Abstract
Up-regulation of cyclooxygenase-2 (COX-2) and overproduction of prostaglandins have been implicated in the initiation and/or progression of colon cancer. However, it is uncertain in which cells and how COX-2 is induced initially in the tumor microenvironment. We found that a conditioned medium of the colon cancer cell line, LS 180, contained a factor to induce COX-2 in human peripheral blood mononuclear cells. This factor was purified biochemically and revealed to be mucins. A small amount of mucins (≈100 ng of protein per ml) could elevate prostaglandin E2 production by monocytes. The mucins induced COX-2 mRNA and protein levels of monocytes in a dose- and time-dependent manner, indicating a COX-2-mediated pathway. We also have examined immunohistochemically the localization of COX-2 protein and mucins in human colorectal cancer tissues. It is noteworthy that COX-2-expressing macrophages were located around the region in which mucins were detectable, suggesting that COX-2 also was induced by mucins in vivo. These results suggest that mucins produced by colon cancer cells play a critical role in the initial induction of COX-2 in the tumor microenvironment.
Several epidemiological and chemical studies have shown that nonsteroid antiinflammatory drugs can decrease the incidence and mortality of colorectal cancer and induce the regression of adenomas in patients with familial adenomatous polyposis (1, 2). Nonsteroid antiinflammatory drugs are known to inhibit cyclooxygenase (COX) (3). Two isoforms of COX, i.e., COX-1 and COX-2, which are constitutive and inductive enzymes, respectively, have been identified (4, 5). Expression of COX-2 is up-regulated in various types of cancer including gastrointestinal cancers (6–10). Recent studies have led to the recognition of the importance of COX-2 in colorectal tumorigenesis (11–13). Oshima et al. (14) reported direct evidence that the formation of intestinal polyps in ApcΔ716 knockout mice was dramatically suppressed by crossing them with COX-2 knockout mice. Because such polyps are precursors to cancer, these results indicate that COX-2 acts at a rate-limiting step and contributes at an early stage of colon carcinogenesis. They also showed that COX-2 is not expressed in the colon epithelial cells but in interstitial cells at an early stage, indicating that the interstitial cells are essential for the intestinal polyp formation. There are some reports showing the expression of COX-2 in infiltrated macrophages in tumor tissues (13, 15–17).
Many tumors arising from epithelial tissues produce mucins. They are defined by their characteristic O-glycosylated domains, which contain a repetitive protein backbone with an especially high content of Thr and Ser residues. Upon malignant transformation, many epithelial cells produce mucins in abnormal amounts and/or with abnormal glycosylation patterns (18). A human colon cancer cell line, LS 180, produces MUC2 mucin (19), which has a high number of tandem repeat and is highly glycosylated. Most and about half of O-glycans contained in ovine and bovine submaxillary mucin (OSM and BSM), respectively, are elucidated to be a sialyl Tn antigen (20, 21). It is reported that BSM has a central domain consisting of ≈55 tandem repeats of 329 aa (22). Mucins produced by cancer cells are found in the sera of cancer patients and used as disease markers. There have been many reports that patients with a higher amount of mucins in their bloodstream have a lower 5-year survival rate (23). However, little is known regarding the biological significance of mucins. Mucins readily make contact with various circulating cells of the blood stream in cancer patients or with the infiltrated cells in cancer tissues.
Despite much evidence that COX-2 overexpression is an important early event in adenoma formation and that nonsteroid antiinflammatory drugs have a profound effect on adenoma formation, the mechanism by which COX-2 is induced remains unresolved.
In the present article, we demonstrate that mucins secreted from colon cancer cells could induce COX-2 in monocytes/macrophages.
Materials and Methods
Cells and Materials.
A human colon cancer cell line, LS 180, was obtained from the American Type Culture Collection and cultured in Eagle's MEM supplemented with 10% FCS. In some cases, the cells were cultured in the presence of 5 mM phenyl α-GalNAc. Monocytes were obtained from the buffy coat of healthy human donors. The cells were separated on Ficoll-Isopaque centrifugation according to the manufacturer's instructions. The cells (1 × 107 cells per ml) were suspended in RPMI medium 1640 supplemented with 10% FCS and allowed to attach to the culture plate for 1 h. Nonadherent cells were removed by washing the plate with warm RPMI medium 1640, and adherent cells were collected. BSM was obtained from Roche Diagnostics. OSM was prepared as described (24). Murine mAbs MSW 113, MLS 132, and MLS 128, which recognize sialyl Lewis A, sialyl Tn, and Tn antigens, respectively, were prepared as described (25–27). Goat anti-human COX-1 and -2 antibodies and mouse anti-human CD 68 antibody were obtained from Santa Cruz Biotechnology and DAKO, respectively.
Isolation of Mucins from the Conditioned Medium of LS 180 Cells.
Mucins were detected by a dot blot analysis with MLS 132 mAb. A concentrated conditioned medium of LS 180 cells was subjected to gel filtration on Sepharose 6B (3 × 105 cm). From the excluded fractions, the mucins were purified according to Baeckstrom et al. (28).
Purified mucin (5 μg of protein) was subjected to SDS/PAGE (6% gel) according to Laemmli (29), followed by periodate–Schiff and silver stainings. Another sample was transferred to Zeta-probe membrane, and sialyl Lewis A, sialyl Tn, and Tn antigens borne on the mucin core protein were detected by using a mixture of MSW 113, MLS 132, and MLS 128 mAbs. Purified mucin was labeled with [125I]NaI by the chloramine T method (30).
Secretion of Prostaglandin E2 (PGE2) from Monocytes.
Monocytes (1 × 105 cells) were cultured in RPMI 1640 medium supplemented with 10% FCS or cocultured with LS 180 cells (1 × 105 cells) for 36 h. LS 180 cells were cultured in the presence or absence of 5 mM phenyl α-GalNAc, whose culture supernatants were used as the conditioned medium. Monocytes (1 × 105 cells) were cultured for 36 h in the presence of the conditioned medium treated or not with phenyl α-GalNAc or the equivalent amount of culture medium of LS 180 cells. Each culture supernatant was collected and assayed for PGE2 by ELISA.
RT-PCR of COX-1 and COX-2 mRNAs.
Preparation of total RNA from monocytes (1 × 107 cells) was performed by using Isogen (Nippon Gene, Toyama, Japan) according to the manufacturer's instructions. Reverse transcription and cDNA amplification were performed by using the TaKaRa RNA PCR kit (Takara Shuzo, Otsu, Japan). The forward and reverse primers and the expected product sizes were as follows: COX-1, 5′-TGCCCAGCTCCTGGCCCGCCGCTT-3′ and 5′-GTGCATCAACACAGGCGCCTCTTC-3′, 331 bp; COX-2, 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ and 5′-AGATCATCTCTGCCTGAGTATCTT-3′, 305 bp; and β-actin, 5′-ATGGATGATGATATCGC-3′ and 5′-ATAGGAATCCTTCTGACCCA-3′, 291 bp. COX-1 cDNA was amplified for 30 cycles of denaturation at 94°C for 1 min, annealing at 65°C for 1 min, and extension at 72°C for 45 sec. COX-2 cDNA was amplified for 22 or 30 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 45 sec. β-Actin cDNA was amplified for 27 cycles of denaturation at 94°C for 45 sec, annealing at 52°C for 30 sec, and extension at 72°C for 30 sec. The amplified cDNAs were run on 2% agarose gel with 0.5 μg/ml ethidium bromide and visualized under UV light.
Northern Blot Analysis.
The COX-2 cDNA fragment was prepared as described above. A GAPDH cDNA fragment also was amplified by RT-PCR by using forward primer 5′-CGGGATCCACCACCAGTCCATGCCAT-3′ and reverse primer 5′-CCTTAAGTCCACCACCCTGTTGCTG-3′. pUC 18 ligated with the purified cDNA fragments was introduced into TOP 10F′ cells and amplified according to manufacturer instructions. Purified 310-base BamHI and KpnI DNA fragment (COX-2) and 472-base BamHI and EcoRI DNA fragment (GAPDH) were labeled by using a digoxigenin-labeling kit (Roche Diagnostic) according to the manufacturer's instructions.
Total RNA (10 μg) was subjected to electrophoresis on a 1% denaturing formaldehyde/agarose gel and electrotransferred to Hybond N+ nylon membrane (Amersham Pharmacia). After fixation, the membrane was hybridized with digoxigenin-labeled probes at 44°C (COX-2) and 50°C (GAPDH) for 24 h. After incubation with anti-digoxigenin antibody–alkaline phosphatase conjugate and washing, the membrane was visualized by chemiluminescence according to the manufacturer's instructions.
Binding of 125I-Labeled Mucin to Monocytes and Macrophage Scavenger Receptor (MSR) Transfectants.
Expression of recombinant MSR (group A, type I) in COS 7 cells was carried out as described (31).
The binding of 125I-labeled mucin was assessed by incubating monocytes (1 × 106 cells) or the MSR transfectants (1 × 106 cells) with 125I-labeled mucin (10 ng/ml) at 4°C for 2 h in the presence of various inhibitors. The inhibitors used were BSM (30 μg protein/ml), fucoidan, polyinosinic acid (poly I), polycytidylic acid (poly C) (100 μg/ml), and orosomucoid (2 μg of protein per ml). After extensive washing with 25 mM Hepes buffer, pH 7.2, and 0.15 M NaCl, the cells were solubilized with 1 M NaOH and the radioactivity was determined.
Immunoblotting.
When lysate of monocytes was fractionated to prepare total RNA as described above, another fraction containing proteins was obtained. After dialysis against 50 mM Tris⋅HCl buffer, pH 7.5, containing 0.2 M NaCl, 1% Triton X-100, 2 mM EDTA, and 1 mM PMSF, COX-2 protein was immunoprecipitated by using goat anti-human COX-2 antibody and protein G-Sepharose. The immunoprecipitate was subjected to SDS/PAGE followed by Western blotting. The COX-2 protein was detected by using the same antibody as described above.
Tissue Specimens and Immunohistochemistry.
We examined 15 patients with colorectal cancer (nine males and six females; ages 21–36 years) obtained from surgical resection. Five specimens were obtained from the ascending colon, four from the transverse colon, two from the descending colon, one from the sigmoid colon, and three from the rectum.
Immunohistochemical staining was performed with the Vectastain avidin–biotin peroxidase complex kit (Vector Laboratories) as described (15). Double immunostaining was carried out according to Kawahito et al. (32).
Results and Discussion
PGE2 Secreted from Monocytes Cocultured with LS 180 Cells or Cultured in the Presence of Conditioned Medium of LS 180 Cells.
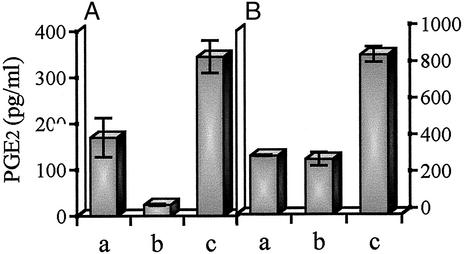
Monocytes were cocultured with colon cancer cells, LS 180, for 36 h. Approximately 1.7-fold PGE2 was secreted by coculture when compared with the separate culture of each cell, as shown in Fig. 1A. To determine whether or not the activating factor is contained in the conditioned medium, monocytes were cultured in the presence of a concentrated conditioned medium of LS 180 cells. The culture medium itself seems to contain a small amount of the activating factor needed to slightly enhance PGE2 production. However, the conditioned medium clearly elevated PGE2 production, indicating that it contained a high level of the activating factor. When the conditioned medium of LS 180 cells was prepared in the presence of 5 mM phenyl α-GalNAc, the activity of the medium was at the control level, suggesting that mucins were relevant to the activity (Fig. 1B).
Figure 1.
PGE2 production in monocytes cocultured with LS 180 cells or cultured in the presence of conditioned medium of LS 180 cells. (A) Monocytes (1 × 105 cells) (lane a) and LS 180 cells (1 × 105 cells) (lane b) were cultured separately for 36 h. The same number of both cells (lane c) was cocultured for 36 h. (B) Monocytes (1 × 105 cells) were cultured for 36 h in the presence of 10% FCS-MEM (lane a) and in the presence of conditioned medium of LS 180 cells treated (lane b) or not (lane c) with 5 mM phenyl α-GalNAc. Each culture supernatant was assayed for PGE2 by ELISA. The data are mean levels (n = 3) ± SD of the secreted PGE2.
Isolation of the Activating Factor from the Conditioned Medium of LS 180 Cells.
To isolate the activating factor, the conditioned medium of LS 180 cells was fractionated by gel filtration on Sepharose 6B. One microgram of protein of each fraction was added to the culture medium of monocytes. The excluded fractions elevated the secretion of PGE2 remarkably, as shown in Fig. 2A.
Figure 2.
Isolation of activating factor from conditioned medium of LS 180 cells. The conditioned medium of LS 180 cells was subjected to gel filtration on Sepharose 6B (3.0 × 105 cm) and then eluted with 25 mM sodium phosphate buffer, pH 7.5, and 0.15 M NaCl. (A) Fractions of 18.6 ml were collected, and absorbance at OD280 was determined. One microgram of protein of each fraction was added to the culture medium of monocytes (1 × 105 cells). After 24 h, secreted PGE2 was assayed by ELISA. (B) One hundred microliters of each fraction was loaded on a nylon membrane. Dot blot analysis was performed by using mAb MLS 132. (C) Purified mucin (5 μg) was subjected to SDS/PAGE with a 6% running gel, followed by silver (lane a) and periodate–Schiff (lane b) staining. Another sample was subjected to Western blotting after SDS/PAGE, and cancer-associated carbohydrate antigens were detected (lane c) as described in Materials and Methods.
A sialyl Tn antigen, one of the typical tumor-associated carbohydrate antigens expressed on mucin core proteins, was detected in the same fractions by dot blot analysis as shown in Fig. 2B, suggesting that the activating factor is a mucin. From the excluded fractions, mucins were purified according to Baeckstrom et al. (28).
The purity of the mucin was examined by SDS/PAGE followed by both protein and carbohydrate stainings (Fig. 2C, lanes a and b). No protein could be detected on silver staining. The same sample was subjected to SDS/PAGE followed by Western blotting. Expression of cancer-associated carbohydrate antigens was demonstrated on the mucins (Fig. 2C, lane c).
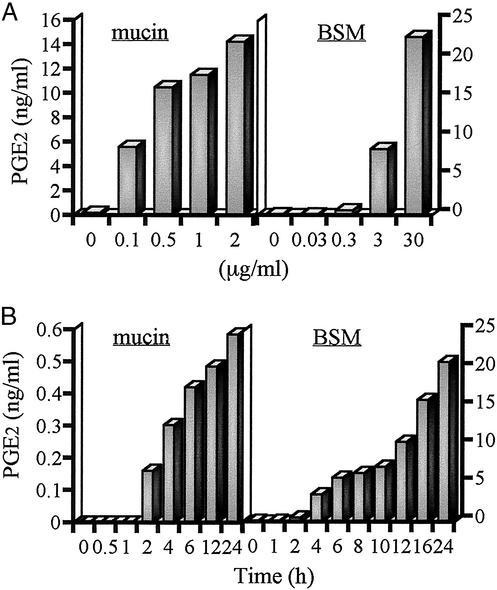
PGE2 Production Enhanced by Mucins or BSM in a Dose- and Time-Dependent Manner.
Mucins and BSM were found to potently increase PGE2 production in monocytes. To examine dose dependency, the monocytes were cultured in the presence of mucins (0–2 μg of protein per ml) or BSM (0–30 μg of protein per ml) for 12 h, and the supernatants were collected for the determination of PGE2 production by ELISA. As shown in Fig. 3A, mucins or BSM enhanced PGE2 production in a dose-dependent manner. A small amount of mucins (100 ng/ml) or BSM (3 μg/ml) could induce the production of PGE2.
Figure 3.
Dose- and time-dependent PGE2 production in monocytes stimulated with mucin or BSM. (A) Monocytes (1 × 105 cells) were cultured in the presence of mucin (0–2 μg of protein per ml) or BSM (0–30 μg of protein per ml) for 12 h. (B) Monocytes (1 × 105 cells) were cultured in the presence of mucin (1 μg of protein per ml) or BSM (30 μg of protein per ml) for the time indicated. Each supernatant was assayed for PGE2 by ELISA.
To study the kinetics of PGE2 production by mucins or BSM, monocytes were incubated with mucins (1 μg/ml) or BSM (30 μg/ml) at different time points (0–24 h), and the culture supernatants were assessed for PGE2 by ELISA (Fig. 3B). Mucins or BSM enhanced PGE2 production as early as 2 h after incubation. The time and dose dependence of PGE2 production suggests a COX-2-mediated pathway.
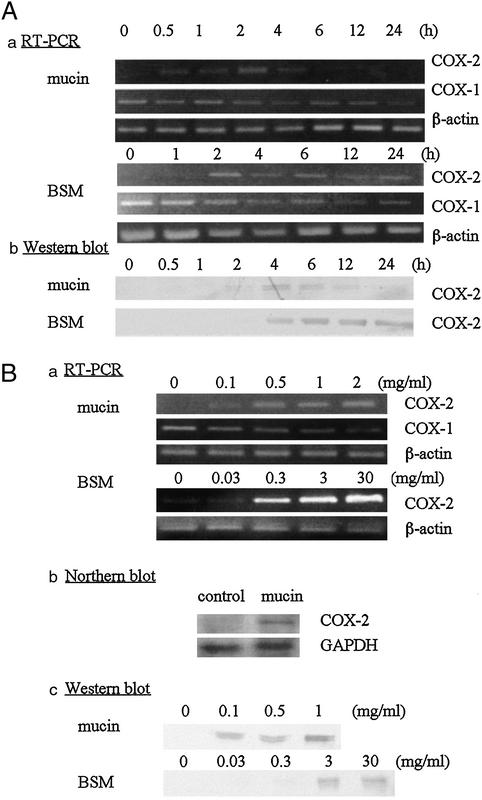
Induction of COX-2 mRNA Expression and Protein Synthesis by Mucins or BSM.
To determine whether mucin- or BSM-induced production of PGE2 was associated with up-regulation of COX-2, COX-2 mRNA and protein levels in monocytes were determined after treatment with mucins or BSM. At different time points (0–24 h), the monocytes were harvested, and total RNA and protein fractions were prepared.
As shown in Fig. 4A, the COX-2 mRNA was not detected in the absence of mucins or BSM, and the level peaked as early as 2 h in response to mucins. In contrast, COX-1 mRNA expression either remained unchanged or declined slightly.
Figure 4.
Induction of COX-2 mRNA and protein in monocytes. (A) Monocytes (5 × 106 cells) were cultured in the presence of mucin (1 μg of protein per ml) or BSM (30 μg of protein per ml) for the time indicated. (a) Total RNA was prepared, and COX-2 cDNA was amplified for 22 cycles and subjected to agarose gel electrophoresis as described in Materials and Methods. (b) COX-2 protein was immunoprecipitated by using anti-human COX-2 antibody and detected as described in Materials and Methods. (Ba) Monocytes (5 × 106 cells) were cultured for 2 h in the presence of mucin (0–2 μg of protein per ml) or BSM (0–30 μg of protein per ml), from which total RNA was prepared. COX-2 cDNA was amplified (mucin-treated monocytes: 22 cycles; BSM-treated monocytes: 30 cycles) and analyzed as described above. (b) Monocytes (2 × 107 cells) were cultured for 2 h in the presence of mucin (1 μg of protein per ml). Total RNA was prepared and Northern blot analysis was performed as described in Materials and Methods. (c) COX-2 protein was prepared from the protein fraction and detected as described above. Representative data of three experiments are shown.
To examine the induction of COX-2 protein in response to mucins or BSM, immunoprecipitation and Western blot analysis were performed. An increase in COX-2 protein synthesis in response to mucins or BSM was demonstrated by the increased intensity of a Mr 70,000 band corresponding to the COX-2 protein. The band was detected as early as 4 h, which is consistent with the fact that the COX-2 mRNA level peaked at 2 h. The doublet seems to be attributed to partial N-glycosylation as reported (33).
The dose dependence of COX-2 mRNA and protein induction was examined. The monocytes were incubated with various concentrations of mucins or BSM for 2 h, and total RNA and protein fractions were prepared. As shown in Fig. 4B, a small amount of mucins or BSM induced COX-2 mRNA, the level of which was increased in a dose-dependent manner. Induction of COX-2 mRNA also was confirmed by Northern blot analysis. A similar dose-dependent induction of COX-2 protein also was demonstrated by Western blot analysis.
Binding of Mucins to Monocytes Through MSR.
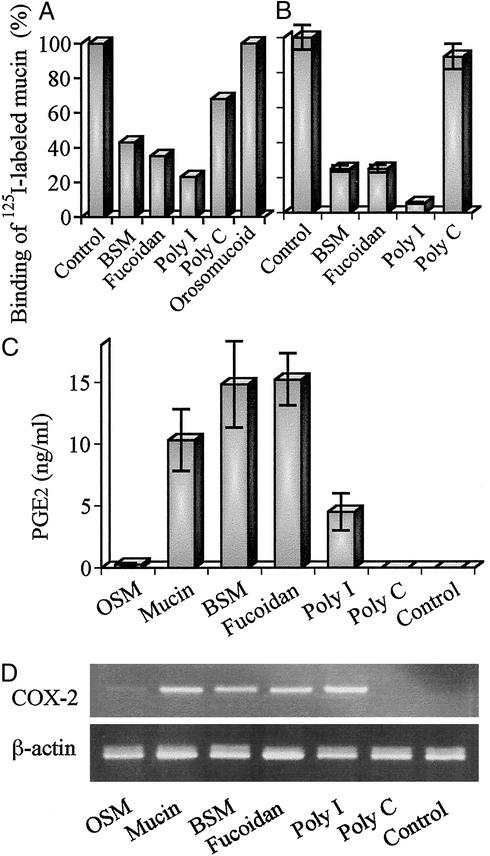
Because monocytes were activated by both mucins and BSM, it appears that the binding sites are carbohydrate moieties but not the core protein. There exist so many types of O-glycans on the mucin core protein that multiple receptors may be responsible for the binding. The mucins contain various tumor-associated carbohydrate antigens such as sialyl Lewis A and sialyl Tn antigens. Treatment with mAbs against these antigens partially reduced the effect (data not shown). Thus, it seems unlikely that specific oligosaccharide chains are responsible for the binding.
Monocytes were incubated with 125I-labeled mucin in the presence or absence of various inhibitors at 4°C for 2 h, and radioactivity of 125I-labeled mucins bound to the cell surface was determined (Fig. 5A). The binding was inhibited effectively by poly I, fucoidan, and BSM but slightly by poly C, suggesting that the MSR is included in the binding proteins. It has been reported that the MSR could recognize a pattern of anionic charges on molecules such as acetyl LDL, poly I, fucoidan (34, 35), and LPS (36). Anionic charges due to sialic acid and sulfate borne on O-glycans of mucins may be recognized by the receptor. We also confirmed that 125I-labeled mucins could bind to the MSR cDNA transfectant. As shown in Fig. 5B, BSM, fucoidan, and poly I, but not poly C, reduced the binding of 125I-labeled mucin to the MSR transfectants, which is consistent with characteristics of the MSR. The effect of other MSR ligands on PGE2 production and induction of COX-2 mRNA was examined (Fig. 5 C and D). Expectedly, in addition to mucin and BSM, fucoidan and poly I, but not poly C, could induce COX-2 mRNA and enhance PGE2 production. OSM had weak activity to induce COX-2 mRNA and enhance PGE2 production. The activity was much lower than that of BSM. Different activity among these mucins seems to be due to a different distribution of anionic charges on the molecule but not to the carbohydrate structure itself. LS 180 cells produce MUC 2 mucin (19), which has a high number of tandem repeats. It is reported that BSM also contains a tandem repeat (22). If the tandem repeat unit has one or more binding sites, the mucin has so many binding sites for the receptor that it could bridge many receptors on the cell surface, probably leading to more extensive activation of the monocytes. In addition, orosomucoid had no effect on the binding of the mucin, suggesting that some siglecs present on the monocyte cell surface are not responsible for the binding of the mucin probably from the masking of siglecs.
Figure 5.
Binding of mucins to monocytes through MSR. (A) Monocytes (1 × 106 cells) were incubated with 125I-labeled mucin at 4°C for 2 h in the presence of various inhibitors. The radioactivity bound to the cell was determined as described in Materials and Methods (duplicate experiments). (B) Binding of 125I-labeled mucin to the MSR cDNA transfectants (1 × 106 cells) was examined as described above (triplicate experiments). (C) Monocytes (1 × 105 cells) were cultured for 36 h in the presence of various ligands. Each culture supernatant was assayed for PGE2 by ELISA. (D) Monocytes (5 × 106 cells) were cultured for 2 h in the presence of various ligands, from which total RNA was prepared. COX-2 and β-actin cDNAs were amplified for 30 and 27 cycles, respectively, and analyzed as described above. The amounts of various ligands used are as follows: OSM and BSM, 30 μg of protein per ml; fucoidan, poly I, and poly C, 100 μg/ml; orosomucoid, 2 μg of protein per ml.
Immunohistochemical Staining of COX-2 in Colorectal Cancer Tissues.
In a previous article (15), we found immunoreactive COX-2 in inflammatory cells, vascular endothelial cells, fibroblasts, and cancer cells. Additionally, we immunohistochemically examined the distribution of cancer-associated carbohydrate antigens such as Tn and sialyl Tn antigens borne on mucin core peptides in colorectal cancer tissues. Itzkowitz et al. (37) reported that Tn and sialyl Tn antigens are expressed in 72–81% and 93–96% of colon cancers, respectively.
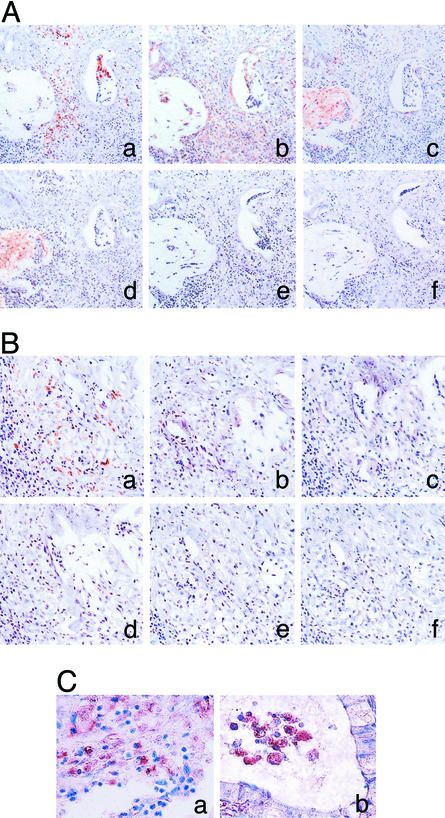
Interestingly, immunoreactive COX-2 was expressed in CD 68-positive cells infiltrated around the region with an expression of mucins (Fig. 6A a–d), whereas COX-2 was not detectable in CD 68-positive cells without mucins in the adjacent tissues (Fig. 6B a–d). Double immunostaining clearly indicates that COX-2 is induced in most of CD 68-positive cells (Fig. 6C a and b).
Figure 6.
Immunostaining for CD 68, COX-2, and cancer-associated carbohydrate antigens in colon cancer tissues. Colon cancer tissues were immunostained by using the Vectastain avidin–biotin peroxidase complex kit as described in Materials and Methods (A and B). (A) COX-2-positive macrophages were located around the region in which carbohydrate antigens were detectable. (B) COX-2 was not expressed in macrophages without carbohydrate antigens around them. (a) CD 68; (b) COX-2; (c) Tn antigen; (d) sialyl Tn antigen; (e) control (mouse IgG); (f) control (goat IgG). (C) The same specimens as in A were double-stained with anti-CD68 and anti-COX-2 antibodies as described in Materials and Methods. Most of CD 68-positive macrophages (brownish-black deposit) were costained with anti-COX-2 antibody (blue deposit). (a) Macrophages in cancer tissues; (b) macrophages in intraluminal space of adenocarcinoma.
The other cells around the CD 68-positive cells also expressed COX-2 (Fig. 6 Ab and C a and b), which probably is induced by mediators secreted from activated macrophages. Immunostaining with normal goat serum and normal mouse IgG was completely negative (Fig. 6A e and f). These immunostaining patterns were observed in other mucin-producing cancer tissues irrespective of differentiation level (unpublished results).
These findings suggest that COX-2 also is induced in vivo in tumor-associated macrophages by mucins secreted from the tumor.
Williams et al. (38) have indicated that tumor growth implanted in COX-2 null mice, but not in COX-1 null or wild-type mice, is attenuated significantly, suggesting that COX-2 in stroma also has an important role in tumor growth. Recently, Sonoshita et al. (39) have demonstrated that COX-2 expression is boosted by PGE2 through the EP2 receptor via a positive feedback loop.
Collectively, these findings strongly suggest that PGE2 exerts a positive effect, inducing the expression of COX-2 in its own and/or nearby cells, resulting in a substantial increase in the capacity of tissues to synthesize and release prostaglandins.
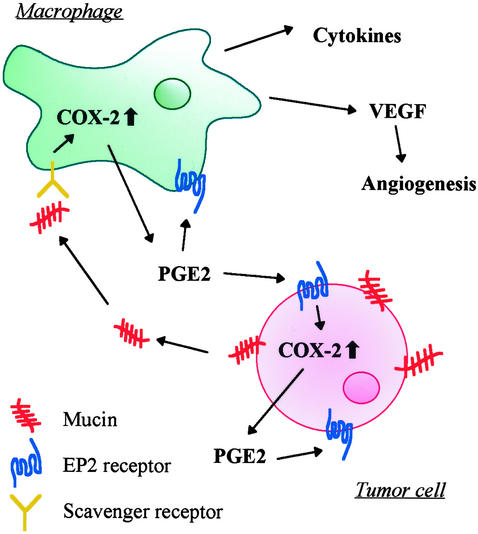
In the present article, we demonstrated that COX-2 in monocytes/macrophages is induced by mucins. We propose the following cascade in the tumor microenvironment as shown in Fig. 7. Mucins are produced by cancer cells. Infiltrated macrophages are activated by the mucins through the MSR. PGE2 secreted from the macrophages binds to EP2 receptor present on cancer cells and/or other cells, and activated cancer cells and macrophages produce vascular endothelial growth factor as reported (39, 40), leading to favorable conditions in epithelial cancer tissues for cancer cell growth.
Figure 7.
Possible role of mucins as chemical mediators in the tumor microenvironment. Mucins produced by colon cancer cells induce COX-2 in macrophages, resulting in PGE2 production. PGE2 functions as a mediator in an autocline and/or paracline fashion, leading to vascular endothelial growth factor (VEGF) production.
Acknowledgments
The buffy coat of human peripheral blood was kindly provided by the Kyoto Red Cross Blood Center. We thank Dr. N. Wakamiya for useful discussion and encouragement. This work was supported by the Foundation for Bio-venture Research Center from the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- COX
cyclooxygenase
- PGE2
prostaglandin E2
- BSM
bovine submaxillary mucin
- OSM
ovine submaxillary mucin
- MSR
macrophage scavenger receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.DuBois R N, Giardiello F M, Smalley W E. Gastroenterol Clin North Am. 1996;25:773–791. doi: 10.1016/s0889-8553(05)70274-0. [DOI] [PubMed] [Google Scholar]
- 2.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 3.Marnett L J. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 4.Smith L L, Garavito M, DoWitt D L. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 5.Eberhart C E, DuBois R N. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 6.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 7.Hwang D, Scollard D, Byrne J, Levine E. J Natl Cancer Inst. 1998;90:445–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- 8.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, Ristrimaki A. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 9.Tucker O N, Dannenberh B J, Yang E K, Zhang F, Tang L, Daly J M, Soslow R A, Masferrer J L, Woerner B M, Koki A T, et al. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 10.Klimp A H, Hollema H, Kemipinga C, Zee A G J, Vries E G E, Daemen T. Cancer Res. 2001;61:7305–7309. [PubMed] [Google Scholar]
- 11.Williams C S, Luongo C, Radhika A, Chang T, Lamps L W, Nanney L B, Beauchamp R D, DuBois R N. Gastroenterology. 1996;111:1134–1140. doi: 10.1016/s0016-5085(96)70083-5. [DOI] [PubMed] [Google Scholar]
- 12.Kawamori T, Rao C V, Seibet K, Reddy B S. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 13.Gupta R A, DuBois R N. Gastroenterology. 1998;114:1095–1098. doi: 10.1016/s0016-5085(98)70330-0. [DOI] [PubMed] [Google Scholar]
- 14.Oshima M, Dinchak J E, Kargman S L, Oshima H, Hancock B, Kwong E, Trzaskos J M, Evans J F, Taketo M M. Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 15.Sano H, Kawahito Y, Wilder R L, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 16.Hull M A, Booth J K, Tisbury A, Scott N, Bonifer C, Markham A F, Coletta P L. Br J Cancer. 1999;79:1399–1405. doi: 10.1038/sj.bjc.6690224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamba H, Ota S, Kato A, Adachi A, Itoyama S, Matsuzaki F. Int J Cancer. 1999;83:470–475. doi: 10.1002/(sici)1097-0215(19991112)83:4<470::aid-ijc6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y S, Gum J, Jr, Brockhausen I. Glycoconjugate J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]
- 19.McCool D J, Forstner J F, Forstner G G. Biochem J. 1994;302:111–118. doi: 10.1042/bj3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigman W, Gottschalk A, editors. Glycoproteins. New York: Elsevier; 1966. pp. 434–445. [Google Scholar]
- 21.Tsuji T, Osawa T. Carbohydrate Res. 1986;151:391–402. doi: 10.1016/s0008-6215(00)90358-6. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Gupta D, Gallagher D, Davis S, Bhavanandan V P. Eur J Biochem. 2000;267:2208–2217. doi: 10.1046/j.1432-1327.2000.01225.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyake M, Taki T, Hitomi S, Hakomori S. N Engl J Med. 1992;327:14–18. doi: 10.1056/NEJM199207023270103. [DOI] [PubMed] [Google Scholar]
- 24.Tettamanti G, Pigman W. Arch Biochem Biophys. 1968;124:41–50. doi: 10.1016/0003-9861(68)90301-9. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa H, Nakada H, Numata Y, Kurosaka A, Fukui S, Funakoshi I, Kawasaki T, Shimada K, Inagaki F, Yamashina I. J Biochem. 1998;104:591–595. doi: 10.1093/oxfordjournals.jbchem.a122516. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka N, Nakada H, Inoue M, Yamashina I. Eur J Biochem. 1999;263:27–32. doi: 10.1046/j.1432-1327.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- 27.Numata Y, Nakada H, Fukui S, Kitagawa H, Ozaki K, Inoue M, Kawasaki T, Funakoshi I, Yamashina I. Biochem Biophys Res Commun. 1990;170:802–808. doi: 10.1016/0006-291x(90)90488-9. [DOI] [PubMed] [Google Scholar]
- 28.Baeckstrom D, Hansson G C, Nilsson O, Johansson C, Gendler S J, Lindholm L. J Biol Chem. 1991;266:21537–21547. [PubMed] [Google Scholar]
- 29.Laemmli U K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Langone J J. Methods Enzymol. 1980;70:350–375. doi: 10.1016/s0076-6879(80)70064-2. [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Fujii H, Kaseyama H, Yamashina I, Nakada H. Biochem Biophys Res Commun. 1999;264:276–280. doi: 10.1006/bbrc.1999.1515. [DOI] [PubMed] [Google Scholar]
- 32.Kawahito Y, Kondo M, Tsubouchi Y, Hashiramoto A, Bishop-Bailey D, Inoue K, Kohno M, Yamada R, Hla T, Sano H. J Clin Invest. 2000;106:189–197. doi: 10.1172/JCI9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith W L, Dewitt D L. Semin Nephrol. 1995;15:179–194. [PubMed] [Google Scholar]
- 34.Kodama T, Reddy P, Kishimoto C, Krieger M. Proc Natl Acad Sci USA. 1988;85:9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 36.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R H. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 37.Itzkowitz S H, Yuan M, Montgomery C K, Kjeldsen T, Takahashi H K, Bigbee W L, Kim Y S. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]
- 38.Williams C S, Tsujii M, Reese J, Dey S K, DuBois R N. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo M M. Nat Med. 2001;7:1048–1051. doi: 10.1038/nm0901-1048. [DOI] [PubMed] [Google Scholar]
- 40.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois R N. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]