Abstract
Donor lymphocyte infusion (DLI) into patients with a relapse of their leukemia or multiple myeloma after allogeneic stem cell transplantation (alloSCT) has been shown to be a successful treatment approach. The hematopoiesis-restricted minor histocompatibility antigens (mHAgs) HA-1 or HA-2 expressed on malignant cells of the recipient may serve as target antigens for alloreactive donor T cells. Recently we treated three mHAg HA-1- and/or HA-2-positive patients with a relapse of their disease after alloSCT with DLI from their mHAg HA-1- and/or HA-2-negative donors. Using HLA-A2/HA-1 and HA-2 peptide tetrameric complexes we showed the emergence of HA-1- and HA-2-specific CD8+ T cells in the blood of the recipients 5–7 weeks after DLI. The appearance of these tetramer-positive cells was followed immediately by a complete remission of the disease and restoration of 100% donor chimerism in each of the patients. Furthermore, cloned tetramer-positive T cells isolated during the clinical response specifically recognized HA-1 and HA-2 expressing malignant progenitor cells of the recipient and inhibited the growth of leukemic precursor cells in vitro. Thus, HA-1- and HA-2-specific cytotoxic T lymphocytes emerging in the blood of patients after DLI demonstrate graft-versus-leukemia or myeloma reactivity resulting in a durable remission. This finding implies that in vitro generated HA-1- and HA-2-specific cytotoxic T lymphocytes could be used as adoptive immunotherapy to treat hematological malignances relapsing after alloSCT.
Treatment of patients with leukemia relapsing after allogeneic stem cell transplantation (alloSCT) by donor lymphocyte infusion (DLI) can induce long-lasting complete remissions through graft-versus-leukemia (GVL) reactivity (1–4). Complete molecular remissions (mCRs) of relapsed chronic myeloid leukemia (CML) in chronic phase have been obtained in 70–80% of treated patients (5–7). In contrast, patients with relapsed acute leukemia or CML in accelerated phase or blast crisis respond in only 20–35% of the cases (3, 7, 8). In a minority of patients with relapsed or persistent multiple myeloma, a graft-versus-myeloma effect after DLI has been demonstrated as well (9–11).
Little is known about the nature and kinetics of antileukemic T cell responses involved in the GVL or graft-versus-myeloma effect after DLI. In patients with relapsed CML after alloSCT who have been treated with low-dose DLI, the time to achieve an mCR may vary from several weeks to 1 year (5, 12). Previously we showed that 5–15 weeks after DLI for relapsed CML significantly increased numbers of cytotoxic T lymphocytes (CTLs) recognizing malignant hematopoietic progenitor cells (HPCs) could be detected in peripheral blood of the recipients (13).
In HLA genotypically identical donor–recipient pairs alloreactive donor T cells may recognize minor histocompatibility antigens (mHAgs) expressed on recipient cells (14). Ubiquitously expressed mHAgs such as HY (15–20), HA-3, HA-4, HA-6, HA-7 (14, 15), and HA-8 (21) may play a role in both graft-versus-host disease (GVHD) and GVL or graft-versus-myeloma effects. In contrast, mHAgs exclusively expressed on recipient cells of hematopoietic origin such as HA-1 and HA-2 (15) or on lineage-specific hematopoietic cells such as HB-1 (23) may result in GVL reactivity in the absence of severe GVHD.
Based on the latter restricted expression, we postulated that DLI for relapsed hematological malignancies from mHAg HA-1− and/or HA-2− donors into HA-1+ and/or HA-2+ recipients may induce mHAg-specific T cell responses associated with a complete remission.
Antigen-specific T cells involved in immune responses in vivo against target antigens of which the peptides of interest have been characterized can be detected by using HLA/peptide tetramers (24–28). Similar to the approach in viral infections it is also possible to measure tissue- or tumor-specific immune responses (29–32). Recently, we demonstrated the presence of increased numbers of mHAg-specific CTLs during GVHD by HLA/HY and -HA-1 tetramer staining (33).
Using HLA-A2/HA-1 or HA-2 peptide tetramers, we have analyzed the kinetics of such T cell responses after DLI in one donor–recipient pair incompatible for both HA-1 and HA-2 and in two HA-1-incompatible donor–recipient pairs. We show that complete hematological responses and conversion from mixed to complete donor chimerism after DLI for relapsed CML and multiple myeloma after alloSCT are associated with a rapid increase in HA-1 and HA-2 tetramer+ T cells in peripheral blood. Thereafter, tetramer+ T cells persisted at a low frequency compatible with immunological memory. To prove their functional relevance in the GVL effect we isolated tetramer+ T cells and showed that these HA-1- or HA-2-specific T cells displayed cytotoxic reactivity against leukemic and normal recipient hematopoietic cells but not to donor cells and were capable of antigen-specific suppression of the leukemic hematopoietic precursor cell growth. Our results illustrate that donor T cells specific for the hematopoiesis-restricted mHAg HA-1 or HA-2 expressed on recipient cells can be involved in the induction of complete remissions of relapsed hematological malignancies after alloSCT.
Case Reports
The three patients received a conditioning regimen consisting of cyclophosphamide and total-body irradiation and were subsequently transplanted with granulocyte colony-stimulating factor-mobilized peripheral blood stem cells (PBSCs) from their HLA-identical donors (Table 1) after informed consent. In vitro Campath-1H was used to deplete the PBSC graft of T cells as GVHD prophylaxis.
Table 1.
Tissue typing of the donors and patients
| Donor–patient pair | Sex | HLA-A and -B type | HA-1 | HA-2 | HY |
|---|---|---|---|---|---|
| 1 | F/F* | A2 A24 B8 B60 | −/+† | −/+† | −/−† |
| 2 | F/M | A2 A3 B7 B44 | −/+ | +/+ | −/+ |
| 3 | M/F | A2 A3 B7 B60 | −/+ | +/+ | +/− |
F, female; M, male.
Donor/patient.
Patient 1 was a 47-year-old woman with CML in chronic phase who experienced GVHD grade I of the skin after transplantation. Nine months after alloSCT a cytogenetic relapse was detected. Retrospective molecular analysis of stored samples showed the presence of BCR/ABL transcripts at 3 and 6 months after transplantation. In preparation to DLI she was treated with 3 × 106 units of α-IFN daily s.c. Four weeks later the CML had progressed to a hematological relapse, and 107 donor mononuclear cells (MNCs) per kg body weight (BW) were administered. After DLI she achieved an mCR that was associated with a transient pancytopenia and conversion to 100% donor chimerism. The DLI was complicated by grade I GVHD of the skin and mouth for which no systemic immunosuppressive treatment was necessary. Presently, 3 years after DLI, she is in good clinical condition without GVHD and in mCR.
Patient 2 was a 46-year-old male patient with CML in chronic phase who experienced no GVHD after transplantation. Follow-up studies after alloSCT were repeated every 3 months for 1.5 years and showed a complete cytogenetic remission as tested by classical cytogenetic analysis and BCR/ABL fluorescence in situ hybridization. However, chimerism analysis always showed partial donor chimerism. Eighteen months after alloSCT a molecular relapse was detected rapidly followed by a lymphatic blast crise. After treatment with cytarabine and daunorubicin a molecular relapse persisted. Two months after chemotherapy he received 108 donor MNCs per kg BW combined with 3 × 106 units of α-IFN daily s.c. Eight weeks after DLI an mCR and conversion to 100% donor chimerism was obtained. However, GVHD grade II developed, which was treated with 1 mg prednisolone/kg BW. During the immunosuppressive treatment BCR/ABL transcripts became positive again. Chimerism analysis persistently showed 100% donor cells. Six months after DLI a severe cardiomyopathy with pulmonary and pericardial fluid retention developed. Several months later he died as a consequence of the cardiac dysfunction.
Patient 3 was a 48-year-old woman with multiple myeloma who developed GVHD grade II of the skin after transplantation, which was treated with prednisolone and cyclosporin A. The M-protein level decreased after transplantation to 3 g/liter, but scattered myeloma cells persisted in the bone marrow. Despite the discontinuation of immunosuppressive treatment 1.5 years after transplantation, the M-protein level increased, and bone marrow examination revealed the presence of 2% myeloma cells and mixed donor chimerism. She was treated with 3 × 106 units of α-IFN/day s.c. 2 weeks later followed by infusion of 107 donor MNCs per kg BW. This resulted in disappearance of the M protein, disappearance of the malignant plasma cells from the bone marrow, and conversion to 100% donor chimerism. After DLI grade II GVHD developed, which was treated with 1 mg prednisolone/kg BW and cyclosporin A, which could be tapered slowly without recurrence of malignant cells. Presently, >2 years after DLI she is in complete remission and in good clinical condition without GVHD.
Materials and Methods
Blood and Bone Marrow Samples.
To study the phenotype and functional characteristics of peripheral blood MNCs and bone marrow MNCs, heparinized samples were collected after informed consent at regular intervals after alloSCT and DLI, isolated, and cryopreserved for future use. Immediately before use the MNCs were thawed and resuspended in Iscove's modified Dulbecco medium plus 10% pooled human serum.
HLA Class I–mHAg Peptide Tetrameric Complexes.
Tetramers were constructed as described (34) with minor modifications and consisted of the hematopoiesis-restricted mHAg HA-1 or HA-2 peptides bound to HLA-A2 molecules. In addition, tetramers were made of the male-specific HY peptide derived from the SMCY gene (16) associated with HLA-A2 or HLA-B7 molecules.
Phenotype Determination.
Phenotypic analysis was performed by staining the cells with phycoerythrin (PE)-labeled tetramers and counterstaining them with anti-CD8/Cy5 or with anti-CD4/Cy5 together with anti-HLA-DR/FITC monoclonal antibodies (Becton Dickinson) and analyzed by using a FACSCalibur (Becton Dickinson). In addition, samples were stained with anti-CD45/Cy5 and -CD14/PE or -CD19/PE monoclonal antibodies. To calculate the absolute number of CD8+ T cells in the peripheral blood samples, the percentage CD8+ T cells was divided by the percentage of CD45+/CD14− lymphocytes and multiplied by the lymphocyte count derived from the white blood cell differential count. Within the CD8+ T cell population the percentage of tetramer+ T cells was determined.
Detection of BCR/ABL+ Cells.
Real-time BCR/ABL PCR analysis was performed on an ABI/Prism 7700 sequence-detector system (Applied Biosystems, Foster City, CA) as described (35). The limit of detection for this assay is a BCR/ABL to porphobilinogen deaminase ratio of 10−5. BCR/ABL fluorescence in situ hybridization was performed as described (36) by using locus-specific identifier BCR/ABL extra signal probes (Vysis, Downers Grove, IL). Classical cytogenetic analysis was performed by GTG-banding.
Chimerism Analysis.
Chimerism analysis of blood and bone marrow samples from patients who were transplanted with donors of the opposite sex was performed by fluorescence in situ hybridization using X and Y chromosome-specific probes as described (36) with minor modifications. A chromosome X centromere-specific DNA probe (37) and a chromosome Y heterochromatic-specific DNA probe were used (38). Chimerism studies on cells from patients with a donor of the same sex were performed by PCR analysis with primers specific for selected polymorphic short tandem repeats using the AmpFLSTR Profiler Plus ID amplification kit (Applied Biosystems) and a GeneAmp 9700 thermocycler (Applied Biosystems) using AmpliTaq Gold DNA polymerase (Applied Biosystems). PCR products were analyzed by using the ABI PRISM 310 Genetic Analyzer and GENESCAN 2.1 analysis software (Applied Biosystems).
Generation of mHAg-Specific CTL Clones.
To isolate HA-1 and HA-2 tetramer+ T cells, peripheral blood MNCs from patient 1, collected 7 or 12 weeks after DLI, were stained at 4°C for 2 h with the relevant tetramer and sorted at a concentration of one cell per well by using a FACSVantage (Becton Dickinson). Tetramer+ T cells were cultured in Iscove's modified Dulbecco medium plus 10% human serum and stimulated nonspecifically with irradiated allogeneic feeder cells, phytohemagglutinin, and 120 units/ml IL-2 until enough HA-1- or HA-2-specific effector T cells could be harvested to perform functional assays.
Functional Assays.
51Cr-release assays were performed as described (39). The liquid HPC growth-inhibition assay was performed as described (13). Effector cells were irradiated with 25 Gy before use preventing their proliferation in the liquid HPC growth-inhibition assay. Recipient malignant HPCs collected before transplantation and before DLI were used as target cells as well as HPCs from unrelated HLA-A2+, HA-1+, and/or HA-2+ CML patients. In addition to the HA-1- and HA-2-specific CTL clones, an HLA-A1-restricted HY-specific CTL clone was used as a negative control, and an allospecific anti-HLA-A2 CTL clone (MBM-13) was used as a positive control. The IFN-γ-capture assay was performed as described (40).
Results
Phenotype, Disease Marker, and Chimerism Analysis.
Case 1.
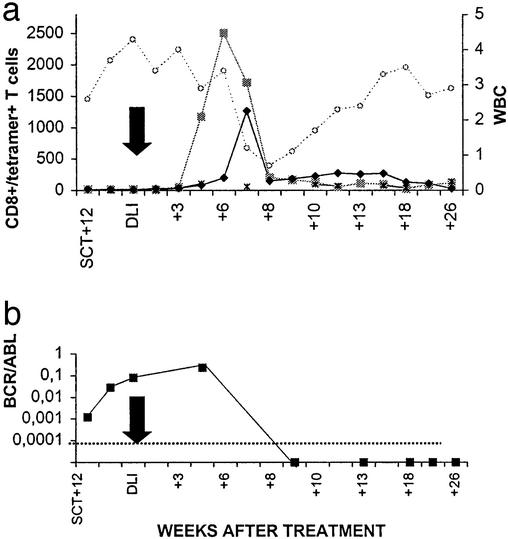
In this donor–patient combination both HA-1 and HA-2 disparity was present in the GVL/GVH direction. Before DLI the level of HA-1- and HA-2-specific CTLs per ml blood as detected by tetramer staining was low (≤0.02%). A rapid increase to >2,500 HA-2 tetramer+ T cells per ml was detected 5–7 weeks after DLI (Fig. 1a). At a somewhat slower rate, HA-1 tetramer+ T cells also started to rise 5 weeks after DLI and reached a level of 1,265 cells per ml 7 weeks after DLI. Nonspecific T cell activation or staining with tetramers after DLI was excluded because the number of HLA-A2 restricted HY-specific T cells did not change in this female-to-female transplantation (Fig. 1a). The peak of HA-1 and HA-2 tetramer+ T cells was followed immediately by disappearance of malignant recipient white blood cells resulting in a transient pancytopenia, disappearance of BCR/ABL transcripts from blood and bone marrow (Fig. 1b), and conversion to 100% donor chimerism. After entering an mCR the number of tetramer+ T cells decreased, but in contrast to the period before DLI, HA-1- and HA-2-specific T cells remained clearly detectable (Fig. 1a).
Figure 1.
HA-1 and HA-2 tetramer+ T cells in peripheral blood from patient 1 in relation to clinical effect. (a) CD8+/tetramer+ T cells are expressed as absolute numbers per milliliter in peripheral blood (left y axis). ■, CD8+/HA-2 tetramer+ T cells; ♦, CD8+/HA-1 tetramer+ T cells; *, CD8+/HY-A2 tetramer+ T cells; ○, white blood cell count (WBC) × 106 per ml in peripheral blood (expressed on the right y axis). The black arrow depicts the moment of DLI. (b) BCR/ABL (■) is expressed as the ratio of BCR/ABL/household gene porphobilinogen deaminase mRNA. The lower level of detection is ≤10−5.
Case 2.
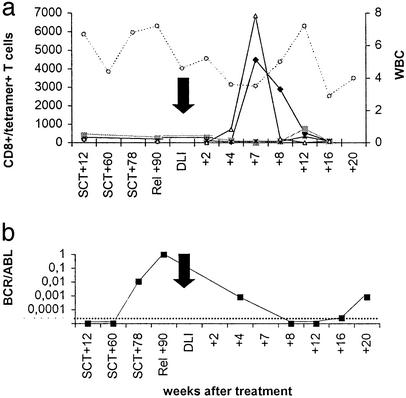
In this donor–patient combination HA-1 and HY disparities were present in the GVL/GVH direction. Before DLI the level of HA-1-specific CTLs per ml of blood as detected by tetramer staining was low (≤0.02%). Seven weeks after DLI the number of HA-1 tetramer+ T cells rapidly increased to >4,400 cells per ml (Fig. 2a). In this female-to-male transplantation an increase of HLA-B7-restricted HY-specific T cells to >6,800 cells per ml was found. HLA-A2/HY-specific T cells were not detectable (Fig. 2a). Nonspecific T cell activation or staining with tetramers after DLI was excluded because the number of HA-2 tetramer+ T cells did not change in this HA-2+ donor–recipient pair (Fig. 2a). Immediately after the HA-1 tetramer+ T cell peak no BCR/ABL transcripts were detectable anymore (Fig. 2b), and complete donor chimerism developed. The patient developed grade II GVHD after DLI and was treated with 1 mg prednisolone/kg BW. Although testing revealed persistent complete donor chimerism in the bone marrow, during the immunosuppressive treatment HA-1 tetramer+ T cells disappeared from the blood, coinciding with reappearance of BCR/ABL transcripts compatible with a molecular relapse.
Figure 2.
HA-1 and HY/B7 tetramer+ T cells in peripheral blood from patient 2 in relation to clinical effect. See the legend to Fig. 1 for a description of symbols and abbreviations used. ▵, CD8+/HY-B7 tetramer+ T cells.
Case 3.
In this donor–patient combination HA-1 disparity was present in the GVL/GVH direction. Before DLI the level of HA-1-specific CTLs per ml blood was low (≤0.02%). Seven weeks after DLI the number of HA-1 tetramer+ T cells increased rapidly to >2,500 cells per ml (Fig. 3a). In this male-to-female transplantation nonspecific T cell activation or staining with tetramers after DLI was excluded by using HLA-B7/HY tetramer staining (Fig. 3a). Four weeks after the tetramer+ T cell peak the M protein decreased to undetectable levels (Fig. 3b), and bone marrow aspirates showed an absence of myeloma cells. The HA-1-specific T cell peak was followed by conversion to persistent complete donor chimerism. Because this patient suffered from grade II GVHD after DLI, she was treated with 1 mg prednisolone/kg BW and cyclosporin A. In contrast to patient 2, HA-1 tetramer+ T cells were still detectable during this treatment on weeks 11 and 12.
Figure 3.
HA-1 tetramer+ T cells in peripheral blood from patient 3 in relation to clinical effect. (a) See the legends to Figs. 1 and 2 for a description of symbols and abbreviations used. (b) ♦, M protein is expressed in grams/liter in peripheral blood. The lower level of detection is <1 g/liter.
The phenotype of the HA-1- or HA-2-specific T cells was similar in all three patients, expressing CD3, CD8, and HLA-DR.
Generation of HA-1- or HA-2-Specific T Cell Clones.
To analyze the functional capacity of the HA-1 and HA-2 tetramer+ T cells to lyse the leukemic target cells from the patient but not donor hematopoietic cells, HA-1 and HA-2 tetramer+ T cells were isolated directly from peripheral blood from patient 1. The tetramer+ T cells were clonally isolated by using single-cell sorting and thereafter nonspecifically expanded. Seven T cell clones staining brightly with the HA-1 tetramer and 24 intermediately staining T cell clones were isolated. In addition, 70 clones staining brightly with the HA-2 tetramer were isolated.
Functional Studies.
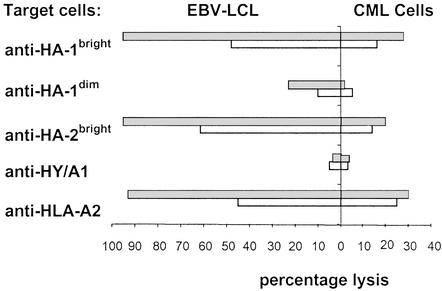
Four of the seven brightly staining HA-1-specific CTL clones could be expanded until enough cells were obtained for a 51Cr-release assay. These clones displayed a mean percentage of 46% lysis (range 35–64%) of recipient Epstein–Barr virus (EBV)-lymphoblastoid cell lines (LCLs) but not donor EBV-LCLs in the 51Cr-release assay. Only one HA-1bright-specific CTL clone could be tested against CML cells from the recipient in a 51Cr-release assay and exhibited 16% lysis of the predominantly mature myeloid cells. Extending the incubation period from 4 to 20 h increased the percentage lysis from 16% to 28% (Fig. 4). Twenty-two of the intermediately HA-1 tetramer-staining CTL clones lysed recipient EBV-LCLs (mean 22%; range 14–32%) after a prolonged incubation period of 20 h. The HA-1dim CTL clones exhibited no cytotoxicity toward the CML cells even after prolonging the incubation period. The seventy HA-2bright CTL clones showed a mean percentage lysis of 65% (range 28–83%) of recipient EBV-LCLs. Of these HA-2-specific CTL clones, four were tested against recipient CML cells in a 51Cr-release assay and lysed those target cells to the same extent as the HA-1-specific CTL clones (mean 13%; range 8–16%). Representative examples of each type of HA-1- or HA-2-specific CTL clone are shown in Fig. 4. As a negative control an HLA-A1-restricted HY-specific CTL clone was used and showed no lysis of the female target cells. As a positive control the HLA-A2 allospecific CTL clone MBM-13 was used and recognized cells from both the donor and recipient.
Figure 4.
Percentage lysis of EBV-LCLs and CML cells from patient 1 by different CTL clones as measured in the 51Cr-release assay. (Left) Lysis of EBV-LCLs. (Right) Lysis of CML cells. Gray bars, 20-h incubation; white bars, 4-h incubation.
Alternatively, functionality of responder T cells present in peripheral blood at the moment of the tetramer+ T cell peak in the three patients was analyzed by the IFN-γ-capture assay. After 18 h of stimulation, 0.21% of T cells from patient 1, 0.68% of T cells from patient 2, and 0.21% of T cells from patient 3 produced IFN-γ. The percentages of CD8+/tetramer+ T cells measured in peripheral blood from patients 1–3 at the same moment were 0.43% (HA-2), 1.57% (HA-1), and 0.21% (HA-1), respectively.
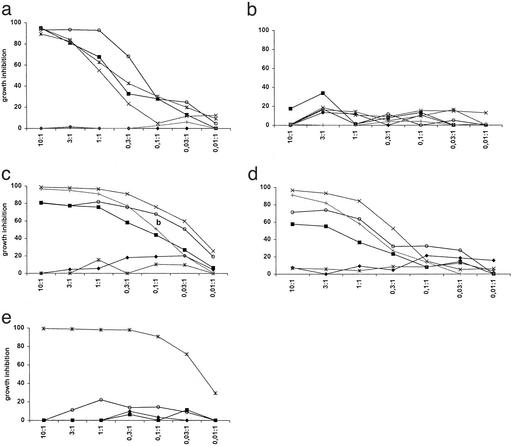
We have demonstrated that remissions obtained in patients with CML after DLI were associated with an increased frequency of T cells recognizing leukemic progenitor cells (13). Therefore, we analyzed one high and one intermediate affinity mHAg HA-1-specific T cell clone and two high-affinity mHAg HA-2-specific T cell clones for their capacity to inhibit the growth of leukemic progenitor cells from the mHAg+ patient 1. The high-affinity anti-HA-1 or HA-2 donor T cells strongly inhibited in a dose-dependent way the growth of leukemic precursor cells from the recipient, collected before transplantation, but not of normal donor progenitor cells (Fig. 5 a, c, and d). Malignant progenitor cells from other HLA-A2+ CML patients expressing the relevant mHAg were also recognized, in contrast to CML precursor cells from mHAg-negative HLA-A2+ patients (Fig. 5 a, c, and d). The recognition of target cells was antigen-specific as demonstrated by the absence of recognition of target cells by an irrelevant HLA-A1-restricted HY-specific CTL clone (Fig. 5e). In these control experiments only cells from the HLA-A1+ male CML patient Y were recognized. As a positive control the HLA-A2 allospecific CTL clone MBM-13 recognized cells from all individuals expressing the HLA-A2 antigen (data not shown). The low-affinity HA-1-specific T cell clone only inhibited to a very limited extent the growth of malignant recipient precursor cells (Fig. 5b). Thus, high-affinity mHAg HA-1- and HA-2-reactive CTLs isolated from peripheral blood of patient 1 specifically lysed both normal and malignant recipient hematopoietic cells but not donor cells.
Figure 5.
Percentage of growth inhibition by different CTL clones in the HPC growth-inhibition assay. Effector cells: (a) high-affinity anti-HA-1 CTL clone; (b) intermediate affinity anti-HA-1 CTL clone; (c and d) high-affinity anti-HA-2 CTL clones; (e) control anti-HLA-A1/HY CTL clone. Target cells: ♦, donor HPCs; ■, recipient CML HPCs before transplantation; ○, recipient CML HPCs at relapse; x, unrelated CML patient X; *, unrelated CML patient Y; +, unrelated CML patient Z. On the x axis the E/T ratio is shown. Tissue typing: CML-X ♀, HLA-A2, HA-1-positive, HA-2-positive; CML-Y ♂, HLA-A1, -A2, HA-1-positive, HA-2-negative; and CML-Z ♂, HLA-A2, HA-1-negative, HA-2-positive.
Discussion
Cellular immunotherapy is a potent treatment modality for patients with relapsed hematological malignancies after alloSCT. The curative effect of DLI has been illustrated by the induction of long-term complete remissions after DLI (1–4).
We have demonstrated that the response to DLI is associated with an increased frequency in peripheral blood of T cells capable of recognizing leukemic precursor cells (13). However, the molecular target structures for the effector T cells in this assay were unknown. Alloreactive T cells with specificity for mHAgs play an essential role in alloSCT, both in the GVH direction (14, 41) and in the host-versus-graft direction (42–44). mHAgs broadly expressed on recipient cells are target molecules in both GVHD and GVL reactions. In contrast, T cells recognizing hematopoiesis-restricted mHAgs may induce a relative specific immune response against malignant hematopoietic recipient cells and induce no or only minor GVHD. Recently the differential recognition of skin tissue by T cells recognizing broadly versus hematopoiesis-restricted mHAgs has been demonstrated and discussed (45).
In the present study we show in mHAg HA-1 and/or HA-2 incompatible donor–recipient pairs a direct association between the emergence of mHAg HA-1 or HA-2 tetramer+ CTLs and the complete disappearance of malignant recipient cells, conversion to complete donor chimerism, and preservation of donor hematopoiesis. The magnitude of the HA-1- or HA-2-specific immune response after DLI did not correlate with the severity of GVHD as demonstrated by the limited GVHD observed in patient 1. In all three patients, however, additional activated T cells were present that may have contributed to GVHD or the GVL effect in these patients. For instance, in patient 2 GVHD was accompanied by the emergence of mHAg HY/B7-specific T cells, which may react directly with skin tissue (45). Thus, HA-1- and HA-2-specific T cells may not have been the sole T cell population responsible for the GVL effect. Using the IFN-γ-capture assay we demonstrated the presence of functional T cells that were reactive with recipient cells. The percentages of tetramer+ T cells and IFN-γ producing T cells were in the same order of magnitude. Because the percentages of activated CD8+ T cells were 30–78% of all CD8+ T cells in the three patients, the IFN-γ-capture assay underestimates the actual number of recipient-reactive donor T cells. This discrepancy may be explained by the fact that the IFN-γ-capture assay is a functional assay and depends on the capability of T cells to become activated and release cytokines during a relatively short incubation period. Differential kinetics of IFN-γ production after activation also may be a cause of the underestimation of the alloreactive T cell frequency. Furthermore, some activated T cells may have preferentially produced other cytokines such as IL-4.
The direct involvement of HA-1- and HA-2-specific CTLs in the GVL response was demonstrated by complete suppression of leukemic progenitor cell growth in the absence of donor HPC growth inhibition. As reported previously, this assay is much more sensitive than the 51Cr-release assay (13). The relatively low percentage of lysis in the 51Cr-release assay compared with the degree of leukemic progenitor cell-growth inhibition is not due to the fact that cytotoxic effector cells are unable to recognize these mature myeloid cells but that mature myeloid cells are relatively insensitive to lysis. Leukemic progenitor cells, however, are very sensitive to T cell-mediated killing.
Because the mHAgs HA-1 and HA-2 have a hematopoiesis-restricted tissue distribution and HA-1- and HA-2-specific donor T cells may be capable of eradicating hematological malignancies in vivo, HA-1 and HA-2 peptides may be used to generate mHAg-specific donor-derived CTLs in vitro. Such CTLs may be used to treat HA-1+ or HA-2+ patients who suffer from a relapse of their disease after alloSCT (22).
In conclusion, using HLA-A2/HA-1 and HA-2 peptide tetrameric complexes we demonstrate a profound increase in CD8+ T cells specific for the hematopoiesis-restricted mHAgs HA-1 and HA-2, which is followed immediately by complete disappearance of BCR/ABL+ cells or of the M protein and myeloma cells and conversion to full donor chimerism resulting in an ongoing complete remission of the relapsed hematological malignancies after alloSCT. In addition, antigen specifically isolated HA-1- and HA-2-reactive T cells inhibited the in vitro growth of malignant BCR/ABL+ HPCs. Herewith, evidence of the type of effector T cells and the molecular nature of their target antigens involved in the GVL effect is provided. This implies that in vitro-generated HA-1- and HA-2-specific CTLs may be used for adoptive immunotherapy to treat relapsed hematological malignancies after alloSCT.
Acknowledgments
This work was supported by grants from the J. A. Cohen Institute for Radiopathology and Radiation Protection and the Dutch Cancer Society.
Abbreviations
- alloSCT
allogeneic stem cell transplantation
- DLI
donor lymphocyte infusion
- GVL
graft-versus-leukemia
- CML
chronic myeloid leukemia
- CTL
cytotoxic T lymphocyte
- HPC
hematopoietic progenitor cell
- mHAg
minor histocompatibility antigen
- GVHD
graft-versus-host disease
- MNC
mononuclear cell
- BW
body weight
- mCR
complete molecular remission
- EBV
Epstein–Barr virus
- LCL
lymphoblastoid cell line
References
- 1.Kolb H J, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 2.Kolb H J, Schattenberg A, Goldman J M, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 3.Collins R H, Shpilberg O, Drobyski W R, Porter D L, Giralt S, Champlin R, Goodman S A, Wolff S N, Hu W, Verfaillie C, et al. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 4.Porter D L, Collins R H, Jr, Hardy C, Kernan N A, Drobyski W R, Giralt S, Flowers M E, Casper J, Leahey A, Parker P, et al. Blood. 2000;95:1214–1221. [PubMed] [Google Scholar]
- 5.Dazzi F, Szydlo R M, Craddock C, Cross N C, Kaeda J, Chase A, Olavarria E, van Rhee F, Kanfer E, Apperley J F, et al. Blood. 2000;95:67–71. [PubMed] [Google Scholar]
- 6.Porter D L, Collins R H, Jr, Shpilberg O, Drobyski W R, Connors J M, Sproles A, Antin J H. Biol Blood Marrow Transplant. 1999;5:253–261. doi: 10.1053/bbmt.1999.v5.pm10465105. [DOI] [PubMed] [Google Scholar]
- 7.Mackinnon S, Papadopoulos E B, Carabasi M H, Reich L, Collins N H, Boulad F, Castro-Malaspina H, Childs B H, Gillio A P, Kernan N A, et al. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 8.Levine J E, Braun T, Penza S L, Beatty P, Cornetta K, Martino R, Drobyski W R, Barrett A J, Porter D L, Giralt S, et al. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 9.Lokhorst H M, Schattenberg A, Cornelissen J J, van Oers M H, Fibbe W, Russell I, Donk N W, Verdonck L F. J Clin Oncol. 2000;18:3031–3037. doi: 10.1200/JCO.2000.18.16.3031. [DOI] [PubMed] [Google Scholar]
- 10.Salama M, Nevill T, Marcellus D, Parker P, Johnson M, Kirk A, Porter D, Giralt S, Levine J E, Drobyski W, et al. Bone Marrow Transplant. 2000;26:1179–1184. doi: 10.1038/sj.bmt.1702685. [DOI] [PubMed] [Google Scholar]
- 11.Kroger N, Kruger W, Renges H, Zabelina T, Stute N, Jung R, Wittkowsky G, Kuse R, Zander A. Br J Haematol. 2001;112:421–423. doi: 10.1046/j.1365-2141.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- 12.Bar B M, Schattenberg A, Mensink E J, Geurts v K, Smetsers T F, Knops G H, Linders E H, de Witte T. J Clin Oncol. 1993;11:513–519. doi: 10.1200/JCO.1993.11.3.513. [DOI] [PubMed] [Google Scholar]
- 13.Smit W M, Rijnbeek M, van Bergen C A, Fibbe W E, Willemze R, Falkenburg J H. Proc Natl Acad Sci USA. 1998;95:10152–10157. doi: 10.1073/pnas.95.17.10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulmy E. Curr Opin Immunol. 1996;8:75–81. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 15.de Bueger M, Bakker A, van Rood J J, Van der W F, Goulmy E. J Immunol. 1992;149:1788–1794. [PubMed] [Google Scholar]
- 16.Wang W, Meadows L R, den Haan J M, Sherman N E, Chen Y, Blokland E, Shabanowitz J, Agulnik A I, Hendrickson R C, Bishop C E, et al. Science. 1995;269:1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- 17.Meadows L, Wang W, den Haan J M, Blokland E, Reinhardus C, Drijfhout J W, Shabanowitz J, Pierce R, Agulnik A I, Bishop C E, et al. Immunity. 1997;6:273–281. doi: 10.1016/s1074-7613(00)80330-1. [DOI] [PubMed] [Google Scholar]
- 18.Vogt M H, de Paus R A, Voogt P J, Willemze R, Falkenburg J H. Blood. 2000;95:1100–1105. [PubMed] [Google Scholar]
- 19.Vogt M H, Goulmy E, Kloosterboer F M, Blokland E, de Paus R A, Willemze R, Falkenburg J H. Blood. 2000;96:3126–3132. [PubMed] [Google Scholar]
- 20.Warren E H, Gavin M A, Simpson E, Chandler P, Page D C, Disteche C, Stankey K A, Greenberg P D, Riddell S R. J Immunol. 2000;164:2807–2814. doi: 10.4049/jimmunol.164.5.2807. [DOI] [PubMed] [Google Scholar]
- 21.Brickner A G, Warren E H, Caldwell J A, Akatsuka Y, Golovina T N, Zarling A L, Shabanowitz J, Eisenlohr L C, Hunt D F, Engelhard V H, et al. J Exp Med. 2001;193:195–206. doi: 10.1084/jem.193.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Blood. 1999;93:2336–2341. [PubMed] [Google Scholar]
- 23.Dolstra H, Fredrix H, Maas F, Coulie P G, Brasseur F, Mensink E, Adema G J, de Witte T M, Figdor C G, van de Wiel-van Kemenade E. J Exp Med. 1999;189:301–308. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman J D, Moss P A, Goulder P J, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 25.Callan M F, Tan L, Annels N, Ogg G S, Wilson J D, O'Callaghan C A, Steven N, McMichael A J, Rickinson A B. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X, Demoitie M A, Donahoe S M, Ogg G S, Bonhoeffer S, Kakimoto W M, Gillespie G, Moss P A, Dyer W, Kurilla M G, et al. J Infect Dis. 2000;181:165–175. doi: 10.1086/315201. [DOI] [PubMed] [Google Scholar]
- 27.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, et al. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 28.Gratama J W, van Esser J W, Lamers C H, Tournay C, Lowenberg B, Bolhuis R L, Cornelissen J J. Blood. 2001;98:1358–1364. doi: 10.1182/blood.v98.5.1358. [DOI] [PubMed] [Google Scholar]
- 29.Molldrem J J, Lee P P, Wang C, Felio K, Kantarjian H M, Champlin R E, Davis M M. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 30.Clark R E, Dodi I A, Hill S C, Lill J R, Aubert G, Macintyre A R, Rojas J, Bourdon A, Bonner P L, Wang L, et al. Blood. 2001;98:2887–2893. doi: 10.1182/blood.v98.10.2887. [DOI] [PubMed] [Google Scholar]
- 31.Karanikas V, Colau D, Baurain J F, Chiari R, Thonnard J, Gutierrez-Roelens I, Goffinet C, Schaftingen E V, Weynants P, Boon T, et al. Cancer Res. 2001;61:3718–3724. [PubMed] [Google Scholar]
- 32.Yamshchikov G, Thompson L, Ross W G, Galavotti H, Aquila W, Deacon D, Caldwell J, Patterson J W, Hunt D F, Slingluff C L., Jr Clin Cancer Res. 2001;7:909s–916s. [PubMed] [Google Scholar]
- 33.Mutis T, Gillespie G, Schrama E, Falkenburg J H, Moss P, Goulmy E. Nat Med. 1999;5:839–842. doi: 10.1038/10563. [DOI] [PubMed] [Google Scholar]
- 34.Burrows S R, Kienzle N, Winterhalter A, Bharadwaj M, Altman J D, Brooks A. J Immunol. 2000;165:6229–6234. doi: 10.4049/jimmunol.165.11.6229. [DOI] [PubMed] [Google Scholar]
- 35.Mensink E, van de Locht A, Schattenberg A, Linders E, Schaap N, Geurts van Kessel A, de Witte T. Br J Haematol. 1998;102:768–774. doi: 10.1046/j.1365-2141.1998.00823.x. [DOI] [PubMed] [Google Scholar]
- 36.Beverstock G C, de Meijer P H, ten Bokkel H D, Pruijt J F, den Ottolander G J, Wessels H W, Mollevanger P. Cancer Genet Cytogenet. 1996;89:132–135. doi: 10.1016/0165-4608(95)00316-9. [DOI] [PubMed] [Google Scholar]
- 37.Willard H F, Smith K D, Sutherland J. Nucleic Acids Res. 1983;11:2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooke H J, Schmidtke J, Gosden J R. Chromosoma. 1982;87:491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- 39.Goulmy E, Termijtelen A, Bradley B A, van Rood J J. Tissue Antigens. 1976;8:317–326. doi: 10.1111/j.1399-0039.1976.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 40.Becker C, Pohla H, Frankenberger B, Schuler T, Assenmacher M, Schendel D J, Blankenstein T. Nat Med. 2001;7:1159–1162. doi: 10.1038/nm1001-1159. [DOI] [PubMed] [Google Scholar]
- 41.Goulmy E, Schipper R, Pool J, Blokland E, Falkenburg J H, Vossen J, Grathwohl A, Vogelsang G B, van Houwelingen H C, van Rood J J. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 42.Goulmy E, Termijtelen A, Bradley B A, van Rood J J. Nature. 1977;266:544–545. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- 43.Voogt P J, Fibbe W E, Marijt W A, Goulmy E, Veenhof W F, Hamilton M, Brand A, Zwann F E, Willemze R, van Rood J J. Lancet. 1990;335:131–134. doi: 10.1016/0140-6736(90)90003-n. [DOI] [PubMed] [Google Scholar]
- 44.Marijt W A, Kernan N A, Diaz-Barrientos T, Veenhof W F, O'Reilly R J, Willemze R, Falkenburg J H. Bone Marrow Transplant. 1995;16:125–132. [PubMed] [Google Scholar]
- 45.Dickinson A M, Wang X N, Sviland L, Vyth-Dreese F A, Jackson G H, Schumacher T N, Haanen J B, Mutis T, Goulmy E. Nat Med. 2002;8:410–414. doi: 10.1038/nm0402-410. [DOI] [PubMed] [Google Scholar]