Abstract
Fast cholinergic neurotransmission between superior cervical ganglion neurons (SCGNs) in cell culture is initiated by N-type Ca2+ currents through Cav2.2 channels. To test the ability of different Ca2+-channel subtypes to initiate synaptic transmission in these cells, SCGNs were injected with cDNAs encoding Cav1.2 channels, which conduct L-type currents, Cav2.1 channels, which conduct P/Q-type Ca2+ currents, and Cav2.3 channels, which conduct R-type Ca2+ currents. Exogenously expressed Cav2.1 channels were localized in nerve terminals, as assessed by immunocytochemistry with subtype-specific antibodies, and these channels effectively initiated synaptic transmission. Injection with cDNA encoding Cav2.3 channels yielded a lower level of presynaptic labeling and synaptic transmission, whereas injection with cDNA encoding Cav1.2 channels resulted in no presynaptic labeling and no synaptic transmission. Our results show that exogenously expressed Ca2+ channels can mediate synaptic transmission in SCGNs and that the specificity of reconstitution of neurotransmission (Cav2.1 > Cav2.3 ≫ Cav1.2) follows the same order as in neurons in vivo. The specificity of reconstitution of neurotransmission parallels the specificity of trafficking of these Cav channels to nerve terminals.
Electrophysiological and pharmacological studies have defined a diverse array of native Ca2+ currents having different functions in neurons (1, 2). Voltage-gated Ca2+ channels are complexes of a pore-forming α1 subunit with associated α2δ, β, and γ subunits (3–5). α1 subunits can be divided into three families based on structure and function (6). Ca2+ channels having α1 subunits of the Cav1 family conduct L-type Ca2+ currents (7, 8), which are important for Ca2+-dependent regulation of enzyme activity and gene transcription in neurons (9–11). Ca2+ channels having α1 subunits of the Cav2 family conduct N-, P/Q-, and R-type Ca2+ currents (12–15), which initiate synaptic transmission in nerve terminals and integrate synaptic signals in cell bodies and dendrites of neurons (16–20). Ca2+ channels having α1 subunits of the Cav3 family generate T-type Ca2+ currents, which shape action potentials and generate repetitive firing patterns in neuronal cell bodies and dendrites (21–23).
In the central nervous system, excitatory neurotransmitter release is mediated cooperatively by N-, P/Q-, and R-type Ca2+ currents (1, 18–20, 24). In contrast, neurotransmission in sympathetic ganglion neurons is initiated exclusively by N-type Ca2+ currents (25, 26). These neurons have substantial N-type and L-type Ca2+ currents in their cell bodies (27, 28), but P/Q-type Ca2+ currents sensitive to ω-agatoxin IVA are not observed (29, 30). The molecular basis for the specific role of Cav2 channels in fast neurotransmission is not well understood. Methods to analyze the function of exogenously expressed Ca2+ channels in synaptic transmission would be valuable in addressing these questions. Here we describe functional expression of exogenous Cav1.2, Cav2.1, and Cav2.3 channels in superior cervical ganglion neurons (SCGNs) and show that Cav2.1 is most effective in nerve-terminal localization and synaptic transmission, Cav2.3 is less effective, and Cav1.2 is ineffective. Evidently, these Cav2 subunits contain the information within them for selective expression, targeting to nerve terminals, and function in synaptic transmission, whereas Cav1.2 does not.
Experimental Procedures
Preparation of Ca2+-Channel cDNAs.
cDNAs encoding the rat brain Ca2+-channel subunits α12.1 [rbA-I (31)] and α12.3 [rbE-II (14)] were subcloned into the vertebrate expression vector pMT2 [Genetics Institute, Cambridge, MA (31)]. The rat brain α11.2 [rbC-II (7, 32)] was constructed in pZEM229. For SCGN-expression studies, α1 subunit cDNA was prepared via Quantum Maxiprep (Bio-Rad) and resuspended in distilled deionized water. Plasmid DNA for all constructs was prepared with Quantum Maxiprep (Bio-Rad). For SCGN-expression experiments, cDNA concentrations of 3–6 mg/ml in sterile distilled deionized water were used, with shipment on dry ice and storage at −80°C until use.
cDNA Injection.
SCGNs cells from 7-day-postnatal rats were prepared as described (25, 33). cDNA constructs in distilled deionized water were dissolved in the suction pipette solution (150 mM potassium acetate/5 mM MgATP/10 mM Hepes, pH 7.35) (25, 33). The cDNAs were introduced into the nucleus of SCGNs by diffusion from a glass pipette (40- to 60-MΩ tip resistance) with hand pressure applied to a syringe connected to the injection pipette. The concentration of cDNAs in the injection pipette was 0.275 mg/ml. Dextran fluorescein (10 kDa, Molecular Probes) was introduced in the pipette solution to monitor cDNA entry into nuclei (34): 5% for α12.1 cDNA or 7.5% for other cDNAs. After incubation at 37°C/5% CO2 in minimal essential medium supplemented with 10% FCS/5% horse serum/1% penicillin-streptomycin/25 ng/ml nerve growth factor for 20–24 h, injected neurons were identified with an inverted microscope (Diaphot 300, Nikon) equipped with an epifluorescence unit and Arugas/HiSCA (Hamamatsu Photonics, Ichinocho, Japan).
Antibodies.
CNA4 is a glutathione S-transferase fusion protein containing amino acids 410–482 from the intracellular loop between domains I and II of Cav2.1, a sequence common to several Cav2.1 isoforms. Production and purification of CNA4 fusion protein and anti-CNA4 antibodies were performed as described (35). The anti-CNA5 antibody recognizes the intracellular loop between domains II and III of the α1 subunit of the rbA isoform of Cav2.1 channels (35). These antibodies and anti-CNB2 against Cav2.2, anti-CNC1 against Cav1.2, and anti-CNE1 antibodies against Cav2.3 have been characterized previously by immunoblotting and immunocytochemistry and shown to specifically recognize these Ca2+-channel isoforms (35–40). The antisynaptophysin antibody was purchased from Chemicon, and Cy5-labeled avidin D was obtained from Jackson ImmunoResearch.
Immunocytochemistry.
SCGNs were cultured and injected in Tokyo, fixed in acetone for 5 min or 4% paraformaldehyde for 30 min, rinsed three times in PBS for 5 min, frozen for shipment to Seattle on dry ice, and stored at −80°C. The SCGN cultures were allowed to warm to room temperature before processing. Both the SCGN and tsA-201 cell cultures were rinsed with 0.1 M phosphate buffer for 5 min, fixed in 4% paraformaldehyde for 20 min, rinsed in 0.1 M phosphate buffer for 5 min, rinsed in 0.1 M Tris buffer for 15 min, rinsed in Tris-buffered saline (TBS) for 15 min, and then blocked for 1 h by using TBS containing 5% nonfat milk and 5% normal goat serum. The cultures were incubated with anti-CNA4 (diluted 1:15), anti-CNA5 (diluted 1:15), anti-CNB2 (diluted 1:15), anti-CNC1 (diluted 1:15), or anti-CNE1 antibodies (diluted 1:15) overnight at room temperature. The antibodies were diluted in TBS containing 5% nonfat milk, 5% normal goat serum, and 0.03% Triton X-100. The cultures then were rinsed in TBS for 30 min, incubated in biotinylated goat anti-rabbit IgG (Vector Laboratories, diluted 1:300) for 2 h at room temperature, rinsed in TBS for 30 min, incubated in avidin D Texas red or avidin D fluorescein (Vector Laboratories, diluted 1:300) for 2 h at room temperature, rinsed, coverslipped by using Vectashield (Vector Laboratories), and viewed by using a Bio-Rad MRC 600 confocal microscope. For double-labeling experiments the procedures are the same as described above except the cells were incubated with anti-CNA5 and antisynaptophysin (diluted 1:200) simultaneously. After rinsing, the cells were incubated in biotinylated anti-mouse IgG and anti-rabbit IgG Texas red (diluted 1:100), rinsed, incubated in avidin D Cy5 (diluted 1:300) and anti-rabbit IgG Texas red, and then rinsed and coverslipped as described above. Images were analyzed by using the METAMORPH (Universal Imaging, Downingtown, PA) image-analysis program and processed in PHOTOSHOP (Adobe Systems, Mountain View, CA) and CANVAS 5.03 (Deneba, Miami). Control cultures included ones in which the primary antibody was omitted or replaced by normal rabbit serum. In both cases, no specific staining was observed.
Ca2+ Currents in SCGNs.
Whole-cell patch-clamp recordings from cell bodies of SCGNs, 10–14 days in culture, were obtained by using standard techniques as described (25). The currents were recorded with a Nihon Kohden CEZ whole-cell clamp amplifier and analyzed with the pCLAMP system (Axon Instruments, Foster City, CA) in the presence of 1.5 μM tetrodotoxin (Sigma) and 10 μM nifedipine (Sigma) to block Na+ currents and L-type Ca2+ currents, respectively. The external solution contained 136 mM tetraethylammonium chloride, 5.94 mM CsCl, 10 mM CaCl2, 1.2 mM MgCl2, 11 mM glucose, and 10 mM Hepes (pH 7.4). Patch pipettes were filled with a solution consisting of 130 mM cesium acetate, 5 mM MgATP, 10 mM Hepes, and 10 mM EGTA (pH 7.3). Pipette resistance was 1.5–2.0 MΩ. ω-Agatoxin IVA and ω-conotoxin GVIA were obtained from Peptide Institute (Osaka).
Synaptic Transmission Between SCGNs.
Conventional intracellular recordings were made from two neighboring neurons, cultured for 4–6 weeks, by using microelectrodes filled with 1 M potassium acetate (70–80 MΩ). Presynaptic neurons were selected by the fluorescence of dye in their nuclei. Excitatory postsynaptic potentials (EPSPs) were recorded from a neighboring neuron when action potentials were generated in the presynaptic neuron by passage of current through an intracellular recording electrode. EPSPs were recorded once every 20 s. Experiments were carried out at room temperature (25–27°C). Neurons were superfused with modified Kreb's solution consisting of 136 mM NaCl, 5.9 mM KCl, 8 mM CaCl2, 1.2 mM MgCl2, 11 mM glucose, and 3 mM Na-Hepes (pH 7.4). Superfusion was stopped after the application of Ca2+-channel blockers. Electrophysiological data were collected and analyzed by using software written by the late Ladislav Tauc (Centre National de la Recherche Scientifique, Gif sur Yvette, France). The peak amplitudes of EPSPs were measured and averaged. The resultant values were smoothed by an eight-point moving average algorithm and plotted against recording time with the normalized average EPSP amplitudes.
Results
Ca2+-Channel Pharmacology.
Our experiments take advantage of the specific pharmacology of voltage-gated Ca2+ channels (reviewed in refs. 1, 2, and 5). Cav1.2 channels mediate L-type Ca2+ currents that are specifically blocked by dihydropyridines such as nifedipine. Cav2.2 channels mediate N-type Ca2+ currents that are blocked with high affinity and specificity by ω-conotoxin GVIA. Cav2.1 channels mediate P/Q-type Ca2+ currents that are blocked by ω-agatoxin IVA. Cav2.3 channels mediate R-type Ca2+ currents that are preferentially blocked by the synthetic spider toxin SNX-482 or Ni2+, but SNX-482 does not block all R-type Ca2+ currents, and Ni2+ is not highly selective among channel types. In the experiments described below, we use primarily ω-conotoxin GVIA and ω-agatoxin IVA to define the contribution of endogenous Cav2.2 channels and exogenously expressed Cav2.1 channels to synaptic transmission between SCGNs.
Expression of Exogenous Ca2+ Channels in Cultured SCGNs.
Expression of endogenous Ca2+ channels in cultured SCGNs was assessed by immunocytochemistry with subunit-specific antibodies (24, 35, 38–40). Cav1.2, Cav2.2, and Cav2.3 channels were observed in the cell bodies of these neurons (Fig. 1 B–D), consistent with electrophysiological recordings of L-, N-, and R-type Ca2+ currents in these cells. Only Cav2.2 channels were observed in punctate clusters on cell bodies, as expected for the specific role of these channels in nerve terminals where they initiate transmitter release (Fig. 1B). In contrast, Cav2.1 channels, which mediate P/Q-type Ca2+ currents, were not observed in these immunocytochemical studies of injected neurons (Fig. 1A).
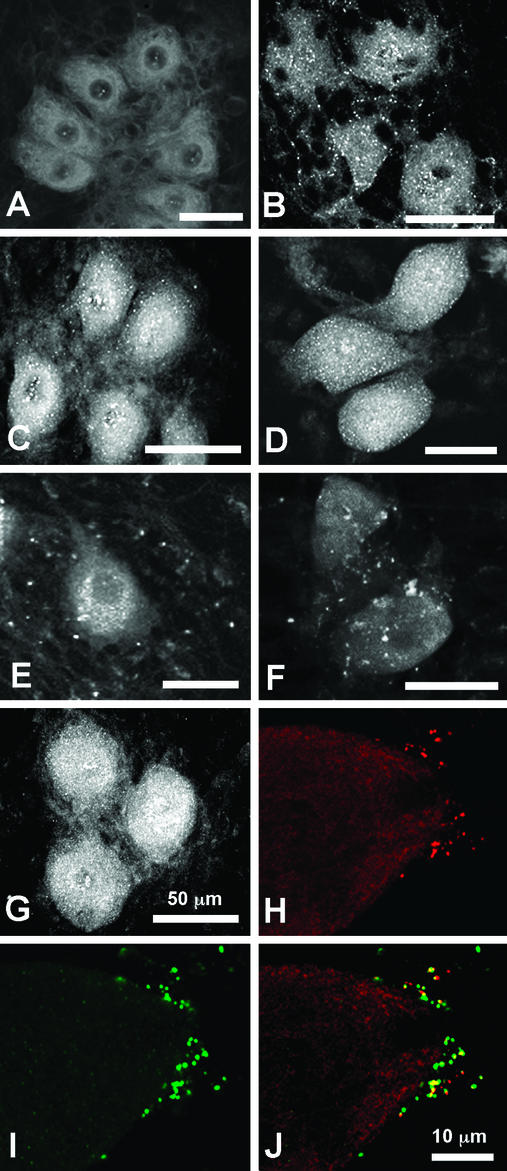
Figure 1.
Expression of Ca2+ channels in control and injected SCGNs. Nuclei of selected SCGNs were injected with cDNA encoding Ca2+-channel α1 subunits along with dextran fluorescein 20–24 h before fixation. Cultures of SCGNs were fixed and processed for immunocytochemistry as described in Experimental Procedures. (A) Control culture stained with anti-CNA5 against Cav2.1. (B) Control culture stained with anti-CNB1 against Cav2.2. (C) Control culture stained with anti-CNC1 against Cav1.2. (D) Control culture stained with anti-CNE1. (E) Culture with neurons injected with Cav2.1 cDNA and stained with anti-CNA5. (F) Culture with neurons injected with Cav2.3 cDNA stained with anti-CNE2. (G) Culture with neurons injected with Cav1.2 cDNA and stained with anti-CNC1. (H and I) A culture with neurons injected with Cav2.1 cDNA and double-labeled with anti-CNA5 (H, red) and anti-synaptophysin (I, green). (J) Merged image of H and I. Regions of overlap appear yellow and demonstrate that the majority of anti-CNA5-positive puncta are associated with synaptic sites.
To detect expression of exogenous Ca2+ channels in nerve terminals of cultured SCGNs, we examined the distribution of Cav1.2, Cav2.1, and Cav2.3 channels in neurons injected with cDNAs encoding these α1 subunits [rbA-I (31), rbE-II (14), and rbC-II, (7)]. The cDNAs were introduced, along with dextran fluorescein as a visual marker, into the nuclei of SCGNs. Twenty to 24 h later, the cultures were fixed with acetone or 4% paraformaldehyde for immunofluorescent staining with subunit-specific antibodies. Cell bodies of injected neurons express high amounts of all three α1 subunits (data not shown). In addition, the neighboring neurons to Cav2.1-injected SCGNs have many immunoreactive puncta representing immunostained nerve terminals with clusters of Cav2.1 channels (Fig. 1E, compare with Fig. 1A). Cav2.3-injected neurons have fewer nerve terminals that are immunostained (Fig. 1F), and Cav1.2-injected neurons do not have any terminals that are immunostained (Fig. 1G).
Cav2.1-immunostained puncta were shown to be nerve terminals by coimmunostaining with antibodies against the synaptic vesicle protein synaptophysin. Adjacent to the cell body shown in Fig. 1 H–J is an injected SCGN expressing Cav2.1, as indicated by labeling with fluorescein dextran and anti-CNA5 (data not shown). Double immunolabeling showed that Cav2.1-stained puncta on adjacent neurons (Fig. 1H) overlapped or were closely colocalized with synaptophysin-stained puncta (Fig. 1I). Merged images showed yellow puncta having similar stain intensity for the two proteins as well as red Cav2.1-stained puncta that were partially overlapping or closely colocalized with green synaptophysin-stained puncta (Fig. 1J). The results of Fig. 1 show that the relative ability of expressed α1 subunits to be transported to nerve terminals is Cav2.1 > Cav2.3 ≫ Cav1.2. Although the levels of staining by different antibodies cannot be compared quantitatively, it appeared that more nerve terminals were immunostained intensely by antibodies against endogenous Cav2.2 (Fig. 1B) than by antibodies against exogenously expressed Cav2.1 or Cav2.3 (Fig. 1 E and F). These immunofluorescent staining results show that exogenous α1 subunits can be expressed in SCGNs and transported to nerve terminals, but they may not be present at the same density as endogenously expressed Cav2.2.
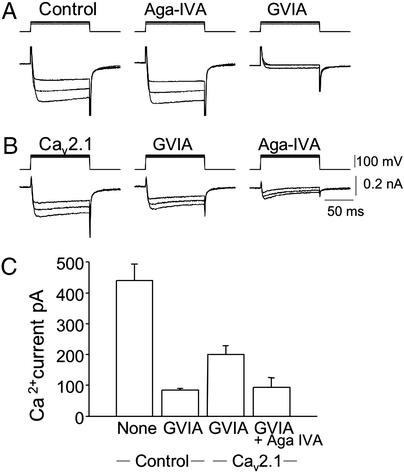
To confirm functional expression of exogenous P/Q-type Ca2+ channels in SCGNs, Ca2+ currents in neuronal cell bodies were measured by whole-cell patch-clamp recordings in the presence of 10 μM nifedipine, an L-type Ca2+-channel blocker. cDNA encoding Cav2.1 channels was injected into the nucleus 20–24 h before Ca2+-current measurement (Fig. 2). In noninjected neurons, the peak Ca2+ currents, 440 ± 53 pA (mean ± SEM, n = 6), were reduced to 85 ± 5.1 pA (n = 6) after bath application of ω-conotoxin GVIA at 5 μM, a saturating concentration (Fig. 2 A and C). In contrast, peak Ca2+ currents of 200 ± 29 pA (n = 7) were recorded in the α12.1-injected neurons in the presence of ω-conotoxin GVIA (Fig. 2 B and C). Thus, ω-conotoxin GVIA-insensitive currents in the α12.1-injected neurons were 2.35-fold larger than in noninjected neurons. Additional bath application of ω-agatoxin IVA, a P/Q-type Ca2+-channel blocker, at 250 nM reduced the ω-conotoxin GVIA-resistant currents in the α12.1-injected neurons to 93 ± 32 pA (n = 7). These results show that functional ω-agatoxin IVA-sensitive P/Q-type Ca2+ channels were expressed in the α12.1-injected neurons, presumably through association with endogenous β, γ, and α2δ subunits, which are known to be expressed in SCGNs.
Figure 2.
Ca2+ currents through P/Q-type Ca2+ channels expressed in cultured SCGNs. cDNA encoding the rat brain Cav2.1 channels was injected into the nuclei of SCGNs after 10–14 days in culture, and Ca2+ currents were measured by whole-cell voltage clamp in the presence of nifedipine (10 μM). (A) Ca2+ currents recorded from a control neuron (Left), 5 min after adding 250 nM agatoxin IVA (Aga-IVA, Center), and 3 min after adding 5 μM ω-conotoxin GVIA (Right). (B) Ca2+ currents recorded with an α12.1-injected neuron (Left), 10 min after adding 5 μM ω-conotoxin GVIA (Center), and 3 min after adding 250 nM agatoxin IVA (Right). (C) Averaged peak amplitudes of Ca2+ currents are presented for six experiments with control neurons and seven experiments with α12.1-injected neurons. Where indicated, 5 μM ω-conotoxin GVIA or 250 nM agatoxin IVA was added.
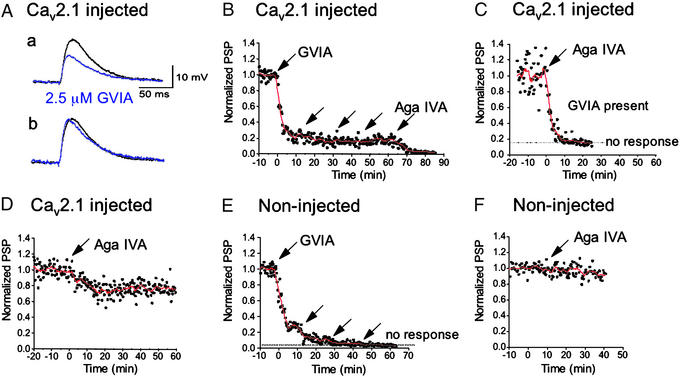
ω-Agatoxin IVA-Sensitive Synaptic Transmission Induced by Expression of Cav2.1 cDNA.
To assess the contribution of newly expressed P/Q-type Ca2+ currents at presynaptic terminals to release of the transmitter acetylcholine, SCGNs in culture for 4–6 weeks were injected with Cav2.1 cDNA, and synaptic transmission was examined 20–24 h later in the presence of N- or P/Q-type Ca2+-channel blockers (Fig. 3) by recording EPSPs evoked by presynaptic action potentials once every 20 s (25, 33, 41). ω-Conotoxin GVIA reduced amplitudes of EPSPs to 23 ± 7% (n = 5) at 10 min after bath application of 2.5 μM toxin to synapses where the presynaptic neurons were injected with α12.1 (Fig. 3 A and B). The rates of rise and decay of the EPSP were very similar, although the rise time was slightly shorter after ω-conotoxin GVIA (Fig. 3A). The addition of three more doses of 2.5 μM ω-conotoxin GVIA had little further effect, reducing EPSPs to 17 ± 4% (n = 5; Fig. 3B). The addition of 250 nM ω-agatoxin IVA inhibited the remaining synaptic transmission (Fig. 3B), demonstrating that the injected P/Q-type Ca2+ channels can mediate synaptic transmission. The EPSPs remaining in the presence of 5 μM ω-conotoxin GVIA were abolished completely by ω-agatoxin IVA at 250 μM (Fig. 3C). In the α12.1-injected neurons, EPSPs were reduced to 71 ± 6% (n = 5) at 15 min by treatment with 250 nM ω-agatoxin IVA without prior treatment with ω-conotoxin GVIA (Fig. 3D). Therefore, ≈30% of the synaptic transmission in injected neurons is initiated by expressed P/Q-type Ca2+ channels. In uninjected neurons, synaptic transmission was inhibited completely by ω-conotoxin GVIA but was unaffected by ω-agatoxin IVA (Fig. 3 E and F). Thus, the agatoxin IVA-sensitive synaptic transmission in injected neurons is mediated entirely by exogenously expressed P/Q-type Ca2+ channels.
Figure 3.
Effects of ω-conotoxin GVIA and ω-agatoxin IVA on synaptic transmission of cultured SCGNs expressing Cav2.1 channels. SCGNs were injected with cDNAs encoding Cav2.1, and injected neurons were selected as presynaptic neurons for measurement of synaptic transmission. Normalized average amplitudes of postsynaptic potentials (EPSPs, ●) and the smoothed values with moving average algorithm (line) were plotted against recording time. An arrow indicates a drop application of ω-conotoxin GVIA producing a concentration of 2.5 μM or of ω-agatoxin IVA (Aga IVA) producing a concentration of 250 nM in the bath. (A) EPSP recordings. (a) EPSPs from one representative experiment with Cav2.1-injected neuron recorded at 5 min before (black trace) and at 10 min after adding 2.5 μM ω-conotoxin GVIA (blue trace) are illustrated. (b) The peak amplitude of EPSP with ω-conotoxin GVIA (blue trace) was normalized to that of the control EPSP before adding ω-conotoxin GVIA (black trace). The time constant (τ) for rate of rise, 5.6 ± 1.0 ms (n = 3), was decreased by ω-conotoxin GVIA to 4.7 ± 0.7 ms. τ for rate of fall, 35.0 ± 11.4 ms (n = 3), was slightly decreased by ω-conotoxin GVIA, 26.5 ± 5.2 ms. (B) EPSPs were recorded from Cav2.1-injected synapses (n = 5). ω-Conotoxin GVIA was applied additively every 15 min. ω-Agatoxin IVA was added in the presence of 10 μM ω-conotoxin GVIA. (C) EPSP amplitudes were recorded from α12.1-expressing synapses in the presence of ω-conotoxin GVIA at 5 μM (n = 6). ω-Agatoxin IVA was applied at t = 0. (D) EPSPs were recorded from α12.1-expressing synapses (n = 5). ω-Agatoxin IVA was applied at t = 0. (E) As a control, ω-conotoxin GVIA was applied additively to control synapses (n = 5). (F) As a control, ω-agatoxin IVA was applied to control synapses at t = 0 (n = 5). PSP, postsynaptic potential.
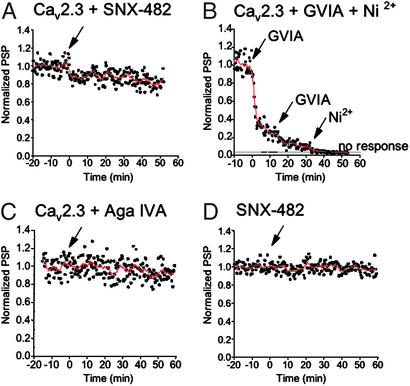
Synaptic Transmission in Neurons Expressing Exogenous Cav2.3 Channels.
R-type Ca2+ currents mediate synaptic transmission less efficiently than N- or P/Q-type Ca2+ currents in synapses where they are expressed together such as the calyx synapses of the medial nucleus of the trapezoid body (20, 24). To examine the ability of exogenously expressed R-type Ca2+ channels to initiate synaptic transmission at SCGN synapses, cDNA encoding rat brain Ca2+ channel α12.3 subunits was introduced into presynaptic neurons, and synaptic transmission was studied 20–24 h later. SNX-482 or Ni2+ was used to block R-type Ca2+ currents (14, 42). EPSP amplitude was reduced to 90 ± 5% (n = 6) at 10 min after bath application of 100 nM SNX-482 in Cav2.3-expressing synapses (Fig. 4A), but the toxin had no effect on nonexpressing synapses (97 ± 5% at 10 min; Fig. 4D). After block of endogenous N-type Ca2+ currents by ω-conotoxin GVIA, ≈10% of the EPSP amplitude remained and could be inhibited by 150 μM Ni2+ (Fig. 4B). In contrast, ω-agatoxin IVA had no effect on neurons injected with Cav2.3 (Fig. 4C). These results indicate that the effect of ω-agatoxin IVA is specific for synaptic transmission at Cav2.1-injected synapses and has no effect on the Cav2.3-injected synapses. Expression of exogenous Cav2.3 channels is less efficient in supporting synaptic transmission than expression of Cav2.1 channels.
Figure 4.
Effects of SNX-482, Ni2+, ω-conotoxin GVIA, and ω-agatoxin IVA (Aga IVA) on synaptic transmission of cultured SCGNs expressing Cav2.3 channels. Synaptic transmission was recorded from pairs of SCGNs as described in Experimental Procedures. An arrow indicates drop application of SNX-482 producing a concentration of 100 nM, that of Ni2+ producing a concentration of 150 μM, that of ω-conotoxin GVIA producing a concentration of 2.5 μM, or that of ω-agatoxin IVA producing a concentration of 250 nM. (A) EPSPs were recorded from α12.3-expressing synapses (n = 6). SNX-482 was applied at t = 0. (B) EPSPs were recorded from α12.3-expressing synapses (n = 7). ω-Conotoxin GVIA and Ni2+ were applied additively. (C) EPSPs were recorded from α12.3-expressing synapses (n = 5). ω-Agatoxin GVIA was applied at t = 0. (D) As a control, EPSPs were recorded from noninjected synapses (n = 6). SNX-482 was added at t = 0. PSP, postsynaptic potential.
Synaptic Transmission in Neurons Expressing Exogenous Cav1.2 Channels.
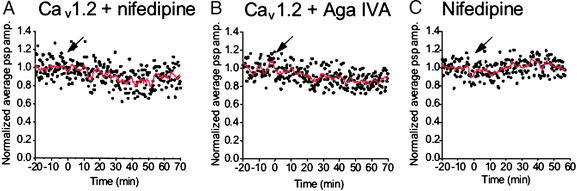
To test the contribution of L-type Ca2+ channels to acetylcholine release at the SCGN synapses, Cav1.2 subunits were expressed in presynaptic neurons. As expected from the immunocytochemical results (Fig. 1), bath application of nifedipine at 2.5 μM did not affect synaptic transmission in the injected synapses (97 ± 7%, n = 4, at 15 min; Fig. 5A) or in control synapses (96 ± 8%, n = 6, at 15 min; Fig. 5C). As a control for toxin specificity, we confirmed that ω-agatoxin IVA (250 nM) produced no effect on synaptic transmission at the Cav1.2-injected synapses (96 ± 6%, n = 6, at 15 min; Fig. 5B). These results provide further evidence that the inhibitory effect of ω-agatoxin IVA on synaptic transmission is specific for the Cav2.1-injected synapses but not for the synapses expressing the other α1 subunits.
Figure 5.
Effects of nifedipine and ω-agatoxin IVA (Aga IVA) on synaptic transmission of cultured SCGNs expressing Cav1.2 channels. Synaptic transmission was recorded from pairs of SCGNs as described in Experimental Procedures. An arrow indicates drop application of nifedipine producing a concentration of 2.5 μM or that of ω-agatoxin IVA producing a concentration of 250 nM. (A) EPSPs were recorded from α11.2-expressing synapses (n = 4). Nifedipine was applied at t = 0. (B) EPSPs were recorded from α11.2-expressing synapses (n = 6). ω-Agatoxin IVA was applied at t = 0. (C) As a control, EPSPs were recorded from control synapses (n = 6). Nifedipine was applied at t = 0. psp, postsynaptic potential; amp., amplitude.
Discussion
SCGNs as Expression Hosts.
SCGNs have been reported as mammalian expression hosts for G proteins and receptors for neurotransmitters (34, 43, 44). Cytoplasmic injection of cRNA (34, 44) or nuclear injection of cDNA (43) results in expression of the gene products within 14–24 h in adult rat SCGNs. In this study, we have demonstrated that pore-forming α1 subunits of Ca2+ channels form functional channels in cultured SCGNs. The exogenous α1 subunits probably associate with endogenous β, γ, and α2δ subunits in presynaptic SCGNs to form functional Ca2+ channels and interact with endogenous SNARE proteins via the synaptic protein interaction (synprint) site to initiate synchronous neurotransmitter release (41). These results demonstrate that it is possible to study functions of Ca2+ channels in nerve terminals by expressing cDNA or cRNA in cultured SCGNs and monitoring synaptic transmission.
Consistent with previous studies of freshly dissociated SCGNs (29, 30), no ω-agatoxin-sensitive P/Q-type Ca2+ current was recorded in cultured SCGNs. In Cav2.1-injected neurons, Ca2+ currents in the presence of the N-type Ca2+-channel blocker ω-conotoxin GVIA and the L-type Ca2+-channel blocker nifedipine were increased ≈2-fold, and the increased Ca2+ current was inhibited by ω-agatoxin IVA, demonstrating that it is mediated by exogenous Cav2.1 channels. In freshly dissociated SCGNs, high-voltage-activated R-type Ca2+ currents resistant to nifedipine, ω-conotoxin GVIA, and ω-agatoxin IVA have been reported (45). We recorded R-type Ca2+ currents with similar pharmacological properties in control neurons, whereas the larger R-type Ca2+ currents in injected SCGNs demonstrated functional expression of Cav2.3 channels.
Exogenously Expressed Cav2.1 and Cav2.3 Channels Can Initiate Synaptic Transmission.
Our experiments show that exogenous α1 subunits expressed in SCGNs in culture by introduction of cDNAs encoding Cav2.1 or Cav2.3 channels into their nuclei are also able to mediate acetylcholine release and synaptic transmission. Synaptic transmission between control SCGNs in culture was blocked completely by ω-conotoxin GVIA at 7.5 μM but was not affected by ω-agatoxin IVA at 250 nM, confirming that transmitter release is mediated specifically by N-type Ca2+ channels. At synapses where cDNA encoding Cav2.1 was introduced into presynaptic neurons, synaptic transmission was sensitive to ω-agatoxin IVA at 250 nM, indicating that P/Q-type Ca2+ channels are also able to mediate acetylcholine release from sympathetic neurons. Approximately 30% of neurotransmitter release was inhibited by ω-agatoxin IVA at 250 nM in these injected neurons. Exogenous R-type Cav2.3 channels but not exogenous L-type Cav1.2 channels, are also able to mediate cholinergic synaptic transmission in these cells. Exogenous R-type Ca2+ channels are less effective than P/Q-type Ca2+ channels in mediating synaptic transmission, as found at the calyx of Held (24).
Reconstitution of Neurotransmission Correlates with Nerve-Terminal Localization.
The specific role of Cav2 channels in fast neurotransmission might be due to their specific trafficking to nerve terminals, specific functional properties and protein–protein interactions within nerve terminals, or both. The results presented here show a close correlation between the ability of different Cav channel subtypes to be localized to nerve terminals and their effectiveness in reconstitution of neurotransmission. Thus, in this cell system, differential trafficking of Cav2 channels to nerve terminals is a key determinant of their effectiveness in synaptic transmission.
Our results indicate that the exogenous P/Q- and R-type Ca2+ channels expressed in SCGNs function in concert with native N-type Ca2+ channels in acetylcholine release, because synaptic transmission was blocked additively by ω-conotoxin GVIA, ω-agatoxin IVA, and Ni+. Evidently, molecular features of Cav2 but not Cav1.2 channels are essential for transport and concentration at nerve terminals of SCGNs. Cholinergic synapses formed by injected SCGNs in culture are an effective system to study the molecular basis for the specific function of Ca2+-channel subtypes in synaptic transmission.
Acknowledgments
We thank Elizabeth M. Sharp for support in the molecular biological experiments. This work was supported by grants from the Human Frontier Science Program, the Japanese Ministry of Education, Science, Sports, and Culture, and the Japan Society for the Promotion of Science (to S.M.), National Institutes of Health Grant NS22625 (to W.A.C., C.T.Y., and R.E.W.), and the Muscular Dystrophy Association (to R.E.W.).
Abbreviations
- SCGN
superior cervical ganglion neuron
- EPSP
excitatory postsynaptic potential
References
- 1.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 2.Tsien R W, Lipscombe D, Madison D, Bley K, Fox A. Trends Neurosci. 1995;18:52–54. [PubMed] [Google Scholar]
- 3.Takahashi M, Seagar M J, Jones J F, Reber B F, Catterall W A. Proc Natl Acad Sci USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann F, Lacinova L, Klugbauer N. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 5.Catterall W A. Cell Calcium. 1998;24:307–323. doi: 10.1016/s0143-4160(98)90055-0. [DOI] [PubMed] [Google Scholar]
- 6.Ertel E A, Campbell K P, Harpold M M, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch T P, Tanabe T, Birnbaumer L, et al. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 7.Snutch T P, Tomlinson W J, Leonard J P, Gilbert M M. Neuron. 1991;7:45–47. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 8.Williams M E, Feldman D H, McCue A F, Brenner R, Velicelebi G, Ellis S B, Harpold M M. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 9.Murphy T H, Worley P F, Baraban J M. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 10.Mermelstein P G, Bito H, Deisseroth K, Tsien R W. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bading H, Ginty D D, Greenberg M E. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 12.Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, et al. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 13.Dubel S J, Starr T V B, Hell J, Ahlijanian M K, Enyeart J J, Catterall W A, Snutch T P. Proc Natl Acad Sci USA. 1992;89:5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 15.Williams M E, Brust P F, Feldman D H, Patthi S, Simerson S, Maroufi A, McCue A F, Velicelebi G, Ellis S B, et al. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 16.Hirning L D, Fox A P, McCleskey E W, Olivera B M, Thayer S A, Miller R J, Tsien R W. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 17.Sather W A, Tanabe T, Zhang J-F, Mori Y, Adams M E, Tsien R W. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Momiyama A. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- 19.Turner T J, Adams M E, Dunlap K. Science. 1992;258:310–313. doi: 10.1126/science.1357749. [DOI] [PubMed] [Google Scholar]
- 20.Wu L G, Borst J G, Sakmann B. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Reyes E, Cribbs L L, Daud A, Lacerda A E, Barclay J, Williamson M P, Fox M, Rees M, Lee J H. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- 22.Lee J-H, Daud A N, Cribbs L L, Lacerda A E, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. J Neurosci. 1999;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huguenard J R. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- 24.Wu L-G, Westenbroek R E, Borst J G G, Catterall W A, Sakmann B. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochida S, Saisu H, Kobayashi H, Abe T. Neuroscience. 1995;65:905–915. doi: 10.1016/0306-4522(94)00508-3. [DOI] [PubMed] [Google Scholar]
- 26.Koh D S, Hille B. Proc Natl Acad Sci USA. 1997;94:1506–1511. doi: 10.1073/pnas.94.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plummer M R, Logothetis D E, Hess P. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 28.Regan L J, Sah D W Y, Bean B P. Neuron. 1991;6:269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- 29.Mintz I M, Adams M E, Bean B P. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Ikeda S R. J Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]
- 31.Stea A, Tomlinson W J, Soong T W, Bourinet E, Dubel S J, Vincent S R, Snutch T P. Proc Natl Acad Sci USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hockerman G H, Johnson B D, Scheuer T, Catterall W A. J Biol Chem. 1995;270:22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- 33.Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. Neuron. 1994;13:1131–1142. doi: 10.1016/0896-6273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda S R, Lovinger D M, McCool B A, Lewis D L. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai T, Westenbroek R E, Rettig J W, Hell J W, Catterall W A. J Cell Biol. 1996;134:511–528. doi: 10.1083/jcb.134.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westenbroek R E, Ahlijanian M K, Catterall W A. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- 37.Westenbroek R E, Hell J W, Warner C, Dubel S J, Snutch T P, Catterall W A. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 38.Hell J W, Westenbroek R E, Warner C, Ahlijanian M K, Prystay W, Gilbert M M, Snutch T P, Catterall W A. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westenbroek R E, Sakurai T, Elliott E M, Hell J W, Starr T V B, Snutch T P, Catterall W A. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokoyama C T, Westenbroek R E, Hell J W, Soong T W, Snutch T P, Catterall W A. J Neurosci. 1995;15:6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mochida S, Sheng Z-H, Baker C, Kobayashi H, Catterall W A. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 42.Newcomb R, Szoke B, Palma A, Wang G, Chen X H, Hopkins W, Cong R, Miller J, Urge L, et al. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 43.Ikeda S R. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 44.Filippov A K, Webb T E, Barnard E A, Brown D A. J Neurosci. 1998;18:5170–5179. doi: 10.1523/JNEUROSCI.18-14-05170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boland L M, Morrill J A, Bean B P. J Neurosci. 1994;14:5011–5027. doi: 10.1523/JNEUROSCI.14-08-05011.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]