pGBT9 bait vector (CLONTECH). DNA fragments encoding htt species were derivatives of cDNAs encoding htt amino acids 1–546 (Centre for Molecular Medicine and Therapeutics, Vancouver). Yeast cells CG1945 were transformed with pGBT9 encoding amino acids 1–152 of mutated htt (81 Glns). A two-hybrid screening was performed as described (25) by mating transformed yeast cells CG1945 with yeast cells Y187 transformed with a random primed C. elegans cDNA activation domain library subcloned into the pACT vector (R. Barstead, Oklahoma Medical Research Foundation, Oklahoma City). Diploid clones were grown on minimal medium lacking Leu, Trp, and His and tested for β-galactosidase activity with filter assays. Independent interactor clones were identified based on a BamHI and EcoRI digestion profile and tested for htt-dependent activation of HIS3 and lacZ reporters in plate assays. Prey cDNAs in true independent interactor clones were sequenced as described (26). Sequence analysis was performed using blast (GenBank, Bethesda) and pfam (www.sanger.ac.uk) searches. Relevant cDNAs also were tested in two-hybrid liquid phase β-galactosidase assays as described (27), and results were analyzed by using ANOVA (statview, Apple Computer).
In Vitro Binding Experiments.
We subcloned DNA fragments encoding full-length CIP4 (P. Aspenström, Ludwig Institute for Cancer Research, Uppsala) into the pGEX vector (Amersham Pharmacia Biotech) to generate GST fusion proteins. GST fusions containing the CIP4 protein were produced in Escherichia coli by using standard procedures. Purified GST-CIP4 fusion (30 μg) was incubated with glutathione agarose beads (150 μl) for 2 h in 1× PBS at room temperature and washed. Protein extracts (60 μg) from lymphoblastoid cell lines of HD family subjects (NIGMS Cell Repository, Coriell Institute for Medical Research, Camden, NJ) were added in 0.1 ml of PBS overnight at room temperature, and the incubation was continued overnight. Beads then were collected by centrifugation and washed three times with 1 ml of PBS to remove unbound proteins. Bound proteins were eluted from the beads, separated by SDS/PAGE, and analyzed by Western blotting using a mAb specific to htt (HU-4C8; 1:2,500; Euromedex, Mundolsheim, France). The CAG repeat size in the htt gene of the individuals tested was determined as described (28).
Brain Tissue Specimens.
Postmortem striatal tissue specimens from 14 adult-onset HD patients (three grade 2, five grade 3, and six grade 4 cases; mean age, 64.2 years; range, 53–78 years) and six age-matched patients without any known neurologic sequella (mean age, 68.1 years; range 62–79 years) were dissected fresh and placed in cold (4°C) 2% paraformaldehyde–lysine–periodate solution for 24–36 h. Brain tissue specimens were received from the Bedford Veterans Administration Medical Center Brain Tissue Archive. The postmortem intervals did not exceed 20 h (mean time, 8.1 h; range, 4–20 h) and were similar for controls and HD patients. CAG repeat length analysis was performed in all of the HD cases (14/14). The range of CAG repeats in the adult-onset HD patients was 41–52. Each HD patient had been clinically diagnosed based on known family history and phenotypic symptoms of HD. The diagnosis of HD was confirmed by neuropathologic examination and graded by severity (3). Striatal tissue blocks were processed as described (10), rinsed in 0.1 M sodium phosphate buffer, and placed in cold cryoprotectant in increasing concentrations of 10% and 20% glycerol/2% DMSO solution for 24–36 h. Frozen serial sections of the striatal tissue block from the anterior commissure to the rostral extent were cut at 50-μm intervals in the coronal plane and placed within a six-well collection container. The cut sections were stored in 0.1 M sodium phosphate buffer/0.08% sodium azide at 4°C for subsequent Nissl and immunohistochemical techniques. The cut sections were stored in 0.1 M sodium phosphate buffer/0.08% sodium azide at 4°C for subsequent immunocytochemistry and a combined colocalization immunofluoresence for ubiquitin and CIP4 antibodies.
Immunocytochemistry.
A rabbit polyclonal antibody specific to CIP4 has been characterized (P. Aspenström, Ludwig Institute for Cancer Research, Uppsala). Immunohistochemical localization of antibodies to CIP4 (dilution, 1:500) was performed by using a previously reported conjugated second antibody method (29). Tissue sections were preincubated in absolute methanol/0.3% hydrogen peroxide solution for 30 min, washed (three times) in PBS (pH 7.4) for 10 min each, placed in 10% normal goat serum (GIBCO) for 1 h, incubated free-floating in primary antiserum at room temperature for 12–18 h (all dilutions of primary antisera above included 0.08% Triton X-100 and 2% normal goat serum), washed (three times) in PBS for 10 min each, placed in periodate-conjugated goat anti-rabbit IgG (1:300 in PBS, Boehringer Mannheim, Indianapolis) or goat anti-mouse IgG (1:300 in PBS, Boehringer Mannheim), washed (three times) in PBS for 10 min each, and reacted with 3,3′ diaminobenzidine HCl (1 mg/ml) in Tris⋅HCl buffer with 0.005% hydrogen peroxide. To complete double immunocytochemical staining, selected striatal tissue sections immunoreacted with CIP4 antisera were not preincubated in absolute methanol/0.3% hydrogen peroxide solution. Specificity for the antisera used in this study was examined in each immunochemical experiment to assist with interpretation of the results. This examination was accomplished by preabsorption with excess target proteins (e.g., homologous CIP4 fusion proteins) and by omission of the primary antibody to determine the amount of background generated from the detection assay. The CIP4 antibody was tested by preadsorption of dilute primary antisera with an excess of appropriate fusion protein (12 μg/ml) for 6 h at room temperature before incubation.
Fluorescent Immunocytochemistry.
Combined immunofluorescence was performed on superior frontal cortex HD tissue specimens (grade 3) to determine whether CIP4 colocalizes to ubiquitin inclusions. The cortex was chosen because there are few htt/ubiquitin aggregates observed within the striatum. Immunofluorescence was performed as described (29) by incubating striatal tissue sections in the CIP4 polyclonal antisera (1:500) and in a ubiquitin mouse mAb (Zymed; 1:250) in Tris⋅HCl buffer containing 0.3% Triton X-100 for 24–72 h at 4°C. Sections were then rinsed (three times) in PBS, incubated in the dark with goat anti-rabbit FITC conjugate for 2 h at 20°C (Boehringer Mannheim; 1:15), rinsed (three times) in PBS, and incubated with goat anti-mouse tetramethylrhodamine B isothiocyanate (TRITC) conjugate (Boehringer Mannheim; 1:10) for 2 h at 20°C. Detection of CIP4 antisera resulted in the presence of green fluorescence, while detection of ubiquitin antisera resulted in the presence of red fluorescence. Sections were wet-mounted and coverslipped with 50% glycerol on completion of the technique. Identical microscopic fields were immediately photographed with a Nikon fluorescent microscope, delineating the location of CIP4 and ubiquitin immunoreactivities within the same striatal section. The fields were merged and colocalization was observed.
Western Blot Analysis of Brain Extracts.
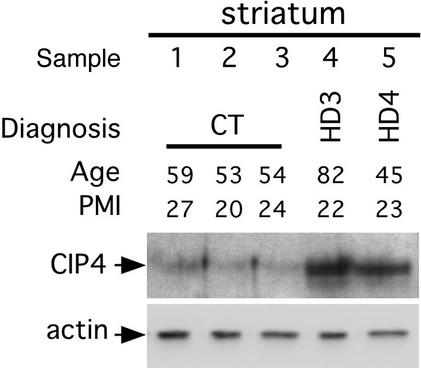
Human tissues were obtained from the Harvard Brain Tissue Resource Center (Belmont, MA). We used brain tissue from three controls (samples 1–3), one HD patient grade 3 (sample 4), and one HD patient grade 4 (sample 5). Samples 1–5 correspond respectively to brain numbers 4741, 4744, 4751, 4797, and 4740. Ages of individuals 1–5 are respectively 59, 53, 54, 82, and 45 years, and postmortem intervals are respectively 27, 20, 24, 22, and 23 h. Brain striatal and cortical samples were homogenized in Nonidet P-40 lysis buffer (20 mM Tris⋅HCl, pH 7.5/150 mM NaCl/2 mM EGTA/1% Nonidet P-40/10 mM β-glycerophosphate/5 mM NaF/1 mM NaPPi/2 mM DTT/1 mM sodium vanadate/100 μM PMSF) and cleared by centrifugation at 6,000 × g (15 min, 4°C). Twenty-five micrograms of homogenates were subjected to Western blot analysis by using either a CIP4 antiserum (1:500; P. Aspenström, Ludwig Institute for Cancer Research, Uppsala) or a β-actin (AC15, 1:5,000; Sigma) antibody.
Death Measurement.
Primary cultures of striatal neurons were prepared from E17 Sprague–Dawley rats and transfected at 4 days in vitro as described (7). After immunostaining, neurons were cytochemically labeled with the DNA dye Hoechst 33258 (2.5 μg/ml, 5 min). Transfected β-galactosidase-positive neurons were analyzed under fluorescence microscopy. For neuronal death assays, striatal neurons were transfected with either CIP4 or the corresponding empty vector. These constructs are cotransfected with β-galactosidase reporter that detects transfected cells. We used a 6:1 ratio (CIP4 or empty vector versus β-galactosidase construct) allowing more than 99% of the β-galactosidase-positive neurons to express CIP4 as checked by subsequent immunostaining. Two days after transfection, neurons were fixed and immunostained as described (7). After immunostaining, neurons were cytochemically labeled with the DNA dye Hoechst 33258 (2.5 μg/ml, 5 min). Transfected β-galactosidase-positive neurons were analyzed under fluorescence microscopy in a blinded manner and scored as healthy or apoptotic by morphological criteria. Neurons were scored as apoptotic only when they had a pyknotic and/or fragmented nucleus and degenerated or absent neurites. Three independent experiments were performed in triplicate and are expressed as the mean value ± SEM. Each bar corresponds to the scoring of about 500 neurons in cell death experiments. Statistical analysis was performed using Fisher's analysis.
Northern Blotting.
The 293 T human epithelial kidney cells were cultured in DMEM supplemented with 10% calf serum (GIBCO) and antibiotics (50 units/ml penicillin/50 μg/ml streptomycin) and transfected with N-terminal htt constructs by the calcium phosphate technique. Forty-eight hours after transfection, total RNA was extracted by using RNeasy Midi Kit (Qiagen, Valencia, CA). RNA was subjected to electrophoresis, through a 1% agarose gel containing formaldehyde, and was transferred onto a nylon-positive membrane (Appligene, Strasbourg, France). The filters were prehybridized at 42°C for 4 h in hybridization buffer containing 50% formamide, 10% dextran sulfate, 1% SDS, 1M NaCl, and salmon sperm. Hybridization was performed overnight in prehybridization buffer containing either a human full-length CIP4 or β-actin cDNA labeled with 32P. Blots then were washed and exposed to Hyperfilm (Amersham Pharmacia Biosciences), and signals were quantified by using NIH IMAGE 1.62. The exon 1 and amino acid 1–480 fragments of htt containing either a normal or mutated polyQ domain have been described (7).
Results
C. elegans K08E3.3b Interacts with htt in Two-Hybrid Tests and Is Homologous to Human CIP4.
The screening of 3.98 × 107 cDNAs by using normal N-terminal htt in a yeast mating-based procedure (mating efficiency, 9%) yielded 46 independent interactor clones. Forty-five clones, represented by multiple cDNAs, activated the HIS and lacZ reporters in the absence of any bait. One clone (represented by a single cDNA) was a true interactor and found to encode amino acids 164–780 of C. elegans predicted protein K08E3.3b (chromosome III). Two-hybrid analysis suggested that binding of htt to K08E3.3b (164–780) is augmented in the presence of an expanded polyQ (Fig. 1). The Pro-rich region in htt is likely to be required for binding to SH3 domain proteins, these interactions being modulated by the length of the adjacent polyQ (30, 31). Consistently, pfam analysis of K08E3.3b (783 aa) revealed a C-terminal SH3 domain, which could mediate the binding to htt, and an N-terminal Fer-CIP4 homology domain. blast (Version 2.2.4) searches revealed a family of strongly related human homologs for K08E3.3b, among which is CIP4 (545 aa; tblastn score: 405 for amino acids 9–545), a protein involved in Cdc42-dependent signal transduction (23). As compared with K08E3.3b, CIP4 shows a well conserved protein structure with the C-terminal SH3 and N-terminal Fer-CIP4 homology domains, suggesting interaction with htt.
Figure 1.
Interaction of C. elegans K08E3.3b (164–780) with human htt in yeast two-hybrid tests. The first 43 (htt43) or 152 (htt152) amino acids of either normal (18 Glns: Q18) or mutated (128 Glns: Q128) htt were tested for interaction with K08E3.3b. In plate assays with selective media lacking Leu (L), Trp (W), and His (H), normal and mutated htt152 bound to K08E3.3b. In liquid phase assays, mutated htt152 bound stronger to K08E3.3b as compared with normal htt152 (ANOVA). No interaction was detected with the Gal4 binding domain alone (−), a random bait protein (lamin C), the first 50 aa of normal ataxin-3 with 23 consecutive Glns (ataxin-3–50Q23), and N-terminal htt lacking the Pro-rich region (htt43Q18 and htt43Q128). Results for β-galactosidase activity are mean values ± SD (n = 15).
CIP4 Interacts in Vitro with Full-Length htt from Lymphoblastoid Cells.
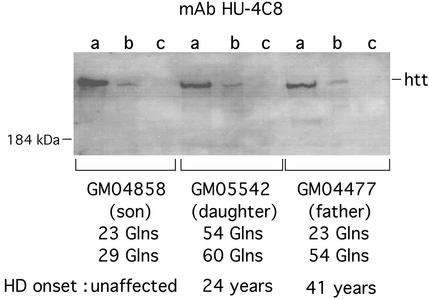
GST fusions purified from E. coli were used to determine whether full-length CIP4 may bind to full-length physiological htt. GST full-length CIP4 was incubated with glutathione agarose beads. Protein extracts from lymphoblastoid cells from three members of an HD-affected Venezuelan family then were added, and similar amounts of bound protein were resolved by SDS/PAGE. Western blots were performed with mAb HU-4C8, which is specific to amino acids 181–810 of htt (Fig. 2). Full-length htt from an unaffected individual GM04858 with two normal polyQ alleles, a HD patient GM05542 homozygous for mutation, and a HD patient GM04477 heterozygous for mutation produced a similar binding signal (a single band corresponding to the two allelic forms of htt), suggesting that CIP4 binds to full-length htt.
Figure 2.
Interaction of full-length htt with GST-CIP4 in vitro. Equivalent amounts of protein extracts from lymphoblastoid cells from three members of an HD-affected family were incubated with purified GST (c) or GST-full length CIP4 (b). The input of protein extracts (a) also was analyzed. Western blotting was performed using anti-htt mAb HU-4C8. Experiments were done in triplicate. The intensity of HU-4C8 signal in GST-CIP4 lanes was similar in the three individuals tested, suggesting that CIP4 binds equally to both normal and mutated full-length htt.
CIP4 Accumulates in Human HD Brain Tissue.
Immunoexpression of CIP4 was present in the nucleus and cytoplasm of neurons in nonneurologic control specimens (Fig. 3). CIP4 immunoreactivity was markedly increased in all HD samples in both neurons and neuropil. The immunocytochemical tissue expression of CIP4 increased with the degree of neuropathologic severity in the neostriatum from HD patients, as compared with nonneurologic control tissue samples (Fig. 3). CIP4 immunoreactivity was increased in neurons within both the caudate nucleus and putamen, in grade 2 HD, with significantly greater intensity of immunoreactivity in grades 3 and 4 HD striatal tissue specimens (Fig. 3). The nuclei were intensely immunoreactive for CIP4, with little or no immunostaining in the nucleolus. Not all striatal neurons expressed CIP4 immunoreactivity. This variable cell labeling did not show dorsoventral differences within the striatum. To further characterize the correlation between CIP4 immunoreactivity and inclusions, double immunofluorescence for CIP4 (FITC) and ubiquitin [tetramethylrhodamine B isothiocyanate (TRITC)] immunoreactivities was performed within the same tissue sections. CIP4 immunoreactivity and ubiquitin-positive inclusions colocalized within the HD tissue specimens (Fig. 4). In contrast, we commonly observed that much of the CIP4 immunofluorescence did not coexist with ubiquitin immunofluorescence. CIP4 immunoreactivity was significantly greater than ubiquitin immunoexpression in the striatum (Fig. 4). To further characterize the increased amount of CIP4 in human HD brains, we performed a Western blot analysis by using an anti-CIP4 antibody to reveal the levels of CIP4 in human brain samples from control (CT) and HD patients (Fig. 5). Anti-β-actin antibody is used as a control for protein levels. Two regions of the brain were analyzed: the cortex (data not shown) and the striatum, the most severely affected brain region in HD. As noted in Fig. 5, individuals are well age-matched and postmortem interval-matched. Interestingly, the levels of CIP4 appear strongly increased in brain extracts from HD patients in comparison with brain extracts from CT individuals (Fig. 5 and data not shown). Altogether these results demonstrate that CIP4 is up-regulated in HD patients in comparison with CT individuals.
Figure 3.
CIP4 immunohistochemistry in the caudate nucleus of normal age-matched nonneurologic control patient (A) and HD patients grades 2, 3, and 4, respectively (B, C, and D). There is light CIP4 immunostaining of neurons and neuropil in the caudate nucleus of a control patient (A). In contrast, there is increased CIP4 immunoreactivity associated with increased severity of HD grade. There is moderate increased immunoreactivity in grade 2 HD (B), with greater tissue immunoreactivity in grade 3 HD (C), and most intense immunoreactivity found in grade 4 HD (D). Increased immunostaining is present in both neurons and neuropil, with more activity found in the nuclei of neurons. (Magnification bar in A = 100 μm, and the same holds for B–D.)
Figure 4.
Combined CIP4 and ubiquitin immunofluorescence in the superior frontal cortex, layer 6, in HD patients. Combined immunofluorescence for CIP4 (red) (A) and ubiquitin (green) (B) immunoreactivities within the same tissue specimen from the superior frontal cortex of a HD patient grade 3 shows colocalization of ubiquitin-positive inclusions and CIP4 immunostaining in the merged figure (yellow) (C).
Figure 5.
CIP4 Western blot from HD brain extracts. Protein extracts were prepared from striatal postmortem samples, resolved by SDS/PAGE, and immunoblotted with anti-CIP4 (Upper) or anti-β-actin (Lower) antibodies. There is increased CIP4 immunoreactivity in grade 3 (sample 4) or grade 4 (sample 5) of HD patients compared with normal individuals (samples 1–3). PMI, postmortem interval in h.
Mutant htt Does Not Up-Regulate CIP4 mRNAs in 293 T Cells.
Overexpression of normal and mutant N-terminal htt in 293 T cells did not result in strong modifications of CIP4 mRNA levels (Fig. 6). There was no up-regulation of CIP4 mRNAs by htt 1–480. It has been suggested that a decreased length of mutant htt may alter gene expression levels more severely (32). However, there was no up-regulation of CIP4 mRNAs by htt exon 1. This finding suggested that increased CIP4 immunoreactivity in HD brain does not appear to result from an early increase in CIP4 synthesis. However, polyQ-dependent gene expression changes may occur in the late phases of HD pathogenesis (17), possibly altering the expression of CIP4.
Figure 6.
Expression of CIP4 mRNAs extracted from 293 T cells. Transfection with cDNAs encoding N-terminal htt species containing either a normal or mutated polyQ domain did not result in the up-regulation of CIP4 mRNA levels. Ratio is (intensity of CIP4 band − background signal)/(intensity of β-actin band − background signal).
Increased Dosage of CIP4 Induces Striatal Cell Death.
The function of CIP4 has been studied in dividing fibroblasts (23, 33). However, the consequences of CIP4 overexpression in postmitotic neurons are unknown. Because CIP4 appears as increased in HD brains, we asked what the effect is of increased level of CIP4 in striatal neurons. We transfected primary cultures of striatal neurons with CIP4 construct or the corresponding empty vector and analyzed cell death (Fig. 7). Interestingly, we found that expression of CIP4 in striatal neurons, the main site of neurodegeneration in HD, induces a statistically significant increase in cell death compared with the control situation. We observed that CIP4 induced cell death at levels similar to, if not greater than, those of mutant htt, and that the toxicity of the empty vector was similar to that of wild-type htt (data not shown). We also cotransfected CIP4 and N-terminal fragments of htt containing the expanded polyQ stretch and could not observe any synergistic effect of the two proteins in inducing cell death (data not shown). Our results indicate that CIP4 is toxic to striatal neurons and suggest that CIP4 may influence HD pathogenesis through accumulation of the protein in striatal cell populations. Additional experiments will be required to establish the mechanism by which this may occur, and to establish whether striatal neurons are preferentially susceptible.
Figure 7.
Cell death in primary cultures of rat striatal neurons expressing CIP4. CIP4 or the corresponding empty vector was transfected in striatal neurons, and percentage of cell death was determined 2 days after transfection. Three independent transfections were performed. The expression of CIP4 significantly increased cell death in comparison with empty vector (Fisher's analysis: ***, P < 0.001 compared with control).
Discussion
Rho GTPases are important regulators of neuronal morphogenesis, including axon growth and guidance and dendrite elaboration and plasticity (34), and the interference of polyQ-expanded htt with these factors may participate in neuronal dysfunction and degeneration in HD. In mice, the early neuropathology of HD may originate from axonal dysfunction and degeneration associated with neuropil aggregates (11). In C. elegans, we have reported previously that the expression of N-terminal mutant htt produces neuronal dysfunction, a phenotype partially associated with aggregates in neuronal processes (35). We also have observed that, although there appears to be no homolog for the htt gene in the nearly complete C. elegans genome (36), htt is able to physically interact with C. elegans proteins, including worm homologs of cAMP-responsive-element-binding protein (CREB)-binding protein (CBP) (unpublished data), a transcriptional regulator involved in HD pathogenesis (14), and CA150, a polymorphic transcriptional regulator that may act as a modifier gene of HD onset age and pathogenesis (28).
We present, herein, neuropathologic and biological evidence for the implication of CIP4 in HD pathogenesis. We identified a C. elegans protein (K08E3.3b) able to bind to N-terminal htt. The CIP4 protein, a human homolog of K08E3.3b, then was found to be associated with HD neuropathology, consistent with the notion that C. elegans is useful in exploring HD mechanisms (28, 35, 37–39). The CIP4 protein indeed appears to bind htt in vitro and to accumulate in HD brain tissue. Whereas CIP4 accumulation in HD striatum seems to be restricted to subpopulations of striatal neurons, a marked overexpression of CIP4 was detected in Western blot from HD whole striatum, suggesting that CIP4 accumulation is a significant event in HD.
In HD brain, all CIP4 did not colocalize with ubiquitin/htt, suggesting that CIP4 may accumulate through abnormal interaction with htt species localized outside ubiquitinated aggregates, possibly htt localized in microaggregates not visible by light microscopy (40). CIP4 is higher in HD brain than in normal brain tissue, and CIP4 immunoreactivity in HD brain increases as the disease grade increases, suggesting that CIP4 may be associated with HD progression. Overexpression of CIP4 was toxic to striatal neurons in culture, supporting the notion that increased cellular CIP4 may contribute to striatal cell death in HD pathogenesis. As for htt, the accumulation of CIP4 in HD brain is likely to be progressive, allowing neuronal cells that have survived and are highly stained for the protein to be detected. In 293 T cells, CIP4 mRNA levels are not up-regulated by normal or mutant N-terminal htt, suggesting that the increased CIP4 immunoreactivity in HD brain may result from accumulation of the protein and not from increased CIP4 synthesis. However, we cannot exclude the possibility that a small increase of CIP4 synthesis not detectable in vitro could lead to protein accumulation over the course of several decades. Studies of the Wiskott–Aldrich syndrome have suggested that CIP4 is a WASP interactor and Cdc42 effector protein involved in cytoskeletal organization (24). In particular, CIP4 appears to be involved in the binding of WASP to microtubules (24). In neuronal cells, an accumulated amount of CIP4 may impair several cellular mechanisms and pathways that involve Cdc42 and/or neuronal WASP (24, 41) such as endocytic pathways (42, 43). In adipocytes, CIP4/2, a splice variant (56 additional amino acids) of CIP4, may play an important role in insulin-stimulated glucose transport by the Glut4 transporter as a downstream effector of the GTPase TC10 (44). Because an insulin signaling pathway to neuronal WASP is required for Glut4 glucose transporter recycling (45), and diabetes mellitus may be associated with HD (46) and mouse models of HD (47), abnormal activity of CIP4 in the presence of polyQ-expanded htt may provide a basis to study the causes for glucose intolerance in HD.
Collectively, these findings characterize CIP4 as a protein likely to be involved in HD pathogenesis, its accumulation, and toxicity in striatal neurons, suggesting that it may interfere with Rho GTPases and signal transduction essential to neuronal survival.
Acknowledgments
We thank C. Wellington and M. Hayden (Centre for Molecular Medicine and Therapeutics, University of British Columbia, Vancouver) for providing a cDNA encoding mutated htt, R. Barstead (Oklahoma Medical Research Foundation, Oklahoma City) for providing a yeast two-hybrid C. elegans cDNA expression library, P. Aspenström (Ludwig Institute for Cancer Research, Uppsala) for providing a CIP4 antiserum and a cDNA encoding CIP4, A. Brice (Institut National de la Santé et de la Recherche Médicale U146, Paris) for providing a cDNA encoding SCA3, and G. Poizat for technical help. We acknowledge the Harvard Brain Tissue Resource Center (Belmont, MA), which is supported in part by Public Health Service Grant MH/NS 31862, for providing human brain tissue for Western analysis. This work was supported by the Hereditary Disease Foundation (Santa Monica, CA), Association Huntington France, Institut National de la Santé et de la Recherche Médicale, and Association pour la Recherche sur le Cancer (ARC), Paris (to C.N.); Centre National de la Recherche Scientifique, Action Thématique et Incitative sur Programme, ARC, Fondation pour la Recherche Medicale/Banque Nationale de Paris Paribas, Paris, and Cure Huntington's Disease Initiative Hereditary Disease Foundation (to F.S.); and National Institutes of Health Grants AG12992, AG13846, AT00613, and NS35255, a Research Enhancement Award Program award, and the Veterans Administration (to R.J.F.). S. Holbert is a postgraduate student at Ecole Pratique des Hautes Etudes and University of Paris 6, Paris, and is supported by the Hereditary Disease Foundation.
Abbreviations
- CIP4
Cdc42-interacting protein 4
- HD
Huntington's disease
- htt
huntingtin
- polyQ
polyglutamine
- WASP
Wiskott–Aldrich syndrome protein
References
- 1.The Huntington's Disease Research Collaborative Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, Martin E, Vonsattel J P, Carraway R, Reeves S A, et al. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 3.Vonsattel J P, Myers R H, Stevens T J, Ferrante R J, Bird E D, Richardson E P., Jr J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Harper P S. Hum Genet. 1992;89:365–376. doi: 10.1007/BF00194305. [DOI] [PubMed] [Google Scholar]
- 5.Ross C A. Neuron. 2002;35:819–822. doi: 10.1016/s0896-6273(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 6.Davies S W, Turmaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 7.Saudou F, Finkbeiner S, Devys D, Greenberg M E. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 8.Reddy P H, Charles V, Williams M, Miller G, Whetsell W O, Jr, Tagle D A. Philos Trans R Soc London B. 1999;354:1035–1045. doi: 10.1098/rstb.1999.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodgson J G, Agopyan N, Gutekunst C A, Leavitt B R, LePiane F, Singaraja R, Smith D J, Bissada N, McCutcheon K, Nasir J, et al. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 10.Kuemmerle S, Gutekunst C A, Klein A M, Li X J, Li S H, Beal M F, Hersch S M, Ferrante R J. Ann Neurol. 1999;46:842–849. [PubMed] [Google Scholar]
- 11.Li H, Li S H, Yu Z X, Shelbourne P, Li X J. J Neurosci. 2001;21:8473–8481. doi: 10.1523/JNEUROSCI.21-21-08473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sisodia S S. Cell. 1998;95:1–4. doi: 10.1016/s0092-8674(00)81743-2. [DOI] [PubMed] [Google Scholar]
- 13.Cha J H. Trends Neurosci. 2000;23:387–392. doi: 10.1016/s0166-2236(00)01609-x. [DOI] [PubMed] [Google Scholar]
- 14.Nucifora F C, Jr, Sasaki M, Peters M F, Huang H, Cooper J K, Yamada M, Takahashi H, Tsuji S, Troncoso J, Dawson V L, et al. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z X, Li S H, Nguyen H P, Li X J. Hum Mol Genet. 2002;11:905–914. doi: 10.1093/hmg/11.8.905. [DOI] [PubMed] [Google Scholar]
- 16.Steffan J S, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol B L, Kazantsev A, Schmidt E, Zhu Y Z, Greenwald M, et al. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 17.Orr H T. Hum Mol Genet. 2002;11:1909–1910. doi: 10.1093/hmg/11.17.1909. [DOI] [PubMed] [Google Scholar]
- 18.Colomer V, Engelender S, Sharp A H, Duan K, Cooper J K, Lanahan A, Lyford G, Worley P, Ross C A. Hum Mol Genet. 1997;6:1519–1525. doi: 10.1093/hmg/6.9.1519. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y F, Deth R C, Devys D. J Biol Chem. 1997;272:8121–8124. doi: 10.1074/jbc.272.13.8121. [DOI] [PubMed] [Google Scholar]
- 20.Song C, Perides G, Liu Y F. J Biol Chem. 2002;277:6703–6707. doi: 10.1074/jbc.M110338200. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y F, Dorow D, Marshall J. J Biol Chem. 2000;275:19035–19040. doi: 10.1074/jbc.C000180200. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Savanenin A, Reddy P H, Liu Y F. J Biol Chem. 2001;276:24713–24718. doi: 10.1074/jbc.M103501200. [DOI] [PubMed] [Google Scholar]
- 23.Aspenström P. Curr Biol. 1997;7:479–487. doi: 10.1016/s0960-9822(06)00219-3. [DOI] [PubMed] [Google Scholar]
- 24.Tian L, Nelson D L, Stewart D M. J Biol Chem. 2000;275:7854–7861. doi: 10.1074/jbc.275.11.7854. [DOI] [PubMed] [Google Scholar]
- 25.Fromont-Racine M, Rain J C, Legrain P. Nat Genet. 1997;16:277–282. doi: 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- 26.Neri C, Albanese V, Lebre A S, Holbert S, Saada C, Bougueleret L, Meier-Ewert S, Le Gall I, Millasseau P, Bui H, et al. Hum Mol Genet. 1996;5:1001–1009. doi: 10.1093/hmg/5.7.1001. [DOI] [PubMed] [Google Scholar]
- 27.Kalchman M A, Koide H B, McCutcheon K, Graham R K, Nichol K, Nishiyama K, Kazemi-Esfarjani P, Lynn F C, Wellington C, Metzler M, et al. Nat Genet. 1997;16:44–53. doi: 10.1038/ng0597-44. [DOI] [PubMed] [Google Scholar]
- 28.Holbert S, Denghien I, Kiechle T, Rosenblatt A, Wellington C, Hayden M R, Margolis R L, Ross C A, Dausset J, Ferrante R J, Neri C. Proc Natl Acad Sci USA. 2001;98:1811–1816. doi: 10.1073/pnas.041566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrante R J, Gutekunst C A, Persichetti F, McNeil S M, Kowall N W, Gusella J F, MacDonald M E, Beal M F, Hersch S M. J Neurosci. 1997;17:3052–3063. doi: 10.1523/JNEUROSCI.17-09-03052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faber P W, Barnes G T, Srinidhi J, Chen J, Gusella J F, MacDonald M E. Hum Mol Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 31.Passani L A, Bedford M T, Faber P W, McGinnis K M, Sharp A H, Gusella J F, Vonsattel J P, MacDonald M E. Hum Mol Genet. 2000;9:2175–2182. doi: 10.1093/hmg/9.14.2175. [DOI] [PubMed] [Google Scholar]
- 32.Chan E Y, Luthi-Carter R, Strand A, Solano S M, Hanson S A, DeJohn M M, Kooperberg C, Chase K O, DiFiglia M, Young A B, et al. Hum Mol Genet. 2002;11:1939–1951. doi: 10.1093/hmg/11.17.1939. [DOI] [PubMed] [Google Scholar]
- 33.Richnau N, Aspenström P. J Biol Chem. 2001;276:35060–35070. doi: 10.1074/jbc.M103540200. [DOI] [PubMed] [Google Scholar]
- 34.Luo L. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 35.Parker J A, Connolly J B, Wellington C, Hayden M, Dausset J, Neri C. Proc Natl Acad Sci USA. 2001;98:13318–13323. doi: 10.1073/pnas.231476398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The C. elegans Sequencing Consortium. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 37.Faber P W, Alter J R, MacDonald M E, Hart A C. Proc Natl Acad Sci USA. 1999;96:179–184. doi: 10.1073/pnas.96.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satyal S H, Schmidt E, Kitagawa K, Sondheimer N, Lindquist S, Kramer J M, Morimoto R I. Proc Natl Acad Sci USA. 2000;97:5750–5755. doi: 10.1073/pnas.100107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morley J F, Brignull H R, Weyers J J, Morimoto R I. Proc Natl Acad Sci USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutekunst C A, Li S H, Yi H, Mulroy J S, Kuemmerle S, Jones R, Rye D, Ferrante R J, Hersch S M, Li X J. J Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlier M F, Nioche P, Broutin-L'Hermite I, Boujemaa R, Le Clainche C, Egile C, Garbay C, Ducruix A, Sansonetti P, Pantaloni D. J Biol Chem. 2000;275:21946–21952. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- 42. Otsuki, M., Itoh, T. & Takenawa, T. T. (December 10, 2002) J. Biol. Chem., 10.1074/jbc.M207433200.
- 43.Velier J, Kim M, Schwarz C, Kim T W, Sapp E, Chase K, Aronin N, DiFiglia M. Exp Neurol. 1998;152:34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Adams R D, Saltiel A R. Proc Natl Acad Sci USA. 2002;99:12835–21840. doi: 10.1073/pnas.202495599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Z Y, Chawla A, Bose A, Way M, Czech M P. J Biol Chem. 2002;277:509–515. doi: 10.1074/jbc.M108280200. [DOI] [PubMed] [Google Scholar]
- 46.Farrer L A. Clin Genet. 1985;27:62–67. doi: 10.1111/j.1399-0004.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 47.Hurlbert M S, Zhou W, Wasmeier C, Kaddis F G, Hutton J C, Freed C R. Diabetes. 1999;48:649–651. doi: 10.2337/diabetes.48.3.649. [DOI] [PubMed] [Google Scholar]