Abstract
Estrogens are neuroprotective against glutamate excitotoxicity caused by an excessive rise in intracellular calcium ([Ca2+]i). In this study, we demonstrate that 17β-estradiol (E2) treatment of hippocampal neurons attenuated the excitotoxic glutamate-induced rise in bulk-free [Ca2+]i despite potentiating the influx of Ca2+ induced by glutamate. E2-induced attenuation of bulk-free [Ca2+]i depends on mitochondrial sequestration of Ca2+, which is blocked in the presence of the combination of rotenone and oligomycin or in the presence of antimycin, which collapse the mitochondrial membrane potential, thereby preventing mitochondrial Ca2+ transport. Release of mitochondrial Ca2+ by carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) after excitotoxic glutamate treatment resulted in a greater [Ca2+]i in E2-treated cells, indicating an E2-induced increase in the mitochondrial calcium ([Ca2+]m) load. The increased [Ca2+]m load was accompanied by increased expression of Bcl-2, which can promote mitochondrial Ca2+ load tolerance. These findings provide a mechanism of E2-induced neuronal survival by attenuation of excitotoxic glutamate [Ca2+]i rise via increased mitochondrial sequestration of cytosolic Ca2+ coupled with an increase in Bcl-2 expression to sustain mitochondrial Ca2+ load tolerance and function.
Neurologic benefits of estrogen replacement therapy in humans include reversal of estrogen deficiency-induced memory dysfunction and reduced risk of Alzheimer's disease (1, 2). Data derived from in vitro analyses indicate that 17β-estradiol (E2) enhances neuronal survival after oxidative stress, excitotoxic insults, and β-amyloid exposure (1–5). Much of the cellular damage caused by these insults can be attributed to dysregulation of calcium (Ca2+) homeostasis (6). Ca2+-induced neurotoxicity is complex and is exemplified by glutamate-induced neurotoxicity, which correlates with the Ca2+ load measured by 45Ca2+ uptake but not with free intracellular Ca2+ ([Ca2+]i) measured by the fluorescent Ca2+ indicator Fura2 (7). These data suggest a role of subcellular Ca2+ sequestration in glutamate neurotoxicity. An organelle critical for Ca2+ buffering in neurons is mitochondria, which can rapidly accumulate Ca2+ (8). Mitochondrial Ca2+ uptake occurs above a threshold of cytosolic Ca2+, and is only slowly released, leading to a net accumulation of mitochondrial Ca2+ ([Ca2+]m) and an alteration of physiological [Ca2+]i transients (9, 10). The large capacity of mitochondria for Ca2+ could provide a neuroprotective mechanism by reducing cytoplasmic free Ca2+ (10). However, excessive [Ca2+]m can lead to detrimental effects, including enhanced free radical production, mitochondrial membrane depolarization, and cell death (11, 12).
Hippocampal neurons pretreated with estrogens and then exposed to excitotoxic glutamate respond with an attenuated rise in [Ca2+]i and increased survival relative to untreated neurons (1, 13, 14). We sought to determine the mechanism by which estrogen can promote intracellular Ca2+ homeostasis and survival in the presence of toxic insults that lead to neuronal death via dysregulation of Ca2+ homeostasis. Because of the Ca2+ buffering capacity of mitochondria, we investigated the role of mitochondria in E2-induced regulation of [Ca2+]i. Because Bcl-2 plays a key role in mitochondrial Ca2+ regulation (15, 16), the effect of E2 on Bcl-2 expression was also investigated. Results of these analyses support the hypothesis that E2-induced neuroprotection is mediated by attenuation of glutamate-induced [Ca2+]i rise via increased mitochondrial sequestration of Ca2+ coupled with increased Bcl-2 expression to promote mitochondrial tolerance of an increased [Ca2+]m load.
Materials and Methods
Chemicals.
Culture materials were from GIBCO/BRL. Chemicals were from Sigma unless otherwise noted. E2 was dissolved in ethanol and diluted in culture medium with final ethanol concentration <0.001%. Fura2-AM and Fura4F-AM were from Molecular Probes. 45Ca2+ was from NEN.
Neuronal Culture.
Primary hippocampal neurons from embryonic day 18 (E18) rat fetuses were cultured as described and generated cultures 98% neuronal in phenotype (13). Briefly, hippocampi were dissected from brains of embryonic rats and dissociated by repeated passage through a series of fire-polished constricted Pasteur pipettes. Cells were plated on poly-d-lysine-coated coverslips (22-mm round or 4-mm2 square gridded) or polyethylenimine-coated six-well plates. Neurons were grown in Neurobasal medium supplemented with 5 units/ml penicillin, 5 mg/ml streptomycin, and B27 supplement. Cultures were maintained at 37°C in humidified 5% CO2 atmosphere for 10–12 days before experimentation.
Measurement of Cytoplasmic Ca2+ by Using Fura2-AM and Fura4F-AM.
Hippocampal neurons were treated with E2 (10 ng/ml) or vehicle control for 48 h before loading in the dark with Fura2-AM (2 μM) or Fura4F-AM (2 μM) in Hanks' balanced salt solution (45 min; 37°C). For the Fura2 studies, [Ca2+]i was determined by comparing the 340/380 ratio to a standard curve as described (13). For the Fura4F studies, the results are reported as the 340/380 ratios. Data are presented as representative traces averaged from at least 10 cells per coverslip. Responses to steroids were quantified as the difference between the average [Ca2+]i (or Fura4F ratio) for 1 min during glutamate exposure [Figs. 1 and 4 A and B, 30–90 sec after glutamate exposure; Figs. 3 and 4C were 120–180 sec after glutamate and carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) exposure, respectively] and the average [Ca2+]i (or Fura4F ratio) for 1 min before exposure. Changes in [Ca2+]i (or Fura4F ratio) are presented as mean ± SEM from 4 independent experiments with at least 30 cells per experiment. For mitochondrial inhibition, Fura4- loaded neurons were incubated in rotenone (2.5 μM) plus oligomycin (5 μg/ml) or antimycin (2.5 μM) for 5 min before imaging. The Ca2+ response to glutamate (200 μM) was determined in the continued presence of mitochondrial inhibitors. Data are presented as representative traces averaged from at least 10 cells per coverslip. Responses to steroids were quantified as the difference between the average Fura4F ratio for 1 min during glutamate exposure and the average Fura4F ratio for 1 min before exposure. Changes in Fura4F ratio are presented as mean ± SEM from four independent experiments with at least 30 cells per experiment. For studies of FCCP-induced release of mitochondrial Ca2+, cells were exposed to glutamate (200 μM) after obtaining baseline [Ca2+]i. After reaching steady-state [Ca2+]i levels, oligomycin (5 μg/ml) was perfused, and 3 min later the cells were exposed to the protonophore FCCP (2.5 μM) and assessed for change in Fura4F ratio.
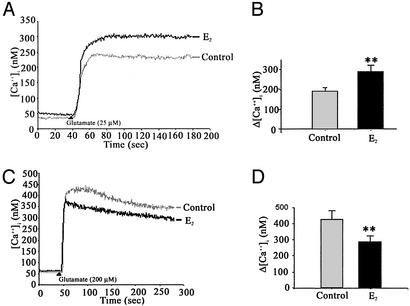
Figure 1.
Dual action of estrogen on the glutamate-induced rise in [Ca2+]i. (A and B) Hippocampal neurons pretreated with E2 (10 ng/ml) for 48 h exhibited a significantly greater response to glutamate (25 μM) than control neurons. (C and D) Hippocampal neurons pretreated with E2 (10 ng/ml) for 48 h exhibited a significantly lower rise in [Ca2+]i in response to glutamate (200 μM) than control neurons. (A and C) Representative tracings of [Ca2+] over time in response to glutamate. (B and D) Quantitative changes in [Ca2+]i in response to glutamate (**, P < 0.01; n = 4 independent experiments with at least 30 neurons per experiment).
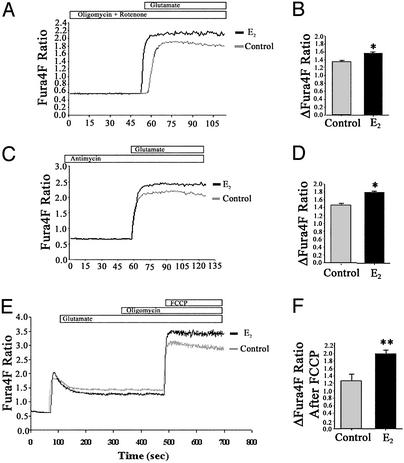
Figure 4.
Estrogen-induced attenuation of the [Ca2+]i rise depends on mitochondrial Ca2+ sequestration and results in increased mitochondrial Ca2+ sequestration. (A and B) Hippocampal neurons were pretreated with E2 (10 ng/ml) or vehicle control for 48 h before imaging [Ca2+]i with Fura4F. Neurons were treated with mitochondrial inhibitors [rotenone (2.5 mM) and oligomycin (5 μg/ml) or antimycin (2.5 μM)] for 5 min before imaging to block mitochondrial [Ca2+]i sequestration. Mitochondrial inhibitors were continuously present during the perfusions. E2-treated neurons exhibited a greater response to glutamate (200 μM) than control neurons in the presence of mitochondrial inhibitors. (A and C) Representative tracings of [Ca2+] over time in response to glutamate. (B and D) Quantitative changes in [Ca2+]i in response to glutamate (*, P < 0.05; n = 3 experiments with at least 30 neurons per experiment). (E and F) Hippocampal neurons treated with E2 (10 ng/ml) for 48 h exhibited a lower rise in [Ca2+]i in response to glutamate (200 μM) than control neurons but exhibited an increased rise in [Ca2+]i after a subsequent exposure to FCCP (1 mM). (E) Representative tracings of [Ca2+] over time in response to glutamate, oligomycin (5 μg/ml), and FCCP. (F) Quantitative changes in [Ca2+]i in response to FCCP defined as the difference between levels after FCCP exposure and levels after glutamate and oligomycin exposure (*, P < 0.05; **, P < 0.01; n = 3 experiments with at least 30 neurons per experiment).
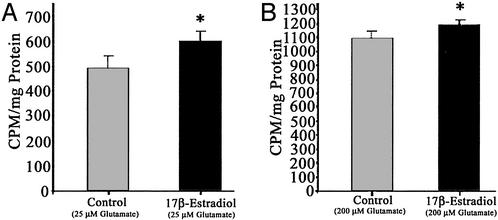
Figure 3.
Estrogen potentiates Ca2+ influx induced by low (25 μM) and high (200 μM) glutamate exposure. Hippocampal neurons pretreated with E2 (10 ng/ml) for 48 h exhibited a significantly greater 45Ca2+ uptake in response to glutamate (25 and 200 μM) than control neurons. (A) Hippocampal neuron cultures pretreated with E2 (10 ng/ml) 48 h before a 10-min exposure to 25 μM glutamate in the presence of 45Ca2+ (*, P < 0.05; n = 3 experiments with six wells per experiment). (B) Primary hippocampal neuron cultures pretreated with E2 (10 ng/ml) 48 h before a 10-min exposure to 200 μM glutamate in the presence of 45Ca2+ (*, P < 0.05; n = 3 experiments with six wells per condition per experiment).
To determine that equal loading of dye occurred in the absence or presence of E2, the procedure of Dineley et al. (16) was used. Neurons loaded with Fura2-AM or Fura4F-AM as above were lysed in low ionic strength buffer (120 mM KCl/10 mM Hepes/0.2 mM EGTA, pH 7.2), centrifuged (10 min at 10,000 × g), and dye concentration was determined in the supernatant under Ca2+-saturated (1 mM Ca2+) and Ca2+-free (1 mM EGTA) conditions. Control and E2-treated neurons loaded equivalent amounts of both Fura2 and -4F as there was no difference in the 340 or 380 fluorescence of the supernatants from control and E2-treated neurons. The respective 340 and 380 Fura2 fluorescence values for control neurons were Ca2+-saturated: 1715.8 ± 168.3, 594.6 ± 40.3; 0 Ca2+: 872.4 ± 22.4, 650.1 ± 33.9. E2-treated neuron values were Ca2+-saturated: 1709.2 ± 151.2, 572.4 ± 40.6; 0 Ca2+: 878.3 ± 18.6, 657.4 ± 36.4. The respective 340 and 380 Fura4F fluorescence values for control neurons were Ca2+-saturated: 1300 ± 103.3, 453.4 ± 30.8; 0 Ca2+: 624.7 ± 19.7, 816.5 ± 8.8. E2-treated neuron values were Ca2+-saturated: 1229.0 ± 86.0, 425.9 ± 25.7; 0 Ca2+: 615.3 ± 21.5, 810.9 ± 12.4. ANOVA indicated no significant difference (P > 0.05) between comparison groups.
Measurement of 45Ca2+ Uptake.
Neurons incubated in Krebs buffer for 10 min at 37°C were exposed to glutamate (25 or 200 μM) plus 1.0 μCi 45Ca2+ per 2 ml (specific activity of 45Ca2+ = 30.7 mC/mg) for 10 min. The cultures were washed twice with Krebs buffer, and 45Ca2+ uptake was terminated by addition of trichloroacetic acid (7%) for 45 min at 4°C. Extracts were counted by liquid scintillation, and NaOH was added to the cultures to solubilize proteins for analysis by the bicinchoninic acid assay kit. Ca2+ uptake is reported as cpm per mg of protein.
Glutamate Toxicity.
Cultures were exposed to E2 (10 ng/ml) or vehicle control 48 h before glutamate exposure for 5 min at 37°C in buffer containing 2 mM KCl, 1 mM MgSO4, 2.5 mM CaCl2, 1 mM NaHPO4, 4.2 mM NaHCO3, 12.5 mM Hepes, 10 mM glucose, 0.1 M NaCl, and 200 μM l-glutamic acid. Cultures were washed and returned to fresh Neurobasal medium. Neuronal cell counts were performed before and 24 h after exposure to excitotoxic glutamate by using gridded coverslips as described (14). One hundred neurons per coverslip were selected for study, and there were three coverslips per condition per experiment for a total of 300 neurons per condition per experiment. Data are presented as mean ± SEM for at least three independent experiments. Neuronal injury was assessed 24 h after excitotoxic glutamate exposure by quantitative measurement of lactate dehydrogenase (LDH) release with a Cytotoxicity Detection kit (Roche Molecular Biochemicals). Data were normalized against the amount of LDH activity released from control cultures receiving glutamate (100%) and were corrected for baseline LDH release from control cells exposed only to buffer. Data are presented as mean ± SEM for at least three independent experiments.
Bcl-2 Expression.
E2 (10 ng/ml) was added to cultures for 48 h, and whole-cell lysates were obtained with lysis buffer (0.005% SDS/0.1% Igepal/0.2 mM sodium orthovanadate/0.2 mM PMSF in PBS). Protein concentration was determined by bicinchoninic acid protein assay (Sigma). Protein was separated on 10% SDS/PAGE, electrotransferred to poly(vinylidene difluoride) membrane (Millipore), and probed with anti-Bcl-2 antibody (1:250; Zymed) followed by horseradish peroxidase-conjugated horse anti-mouse IgG (1:10,000; Vector Laboratories). Bands were visualized by 3,3′,5,5′-Tetramethylbenzidine Peroxidase Substrate kit (Vector Laboratories). Relative levels of Bcl-2 were quantitated by optical density analysis with un-scan-it software (Silk Scientific, Orem, UT). To avoid interassay variations, values were normalized to the control value in each experiment. Data are presented as the mean ± SEM for at least three independent experiments.
Statistics.
Statistically significant differences between groups were determined by an ANOVA followed by a Newman–Keuls post hoc analysis.
Results
17β-Estradiol Potentiates the Glutamate-Induced Ca2+ Influx but Attenuates the Rise in Bulk Cytosolic Ca2+ Induced by Excitotoxic Glutamate.
An issue that remains unresolved is the mechanism by which E2 can exert dual and opposing actions on glutamate-induced [Ca2+]i rise. Prior studies demonstrated that E2 potentiated the neuronal response to glutamate (17, 18), and we recently reported a differential effect of estrogens on the rise in [Ca2+]i that depended on glutamate concentration (13). Accordingly, our first aim was to compare the rise in [Ca2+]i, as measured by Fura2, with the influx of 45Ca2+, under nontoxic and excitotoxic glutamate exposure.
In both control and E2-treated neurons, glutamate induced a rapid increase in [Ca2+]i (Fig. 1). The mean change in [Ca2+]i obtained on stimulation with 25 μM glutamate alone was 131.8 ± 16.5 nM (n = 4; Fig. 1B). Hippocampal neurons pretreated with E2 exhibited a marked (nearly 70%) and significant increase in [Ca2+]i generated by 25 μM glutamate (Fig. 1A) with a mean change in [Ca2+]i of 223.0 ± 69.8 nM (P < 0.05; n = 4 experiments; Fig. 1B).
In the presence of an excitotoxic concentration of glutamate (200 μM), a rapid increase in [Ca2+]i that was larger than that induced by 25 μM glutamate occurred (Fig. 1 C and D). Paradoxically, in contrast to the potentiation of the 25-μM glutamate response, in the presence of 200 μM glutamate E2 significantly attenuated the [Ca2+]i rise induced by excitotoxic glutamate (Fig. 1C). The mean change in [Ca2+]i obtained on stimulation with 200 μM glutamate was 427.9 ± 49.6 nM (n = 4; Fig. 1D). In hippocampal neurons pretreated with E2, the mean change in [Ca2+]i stimulated by 200 μM glutamate was 288.4 ± 31.5 nM, representing an ≈33% decrease in Δ[Ca2+]i (P < 0.05; n = 4; Fig. 1D).
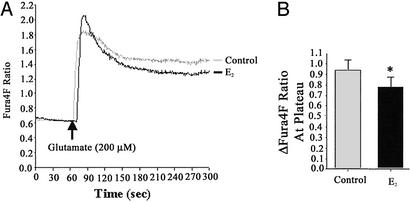
Although the high affinity of Fura2 for Ca2+ provides an excellent strategy for determining changes in Ca2+ transients, the same high affinity is a hindrance when determining maximal elevations of Ca2+ under conditions of exposure to excitotoxic concentrations of glutamate (19, 20). To address this potential problem, we used Fura4F (Kd ≈770 nM), a Ca2+ indicator with an affinity ≈3.4-fold lower than that of Fura2 (Kd ≈224 nM), to confirm that the attenuation of the excitotoxic glutamate-induced Ca2+ rise was not due to a saturation of the high-affinity Fura2. In both control and E2-treated neurons, glutamate induced a rapid increase in the Fura4F fluorescence ratio (Fig. 3). Consistent with the results obtained with Fura2, steady-state [Ca2+]i was significantly lower in E2-treated neurons compared with control neurons. The mean change from baseline to steady-state Fura4F fluorescence ratio obtained on stimulation with 200 μM glutamate was 0.930 ± 0.12 (n = 3; Fig. 3). In hippocampal neurons pretreated with E2, the mean change in the Fura4F fluorescence ratio stimulated by 200 μM glutamate was 0.781 ± 0.18, representing an ≈17% decrease (P < 0.05; n = 3; Fig. 3). Use of the low-affinity dye revealed an initial peak in [Ca2+]i that was followed by a rapid decline to steady-state levels. This initial peak was larger in E2-treated neurons than in control neurons (mean change in Fura4F fluorescence ratios of 1.375 ± 0.09 and 1.192 ± 0.06, respectively).
To determine whether the site of E2 action was at the point of Ca2+ influx, we investigated whether E2 regulated glutamate-induced uptake of Ca2+. Because Fura2 and -4F measures bulk cytoplasmic-free Ca2+ and not total Ca2+ influx, we used 45Ca2+ uptake as a measure of total cellular accumulation of Ca2+. Hippocampal neurons were exposed to E2 (10 ng/ml) or vehicle control for 48 h before monitoring 45Ca2+ uptake in response to 10 min of exposure to glutamate (25 or 200 μM). The accumulation of Ca2+ obtained on stimulation with 25 μM glutamate was 501.3 ± 22.3 cpm/mg of protein (n = 3; Fig. 2A). In hippocampal neurons pretreated with E2, Ca2+ accumulation in response to 25 μM glutamate was significantly enhanced (602.4 ± 16.7 cpm/mg of protein; P < 0.05; n = 3; Fig. 2A). The accumulation of Ca2+ obtained on stimulation with 200 μM glutamate was 1,092.6 ± 31.2 cpm/mg of protein (n = 4; Fig. 2B). Hippocampal neurons pretreated with E2 also showed a significant potentiation of Ca2+ accumulation in response to 200 μM glutamate (1,156.1 ± 24.6 cpm/mg of protein; P < 0.05; n = 3; Fig. 2B).
Figure 2.
Estrogen potentiates the initial rise in Ca2+ but attenuates the steady-state Ca2+ levels induced by high glutamate (200 μM). Hippocampal neurons pretreated with E2 (10 ng/ml) for 48 h exhibited a greater initial response to glutamate (200 μM) followed by a significantly lower steady-state [Ca2+]i compared with control neurons as determined by Fura4F fluorescence. (A) Representative tracings of [Ca2+] over time in response to glutamate. (B) Quantitative changes in [Ca2+]i in response to glutamate (*, P < 0.05; n = 3 independent experiments with at least 30 neurons per experiment).
Estrogen-Induced Attenuation Is Mediated by Mitochondrial Sequestration of Ca2+.
Because [Ca2+]i measured in response to 200 μM glutamate rose above the predicted mitochondrial set point (≈300 nM), [Ca2+]i mitochondrial Ca2+ sequestration should be activated (21, 22). As a means of testing whether E2-mediated attenuation of the glutamate-induced [Ca2+]i rise is associated with an increase in mitochondrial Ca2+ sequestration, we evaluated the effect of mitochondrial depolarization on the [Ca2+]i rise in response to glutamate (200 μM) in hippocampal neurons. Accumulation of Ca2+ within the mitochondrial matrix depends on ΔΨm and the combination of rotenone to inhibit the respiratory chain at complex I and oligomycin to inhibit the mitochondrial ATP synthase completely depolarizes in situ mitochondria, thus effectively blocking mitochondrial Ca2+ accumulation (22). In the presence of rotenone and oligomycin, 200 μM glutamate induced a rapid increase in [Ca2+]i (Fig. 4A) with a mean change in Fura4F fluorescence ratio of 1.294 ± 0.04 (n = 3; Fig. 4B). In the presence of rotenone and oligomycin, the glutamate-stimulated increase in [Ca2+]i was significantly enhanced by pretreatment with E2 (Fig. 4A) with a mean change in Fura4F fluorescence ratio of 1.543 ± 0.05 (P < 0.05; n = 3; Fig. 4B). Similar results were obtained with Fura2 (data not shown).
Although rotenone inhibits respiratory chain complex I, mitochondrial function can be supported by complex II by using succinate as a substrate. Further confounding the analysis, previous reports indicate that oligomycin can interfere with glutamate-stimulated Ca2+ influxes (22, 23). Thus these mitochondrial inhibitors could exert effects beyond inhibiting mitochondrial Ca2+ uptake. To address these pharmacological shortcomings, we used the selective inhibitor of complex III, antimycin, which has been shown to increase the amplitude of the excitotoxic glutamate-induced rise in [Ca2+]i (24). It has been shown that primary hippocampal neurons can maintain their ATP levels in the presence of antimycin for up to 2 h (24), obviating the need for addition of oligomycin, which may interfere with glutamate-stimulated Ca2+ influxes (22, 23). In the presence of antimycin (2.5 μM), glutamate induced a rapid increase in [Ca2+]i that was consistent with the expected value (Fig. 4C) with a mean change in Fura4F ratio of 1.448 ± 0.06 (n = 3; Fig. 4D). In the presence of antimycin, glutamate-stimulated increase in [Ca2+]i was significantly enhanced by pretreatment with E2 (Fig. 4C) with a mean change in Fura4F fluorescence ratio of 1.741 ± 0.09 (P < 0.05; n = 3; Fig. 4D). Similar results were obtained when Fura2 was used instead of Fura4F (data not shown).
Both the oligomycin plus rotenone and antimycin pretreatments abolished the decline in [Ca2+]i, resulting in a sustained Ca2+ level after glutamate stimulation (Fig. 4), indicating that the decline is due to mitochondrial sequestration of intracellular Ca2+. Additionally, the [Ca2+]i levels reached in the presence of the mitochondrial inhibitors is higher than the peak rise observed in their absence. In the absence of mitochondrial inhibitors, the peak rise in Fura4F fluorescence ratio was 1.192 ± 0.08 over baseline (Fig. 2). In presence of oligomycin plus rotenone or antimycin, the rise in Fura4F fluorescence ratio was 1.294 ± 0.04 and 1.448 ± 0.06, respectively (Fig. 4). This rise indicates that mitochondrial sequestration of Ca2+ blunted the peak Ca2+ rise induced by glutamate, as reported (23, 25, 26).
Estrogen Treatment Increases the Mitochondrial Ca2+ Sequestration After Excitotoxic Glutamate Exposure.
To determine whether E2-mediated attenuation of the glutamate-induced rise in [Ca2+]i was associated with an increase in [Ca2+]m, we evaluated the impact of release of [Ca2+]m after exposure to glutamate (200 μM) in hippocampal neurons pretreated with E2 (10 ng/ml). Addition of FCCP (2.5 μM), a protonophore that leads to collapse of the mitochondrial membrane potential and release of [Ca2+]m, resulted in a rapid increase in Fura4F fluorescence over that in response to glutamate alone. Because deenergized mitochondria no longer sequester Ca2+, the magnitude of fluorescence ratio increase was used as an indicator of [Ca2+]m (24, 27). To offset artifacts secondary to depletion of ATP by FCCP, the ATP synthase inhibitor oligomycin was applied before the addition of FCCP. In the presence of oligomycin, glycolysis can maintain the ATP/ADP ratios, which are not affected by the addition of FCCP (27, 28). The addition of FCCP resulted in a greater magnitude of mitochondrial Ca2+ release in E2-treated neurons than in control neurons (Fig. 3E). Similar results were obtained when Fura2 was used instead of Fura4F (data not shown). Calculation of Ca2+ release (defined as the difference between the final fluorescence after FCCP addition and the steady-state fluorescence that occurred during glutamate exposure) indicated a change in Fura4F fluorescence ratio of 1.996 ± 0.13 for E2-treated cells and 1.284 ± 0.19 for control cells (P < 0.01; n = 3; Fig. 4F). The Fura4F ratio change was ≈1.5 times higher in the E2-treated neurons (E2/control = 1.5 ± 0.4; n = 3).
Estrogen Protects Against Excitotoxic Cell Death and Increases Bcl-2 Expression.
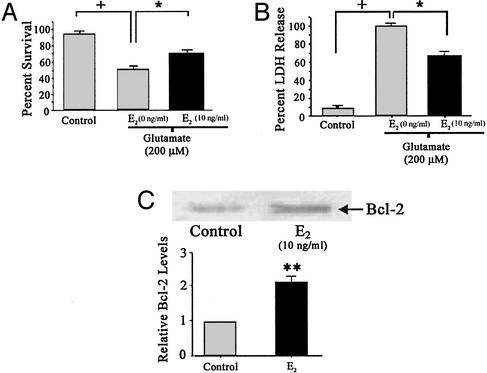
Ca2+ uptake by mitochondria is thought to contribute to toxicity (7, 29). Thus, the increased [Ca2+]m loads induced by E2 treatment would be expected to lead to increased neuronal death, which is antithetical to E2 protection against glutamate excitotoxicity (1, 3). Experiments were performed to determine the protective effect of E2 against glutamate neurotoxicity under the conditions used previously. A 5-min treatment of 200 μM glutamate caused significant cell death as determined by survival cell counts (Fig. 5A) and LDH release (Fig. 5B). A 48-h pretreatment with E2 significantly reduced the amount of neuronal death (Fig. 5A) and the amount of LDH released (Fig. 5B).
Figure 5.
Estrogen protects against glutamate-induced neurotoxicity. Primary hippocampal neuron cultures were pretreated with E2 (10 ng/ml) 48 h before a 5-min exposure to 200 μM glutamate. (A) Viable neurons were counted before and 24 h after a 5-min excitotoxic glutamate (200 μM) exposure. (B) Twenty-four hours after glutamate exposure, culture media was assayed for LDH release from damaged cells. (+, P < 0.05 as compared with control cultures; *, P < 0.05 as compared with vehicle-treated cultures exposed to glutamate alone; n = 4 independent experiments, six wells per condition per experiment). (C) Hippocampal neurons were treated with E2 (10 ng/ml) or vehicle control for 48 h. Representative Western blot showing increased Bcl-2 expression after E2 exposure (Upper). Quantitation of band density normalized to control levels (Lower) (**, P < 0.01; n = 3).
Mitochondria derived from Bcl-2-expressing cells have a higher capacity for Ca2+ uptake and a greater resistance to Ca2+-induced respiratory injury than mitochondria from cells that do not express Bcl-2 (15, 30). Furthermore, studies have reported that E2 increases the expression of Bcl-2 in neuronal cell lines and in rat brain (31–34). To determine whether E2 regulated Bcl-2 expression in parallel to both the sequestration of [Ca2+]m and neuron survival, we determined Bcl-2 expression by Western blot analysis in primary hippocampal neurons. E2 treatment (10 ng/ml; 48 h) resulted in an ≈2-fold increase in Bcl-2 expression that was significantly greater than control (Fig. 5C).
Discussion
In this study, we sought to resolve the paradox of dual regulation of [Ca2+]i by E2 in hippocampal neurons after nontoxic and excitotoxic glutamate exposure. Contrary to the potentiation of the rise in [Ca2+]i seen on exposure to 25 μM glutamate, E2 induced an attenuation of the Ca2+ response to excitotoxic glutamate (200 μM) exposure. As seen in Fig. 1, depending on the glutamate concentration, E2 pretreatment can either potentiate the [Ca2+]i rise in response to synaptically relevant glutamate or attenuate the [Ca2+]i rise in the response to excitotoxic glutamate. Our prior work has shown that the opposing actions are not unique to E2 but are induced by other clinically relevant estrogen replacement therapies, such as conjugated equine estrogens used in the Women's Health Initiative (13). That we were able to demonstrate the attenuation of the Ca2+ rise induced by glutamate by using the lower affinity dye, Fura4F, indicated that the results were not due to saturation artifacts sometimes observed with the high-affinity dye Fura2 for determining [Ca2+] in response to high concentrations of glutamate (19, 20). Although the attenuation of the steady-state glutamate-induced [Ca2+]i was detected with both Fura2 and Fura4F, we were unable to observe the initial peak in [Ca2+]i by using Fura2, possibly due to the differing kinetics of the two dyes. The use of both Ca2+ indicators provided optimal analysis with Fura2, providing a better indication of the magnitude of the E2 effect, whereas Fura4F provided greater detail of the dynamics of Ca2+ signaling.
The initial [Ca2+]i peak that was observed with Fura4F was higher in the E2-treated neurons than in the control neurons, an effect consistent with the E2-mediated potentiation of the 45Ca2+ uptake induced by both 25 and 200 μM glutamate (Fig. 2). These results are consistent with the reported enhancement of N-methyl-d-aspartate (NMDA)-mediated excitatory postsynaptic potentials by E2 (17). As such, differential regulation of the glutamate NMDA receptor activity cannot resolve the paradox of the opposing actions of E2 on glutamate-induced Ca2+ signaling. These results indicate two important features. First, E2 potentiates the rise in [Ca2+]i induced by glutamate irrespective of glutamate concentration. Second, the mechanism of E2-induced attenuation of excitotoxic glutamate rise in [Ca2+]i is downstream to the glutamate receptor. Our data indicate that estrogen-induced reduction in [Ca2+]i after exposure to excitotoxic glutamate is due to a buffering or sequestration mechanism downstream of Ca2+ influx.
Because mitochondrial Ca2+ uptake limits the rise of [Ca2+]i in response to glutamate (26), a likely mechanism underlying the attenuated rise in [Ca2+]i is E2-induced increase Ca2+ sequestration by mitochondria. The use of mitochondrial inhibitors, (i) a combination of rotenone (to inhibit the respiratory chain at complex I) and oligomycin (to inhibit mitochondrial ATP synthase) or (ii) antimycin (to inhibit the respiratory chain at complex III) completely depolarizes in situ mitochondria, effectively blocking mitochondrial Ca2+ accumulation (22, 24). By using this model as a tool, E2-induced attenuation of the [Ca2+]i rise was revealed to depend on mitochondrial sequestration of Ca2+ (Fig. 4). These data were validated by the opposite experiment in which blockade of mitochondrial Ca2+ sequestration resulted in a significant increase in [Ca2+]i after exposure to excitotoxic glutamate in E2-treated neurons. This result is consistent with the E2 potentiation of Ca2+ influx as measured by 45Ca2+ uptake. Together, these data are consistent with the hypothesis that E2-induced attenuation of the [Ca2+]i rise depends on mitochondrial Ca2+ sequestration. Our results obtained in neurons may be applicable to other estrogen-responsive mitochondrial tissues. For example, liver mitochondria isolated from rats treated with E2 show enhanced respiratory substrate-dependent binding of Ca2+ than mitochondria from control rats (35, 36).
Mitochondrial Ca2+ sequestration depends on uptake through the uniporter and efflux due to exchange for Na+ via a mitochondrial Na+/Ca2+ transporter (37). Thus mitochondria behave as buffers of extramitochondrial Ca2+, accumulating the cation whenever its concentration rises above the threshold at which uptake and efflux balance and releasing Ca2+ below this level (9). The data in the present study support a model in which E2 potentiates the Ca2+ influx through the N-methyl-d-aspartate receptor, but only exposure to the higher concentration of glutamate causes [Ca2+]i to exceed the threshold. This model is consistent with the data demonstrating E2 potentiation of the [Ca2+]i rise with 25 μM glutamate (Fig. 1 A and B) and attenuation of the [Ca2+]i rise induced by 200 μM glutamate (Figs. 1 C and D and 3 A and B).
Further supporting a model of E2-induced mitochondrial sequestration of Ca2+, we demonstrated that E2 treatment resulted in increased mitochondrial Ca2+ sequestration in intact neurons after exposure to excitotoxic glutamate. Maintenance of [Ca2+]m levels depends on the proton gradient across the inner membrane, allowing one to use the protonophore FCCP as a tool to dissipate the electrochemical gradient resulting in release of mitochondrial Ca2+ (24, 27, 38). The release of [Ca2+]m is manifested as an increase in [Ca2+]i, which can be detected by calcium indicator dyes (38). Collapse of the mitochondrial membrane potential by FCCP may also reverse ATP synthesis, leading to rapid hydrolysis of cytoplasmic ATP (27), preventing Na+ and Ca2+ extrusion from the cell (39). To prevent artifactual increases in [Ca2+]i due to depletion of ATP by FCCP, the mitochondrial ATP synthase inhibitor oligomycin was added before FCCP exposure. In the presence of oligomycin, neurons can maintain ATP/ADP ratios by glycolysis, and there is no further drop in ATP levels with FCCP administration (27, 28). In the present study, we showed that administration of FCCP, in the presence of oligomycin, after an excitotoxic glutamate stimulus (200 μM) resulted in a significantly greater release of mitochondrial Ca2+ stores from E2-treated cells than from control cells (Fig. 5 E and F). These data indicate that E2-induced increase in mitochondrial Ca2+ sequestration is coupled with an increased [Ca2+]m load.
Although the E2-induced reduction in cytosolic Ca2+ contributes to the neuroprotection, excessive Ca2+ sequestration into mitochondria can lead to mitochondrial dysfunction and subsequent cell death (7, 21, 25, 29). Thus, one would expect an E2-induced increase in mitochondrial Ca2+ sequestration and [Ca2+]m load in response to excitotoxic glutamate to result in increased neuronal death, not increased survival. However, E2 consistently protects against glutamate-induced neurotoxicity. We showed that under the conditions used previously for Ca2+ imaging, E2 protects against glutamate-induced neurotoxicity (Fig. 5), which is consistent with the reported neuroprotective effect of E2 (1–4). If E2 increases mitochondrial Ca2+ sequestration and protects against glutamate excitotoxicity, then it follows that mitochondrial Ca2+ load tolerability has been altered. Preservation of mitochondrial function by E2 is in agreement with reports that show E2 pretreatment maintains ATP production and mitochondrial membrane potential in the face of oxidative stress and mutant presenilin-1 (40, 41). Thus, rather than mediating neuroprotection via reduced cytosolic Ca2+ levels, the more prominent mechanism of E2-induced neuroprotection against glutamate excitotoxicity is via alteration of mitochondrial function, including increased Ca2+ load tolerability.
There is an apparent limit to the amount of Ca2+ that mitochondria can accumulate, illustrated by a decrease in the ratio of Ca2+ released by FCCP to that released by glutamate on higher levels of glutamate exposure (38). One proposed mechanism to increase mitochondrial Ca2+ load tolerability is through increased expression of the antiapoptotic protein Bcl-2. Bcl-2 is localized to the mitochondrial membrane and its expression significantly enhances the capacity for mitochondrial Ca2+ sequestration (15). In addition to increasing the magnitude of Ca2+ sequestered by mitochondria, Bcl-2 enhances the tolerability of mitochondria for increased levels of [Ca2+]i that would otherwise result in dissipation of ΔΨm and cell death (30). Bcl-2 has been identified as an estrogen-responsive gene in reproductive tissues (42), as well as in neurons (31–34). In the current study, we confirmed that E2 induces increased Bcl-2 expression in primary hippocampal neurons. Thus, we hypothesize that by increasing [Ca2+]m uptake capacity, and resultant resistance to Ca2+-induced respiratory inhibition conferred by Bcl-2, E2 limits the loss of viability initiated by excitotoxic glutamate.
Collectively, these data indicate that E2 treatment of primary hippocampal neurons results in increased mitochondrial sequestration of Ca2+ in response to excitotoxic glutamate, which leads to an attenuation of the rise in bulk-free [Ca2+]i. The E2-induced attenuation is correlated with an increase in Bcl-2 expression, which could provide a mechanism by which neurons are protected against deleterious effects of the increased [Ca2+]m induced by E2. Our work is aimed at determining the mechanisms whereby E2 promotes mitochondrial sequestration of Ca2+ and mitochondrial viability in the face of increased Ca2+ load.
Acknowledgments
We thank Drs. David Nichols and Enrique Cadenas for helpful suggestions and critique of this work as it progressed. This study was supported by grants from the National Institutes of Aging (PO1 AG1475: Project 2), the Kenneth T. and Eileen L. Norris Foundation, the L. K. Whittier Foundation (to R.D.B.), and the Alzheimer's Association (NIRG-01-2626) (to J.N.).
Abbreviations
- [Ca2+]i
intracellular Ca2+ concentration
- [Ca2+]m
mitochondrial Ca2+ concentration
- E2
17β-estradiol
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- LDH
lactate dehydrogenase
References
- 1.Brinton R D. Learn Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B S, Alves S E. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 3.Singer C A, Rogers K L, Strickland T M, Dorsa D M. Neurosci Lett. 1996;212:13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Holsboer F. Trends Pharmacol Sci. 1999;20:441–444. doi: 10.1016/s0165-6147(99)01392-9. [DOI] [PubMed] [Google Scholar]
- 5.Green P S, Gridley K E, Simpkins J W. Neurosci Lett. 1996;218:165–168. doi: 10.1016/s0304-3940(96)13148-7. [DOI] [PubMed] [Google Scholar]
- 6.Mattson M P. Exp Gerontol. 1992;27:29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 7.Hartley D M, Kurth M C, Bjerkness L, Weiss J H, Choi D W. J Neurosci. 1993;13:1993–2000. doi: 10.1523/JNEUROSCI.13-05-01993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell P. Biochem Soc Trans. 1976;4:399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls D G. Biochem J. 1978;176:463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholls D, Akerman K. Biochim Biophys Acta. 1982;683:57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- 11.Schinder A F, Olson E C, Spitzer N C, Montal M. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White R J, Reynolds I J. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsen J, Chen S, Brinton R D. Brain Res. 2002;903:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- 14.Brinton R D, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu H P. Neurobiol Aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 15.Murphy A N, Bredesen D E, Cortopassi G, Wang E, Fiskum G. Proc Natl Acad Sci USA. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimiya M, Chang S H, Liu H, Berezesky I K, Trump B F, Amstad P A. Am J Physiol. 1998;275:C832–C839. doi: 10.1152/ajpcell.1998.275.3.C832. [DOI] [PubMed] [Google Scholar]
- 17.Foy M R, Xu J, Xie X, Brinton R D, Thompson R F, Berger T W. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 18.Bi R, Broutman G, Foy M R, Thompson R F, Baudry M. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyrc K, Handran S D, Rothman S M, Goldberg M P. J Neurosci. 1997;17:6669–6677. doi: 10.1523/JNEUROSCI.17-17-06669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stout A K, Reynolds I J. Neuroscience. 1999;89:91–100. doi: 10.1016/s0306-4522(98)00441-2. [DOI] [PubMed] [Google Scholar]
- 21.White R J, Reynolds I J. J Physiol (London) 1997;498:31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budd S L, Nicholls D G. J Neurochem. 1996;67:2282–2291. doi: 10.1046/j.1471-4159.1996.67062282.x. [DOI] [PubMed] [Google Scholar]
- 23.Kannurpatti S S, Joshi P G, Joshi N B. Neurochem Res. 2000;25:1527–1536. doi: 10.1023/a:1026602100160. [DOI] [PubMed] [Google Scholar]
- 24.Wang G J, Thayer S A. J Neurophysiol. 1996;76:1611–1621. doi: 10.1152/jn.1996.76.3.1611. [DOI] [PubMed] [Google Scholar]
- 25.Stout A K, Raphael H M, Kanterewicz B I, Klann E, Reynolds I J. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- 26.Khodorov B, Pinelis V, Storozhevykh T, Yuravichus A, Khaspekhov L. FEBS Lett. 1999;458:162–166. doi: 10.1016/s0014-5793(99)01130-8. [DOI] [PubMed] [Google Scholar]
- 27.Budd S L, Nicholls D G. J Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- 28.White R J, Reynolds I J. J Neurosci. 1995;15:1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormack J G, Halestrap A P, Denton R M. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L, Ling S, Yu X D, Venkatesh L K, Subramanian T, Chinnadurai G, Kuo T H. J Biol Chem. 1999;274:33267–33273. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- 31.Nilsen J, Mor G, Naftolin F. J Neurobiol. 2000;43:64–78. doi: 10.1002/(sici)1097-4695(200004)43:1<64::aid-neu6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 32.Nilsen J, Brinton R D. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Segura L M, Cardona-Gomez P, Naftolin F, Chowen J A. NeuroReport. 1998;9:593–597. doi: 10.1097/00001756-199803090-00006. [DOI] [PubMed] [Google Scholar]
- 34.Dubal D B, Shughrue P J, Wilson M E, Merchenthaler I, Wise P M. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimberg D V, Goldstein S A. Endocrinology. 1967;80:89–98. doi: 10.1210/endo-80-1-89. [DOI] [PubMed] [Google Scholar]
- 36.Kimberg D V, Goldstein S A. J Biol Chem. 1966;241:95–103. [PubMed] [Google Scholar]
- 37.Crompton M, Moser R, Ludi H, Carafoli E. Eur J Biochem. 1978;82:25–31. doi: 10.1111/j.1432-1033.1978.tb11993.x. [DOI] [PubMed] [Google Scholar]
- 38.Brocard J B, Tassetto M, Reynolds I J. J Physiol. 2001;531:793–805. doi: 10.1111/j.1469-7793.2001.0793h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls D G, Budd S L. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Green P S, Simpkins J W. J Neurochem. 2001;77:804–811. doi: 10.1046/j.1471-4159.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- 41.Mattson M P, Robinson N, Guo Q. NeuroReport. 1997;8:3817–3821. doi: 10.1097/00001756-199712010-00031. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira C, Reed J C, Pratt M A. Cancer Res. 1995;55:3902–3907. [PubMed] [Google Scholar]