Abstract
How individual receptive field properties are formed in the maturing sensory neocortex remains largely unknown. The shortening of N-methyl-d-aspartate (NMDA) receptor currents by 2A subunit (NR2A) insertion has been proposed to delimit the critical period for experience-dependent refinement of circuits in visual cortex. In mice engineered to maintain prolonged NMDA responses by targeted deletion of NR2A, the sensitivity to monocular deprivation was surprisingly weakened but restricted to the typical critical period and delayed normally by dark rearing from birth. Orientation preference instead failed to mature, occluding further effects of dark rearing. Interestingly, a full ocular dominance plasticity (but not orientation bias) was selectively restored by enhanced inhibition, reflecting an imbalanced excitation in the absence of NR2A. Many of the downstream pathways involved in NMDA signaling are coupled to the receptor through a variety of protein–protein interactions and adaptor molecules. To further investigate a mechanistic dissociation of receptive field properties in the developing visual system, mice carrying a targeted disruption of the NR2A-associated 95-kDa postsynaptic density (PSD95) scaffolding protein were analyzed. Although the development and plasticity of ocular dominance was unaffected, orientation preference again failed to mature in these mice. Taken together, our results demonstrate that the cellular basis generating individual sensory response properties is separable in the developing neocortex.
Single cells in the brain can possess multiple receptive field properties such as ocular dominance (OD), orientation, and direction selectivity in the primary visual cortex. All these features are shaped by experience during a limited time in early postnatal life (1). The generation of individual properties may result from similar activity-dependent mechanisms (2) but could also be mediated by distinct underlying cellular processes (3). No evidence has been reported to differentiate these possibilities. The N-methyl-d-aspartate (NMDA)-type glutamate receptor (NR) is considered a key player in such Hebbian plasticity because of its calcium permeability (4). Across a variety of brain regions (5–13), synaptic NMDA currents are sharpened over development, which is largely believed to delimit net calcium influx and hence critical periods for circuit refinement (14). Composed of a principal subunit NR1 and different modulatory NR2 partners (4), NMDA current-decay kinetics are truncated by an activity-dependent switch in predominant subunit composition from NR2B to NR2A (7–13).
Indeed, overexpression of the immature NR2B subunit reportedly enhances hippocampal learning in adult mice (15). An alternative mechanism for critical-period plasticity, however, depends on the balance of excitation and inhibition (16, 17), rather than absolute NR function. Recent work in somatosensory cortex in fact indicates that slow kinetics and the absence of NR2A do not affect plasticity (18, 19). Yet this is not surprising, because the critical period tested for barrel cortex appears early during the first week of life, when NR2A expression normally is modest at best (7). Moreover, only anatomical effects of whisker cautery were addressed in vivo (19), which are known to be NR-independent (20). Segregation of axons from the right and left eye by spontaneous waves of retinal activity before vision in the thalamus (21) or tectum (22) is similarly independent of NRs. These morphological events occur neonatally and may reflect more closely mechanisms of initial afferent map formation rather than experience-dependent refinement of established circuits.
Receptive field properties, in contrast, are plastic during later critical periods (23), which must be assessed by electrophysiological measures in vivo. In kitten visual cortex, NR binding and efficacy are correlated with peak sensitivity to monocular deprivation (MD) (1, 24, 25). Immunostaining for the obligatory NR1 subunit in cats and ferrets is consistent with a central role for NRs in promoting and ultimately limiting circuit rearrangements in the developing neocortex (26). However, a specific role for NRs in OD plasticity has eluded pharmacological approaches in vivo because of direct antagonist action on baseline visual responsiveness (1, 27). Here we address the contribution of changing NR kinetics to visual cortical plasticity by using a genetic strategy in mice, which express a robust critical period for MD effects between postnatal days (P)23 and 35 (17, 28).
Global removal of NR1 is neonatal lethal (29), whereas conditional NR1 deletion (20) renders the cortex visually unresponsive as observed for NR antagonists (M.F., T. Iwasato, S. Itohara, and T.K.H., unpublished observations). In contrast, the postnatal time course of NR2A expression within neocortex (7–10, 13, 30–33) makes gene targeting “conditional” to a late time in development without eliminating NRs altogether (34, 35). Our findings distinguish the plasticity of mature, functional circuits by visual input from the early shaping of anatomical connections by whisker cauterization. We show here that the timing of the critical period in visual cortex is normal without NR2A, and the duration of NR-mediated excitation may balance intracortical inhibition for the proper detection of MD. Instead, signaling through an NR2A–postsynaptic density 95-kDa protein (PSD95) complex itself seems essential for the maturation of orientation preference by natural sensory experience.
Methods
Mice carrying a targeted disruption of the “GluRɛ1” (NR2A) gene were generated as described (34). Homozygous mutants derived from multiple backcrossing onto a C57BL/6 background (>14 generations, >99.9% C57BL/6) were used (35), allowing age-matched C57BL/6 mice to serve as controls. For mice lacking PSD95 function as described (36), littermates were compared from heterozygote crossing.
Visual Cortex Electrophysiology in Vivo.
Single-unit recording was performed by standard methods (16, 28). In brief, animals were prepared blind to genotype under Nembutal (50 mg/kg, i.p.) and chlorprothixene (0.2 mg, i.m.) anesthesia supplemented with a mixture of O2 and halothane via a trachea tube as needed. For each animal, five to eight cells (>75 μm apart) were recorded in each of four to six penetrations spaced evenly (>200-μm intervals) across the mediolateral extent of primary visual cortex to map the monocular and binocular zones and avoid sampling bias. Receptive fields of isolated single units were plotted on a tangent screen with moving light-bar stimuli. Cells were assigned OD scores according to the seven-point scale of Hubel and Wiesel (1, 16, 28). A weighted average of the bias toward the contralateral eye [contralateral bias index (CBI)] was calculated for each binocular zone by the formula CBI = [(n1 − n7) + (2/3)(n2 − n6) + (1/3)(n3 − n5)] + N]/2N, where N is the total number of cells and nx is the number of cells with an OD score equal to x. CBI ranges from 0 to 1 for complete ipsilateral/contralateral OD, respectively. Spontaneous or background discharges were subtracted from all visual responses. Cell responsiveness and habituation were rated on a three-point scale (37). Cells were classified as orientation-biased if the firing rate was maximal (>2x) for a given (preferred) orientation versus its orthogonal within the receptive field. Responses of comparable strength for all eight orientations tested (±0, ±30, ±60, and ±90°) were called nonbiased (16, 28, 37).
For brief MD (16, 28), one eyelid was sutured shut under halothane anesthesia for 4–5 days. Long-term MD (>2 weeks) spanned the entire critical period (P17–35). For dark rearing (DR), litters were kept in complete darkness from birth until adulthood (>P45) and then exposed to light for MD. Drug-rescue experiments were performed by intracranial injection (16, 17) (1.5 μl intracerebroventricular per side daily) of diazepam (2 mg/ml, Wako Pure Chemical, Osaka) or vehicle solution (50% propylene glycol, Nacalai Tesque, Kyoto) 1 day before and throughout MD and then recorded blind to treatment. All recordings were obtained contralateral to the deprived eye.
Visual Cortex Electrophysiology in Vitro.
Coronal slices (350 μm) through the binocular zone of mouse visual cortex were prepared at several ages under halothane anesthesia (16), incubated in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (>1 h, 22–25°C) containing 119 mM NaCl, 2.5 mM KCl, 1.3 mM MgSO4, 10 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2, and 11 mM glucose. From single, submerged slices superfused with artificial cerebrospinal fluid (34°C) and picrotoxin (100 μM) or bicuculline methiodide (20 μM), excitatory postsynaptic currents (EPSCs) in layer 2/3 cortical neurons visualized with infrared-Nomarski differential interference contrast optics (Olympus BX-50, Hamamatsu, Ichinocho, Japan) were evoked by a bipolar, focal glass stimulating electrode (artificial cerebrospinal fluid) and recorded by patch electrodes (4–6 MΩ) in whole-cell voltage-clamp mode (Axopatch 1D).
The pipette solution contained 122.5 mM Cs-gluconate, 17.5 mM CsCl, 10 mM Hepes, 0.2 mM EGTA, 8 mM NaCl, 2 mM Mg-ATP, and 0.3 mM Na3-GTP (pH 7.2, 290–300 milliosmolal) (34). Stimulus intensity and location were first set to evoke α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) responses (at −90 mV) from layer 4 that were half the maximum before polysynaptic events became evident (i.e., single peak, constant latency, and no notch on rising phase). Thresholds did not differ between NR2A knockout (KO) and WT. NMDA EPSCs then were isolated by blocking AMPA currents with 6-cyano-7-nitroquinoxaline-2,3-dione (20 μM, Tocris Cookson, Bristol, U.K.), raising the holding potential (to +40 mV), and finally verified by elimination with D-2-amino-5-phosphonovaleric acid (50 μM, Tocris Cookson) superfusion. Ratiometric measurements of NMDA/AMPA currents were made by comparing peak amplitudes at +40 and −90 mV.
Resting membrane potential (WT, −76 ± 1 mV; KO, −75 ± 1 mV), series (RS) (WT, 21 ± 1 MΩ; KO, 19 ± 1 MΩ) and input resistance (RIN) (WT, 144 ± 22 MΩ; KO, 101 ± 13 MΩ) were monitored in all cells (n = 21 each; all P > 0.1). Unstable recording (>10% fluctuation of RS value) during the course of an experiment was rejected from further analysis. Records were filtered (1 kHz), digitized (13 kHz), stored, and analyzed by using EXPERIMENTER'S WORKBENCH (DataWave Technologies, Longmont, CO). Twenty evoked EPSCs were averaged, and decay times were derived by fitting the formula I(t) = If × exp(−t/τf) + Is × exp(−t/τs), where If and Is are the peak amplitude of the fast and slow decay components, respectively, and τf and τs are the respective time constants. For comparison by unpaired t test, weighted mean decay-time constants (±SEM) were calculated by using τw = τf [If/(If + Is)] + τs [Is/(If + Is)].
Biochemical Analysis.
For Western blot analysis, visual cortices from halothane-anesthetized WT mice were dissected and homogenized by sonication (38). Total protein concentrations were determined by colorimetric (Bradford) assay system (Bio-Rad) and used to normalize loading. Protein samples (50 μg per lane) were separated by 7.5% SDS/PAGE and transferred to poly(vinylidene difluoride) membranes (ATTO Corporation, Tokyo). Membranes were incubated with antibodies (1:1,000) to NR2A (39), NR1 (PharMingen), or PSD95 (1:250, Transduction Laboratories, Lexington, KY) before horseradish peroxidase-conjugated secondary antibody (Zymed). Blots were developed to film after ECL treatment (Amersham Pharmacia). Optical density was measured with NIH IMAGE software and normalized to that of the P10 sample present in all immunoblots for comparison across experiments.
Results
The hypothesis that a developmental change in NRs may close critical periods first arose in the visual system through correlative arguments in WT rats (5, 6). We confirmed a late postnatal onset of NR2A protein expression in mouse visual cortex (Fig. 1A; refs. 10 and 30–33) using antibodies verified to be specific in GluRɛ1 (NR2A) KO mice (34, 39). Dramatic up-regulation was already evident before the critical period (P18), refuting a specific role in ending plasticity (40). To further assess a link with MD effects, we examined mature NR2A levels in a mouse model that never enters the critical period due to targeted deletion of the synaptic 65-kDa isoform of glutamic acid decarboxylase (GAD65) (16, 17). These animals displayed normal expression unrelated to their plasticity deficit (>P50; three KO vs. four WT, P > 0.1).
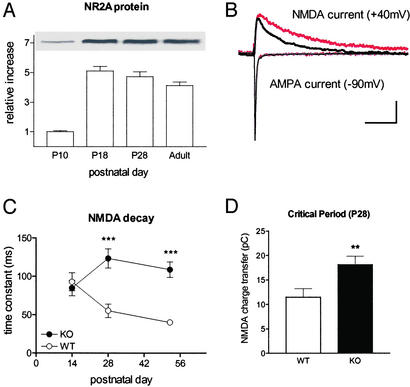
Figure 1.
NR2A deletion prolongs NMDA response in mouse visual cortex. (A) NR2A protein increases dramatically with eye opening (before, P10; after, P18) but well in advance of the critical period (P28) in WT animals (four mice per age normalized to P10 values). (B) Representative traces at P28 from visual cortical neurons in NR2A KO (red) or WT (black) slices. Stimulation was adjusted to evoke half-maximal monosynaptic AMPA response before isolating NMDA currents with γ-aminobutyric acid type A antagonist/6-cyano-7-nitroquinoxaline-2,3-dione (20 μM) and raising membrane potential to +40 mV. Note that AMPA currents recorded first (at −90 mV) were indistinguishable. (Scale, 50 pA, 100 ms.) (C) The decay time constant of NMDA currents is typically shortened by the peak of the critical period in WT mice (P14, six cells, and P28, seven cells; five mice each; P < 0.03). At eye opening (P14) there is no significant difference in kinetics between NR2A KO (five cells, three mice) and WT. By P28 (10 cells, seven KO mice) and beyond (eight cells, seven WT mice), NMDA currents are dramatically longer without NR2A. Adult KO currents (six cells, six mice) are no different from immature WT (P14; P = 0.15). ***, P < 0.001, KO vs. WT by age group (young P14, P14–15; critical period P28, P25–31; adult P52, P43–58). Some error bars (SEM) are smaller than symbol size. (D) Total charge transfer through NRs is enhanced at peak of the critical period in NR2A KO mice. For the same cells as described above at P28: **, P < 0.01.
Supragranular neurons in the binocular zone of WT mice exhibited a correlated shortening of NMDA response with age (Fig. 1C) as reported in rats (6, 8, 10, 12). The NMDA currents in slices from NR2A KO mouse visual cortex remained prolonged well beyond the critical period (Fig. 1 B and C), as anticipated (7–12). Adult KO decay kinetics were similar to immature, precritical-period WT responses (Fig. 1C; P14 WT vs. P52 KO, P = 0.15), indicating that little if any compensation had occurred in the mutant. Consistent with this, no significant change in NR2B or NR1 expression was observed in NR2A KO mice (data not shown and ref. 34).
Extracellular single-unit recording from the same region in vivo revealed robust visual responses. In the absence of NR2A, linear retinotopic maps were observed as in WT mice (regression coefficients, 0.96 ± 0.01 vs. 0.98 ± 0.01, three KO and six WT mice; P > 0.3). Receptive field sizes (7.4 ± 0.5° vs. 6.6 ± 0.2°; P > 0.07), responsiveness (2.96 ± 0.03 vs. 2.93 ± 0.04; P > 0.7), and habituation (1.74 ± 0.07 vs. 1.77 ± 0.17; P > 0.9) all were indistinguishable across groups (88 KO and 151 WT cells, respectively).
The relative strength of input from either eye (OD) was also no different between NR2A KO and WT mice (Fig. 2, no-MD). Brief MD (4–5 days) significantly shifted both distributions in favor of the open eye but only when deprivation started at ≈P25 (Fig. 2, P25 MD vs. adult-MD). Thus, the critical period was not extended into adulthood (>P45) (17, 28) despite prolonged NR current in the absence of NR2A subunits (Fig. 1C).
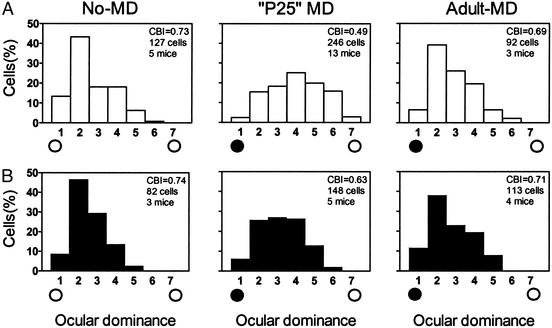
Figure 2.
OD plasticity is weak and restricted in NR2A KO mice. (A) Typically skewed OD histogram of WT mice (No-MD) is shifted by MD toward the open, ipsilateral eye at P25 (open circle, P25-MD; P < 0.0001 vs. no MD, χ2 test) but not in adults (>P45, Adult-MD; P > 0.4 vs. no MD, χ2 test). The CBI of each distribution in the upper-right corner ranges from 0 (ipsi only) to 1 (contra only). (B) NR2A KO similarly loses sensitivity to MD in adulthood (same age groups as described for A: No-MD vs. P25-MD, P < 0.002, or Adult-MD, P > 0.2, χ2 test). The magnitude of shift at P25, however, is significantly weaker than in WT (P < 0.0001, χ2 test).
Surprisingly, the overall magnitude of OD plasticity was reduced (Fig. 2, P25 MD; WT vs. KO, P < 0.0001, χ2 test). One simple explanation might be a reduced number of activated NRs, because the C terminus of the NR2A subunit can interact with postsynaptic scaffolding molecules to cluster receptors at the synapse (41–43). Indeed, this is believed to underlie weak long-term potentiation (LTP) in hippocampal area CA1 of adult NR2A KO mice 9–17 weeks in age (well past the critical period studied here) (34). Despite the elevated induction threshold, however, LTP eventually saturates at the same level as WT with repeated stimulation (35).
This possibility is unlikely here for two reasons. First, we found no difference in NMDA/AMPA ratio in visual cortex at the peak of the critical period (P28, 68 ± 11 vs. 59 ± 4, 7 WT and 10 KO cells, respectively; P = 0.5, t test). In fact, the hippocampal LTP defect is clearly age-dependent, becoming significant after 1 month of age (34, 44). Second, we tested the possibility that 4-day MD was simply insufficient for inducing a full OD shift by maximizing MD duration to span the entire critical period (>2 weeks) (16, 17). Brief MD was saturating in both three WT and four KO animals, because long-term MD produced no further plasticity (CBI = 0.45 ± 0.02 and 0.60 ± 0.02; P < 0.001, t test). Long-term MD also confirms that the sensitivity to eyelid suture had not simply shifted in time.
Alternatively, an appropriate excitatory-inhibitory (E-I) balance within visual cortex may detect deprived-eye input as indicated by impaired plasticity in the absence of γ-aminobutyric acid-synthetic enzyme GAD65 (16). The E-I balance may similarly be tipped in favor of excitation by long-lasting NMDA-mediated EPSCs (Fig. 3A). Because AMPA receptor currents were indistinguishable across genotype (168 ± 14 vs. 195 ± 16 pA, 21 WT and KO cells, respectively; P = 0.2), the net effect of NR2A deletion was significantly increased charge transfer through NR channels (Fig. 1D).
Figure 3.
E-I balance restores full plasticity to NR2A KO mice. (A) Both γ-aminobutyric acid reduction by GAD65 deletion (16) or long-lasting NMDA currents in the absence of NR2A could similarly bias cortical networks in favor of excitation. Enhancing functional inhibition (shaded region) with diazepam may restore the balance needed to detect competition during MD. (B) CBI shift from the nondeprived range (shaded region) is significantly weaker in NR2A KO (filled bars) than WT (open bars; 5 and 13 mice, respectively; ***, P < 0.0001, t test) but fully restored by diazepam (DZ) concomitant with MD during the critical period [vehicle (Veh) and diazepam, four mice each; **, P < 0.01, t test]. (C) Critical-period delay by DR regardless of NR2A expression. DR from birth (DR alone, four WT and three KO mice) retains sensitivity to brief MD in both WT (open bars) and NR2A KO (filled bars) mice (DR+MD, five WT and four KO) when the critical period is normally closed (MD alone, three WT and four KO mice) (17, 28). Shaded region, range of nondeprived, LR adults (>P45).
A clear correlate in vivo was the strong presence of prolonged neuronal discharge in NR2A KO mice (76 ± 2% vs. 2 ± 1% of 113 KO and 127 WT cells; P < 0.01). Disrupting E-I balance yields excess spike firing by single units in all layers that outlasts the visual stimulus, as originally described for GAD65 KO mice (16). In WT animals prolonged discharge is evident only early in life, when inhibition is weak and NR2A expression is rising, but is normally abolished at critical-period onset (17).
Both E-I balance and plasticity are restored in immature WT and GAD65 KO mice by enhancing inhibition with benzodiazepines (16, 17). We similarly attempted to rescue full plasticity in NR2A KO mice by diazepam infusion concomitant with brief MD (Fig. 3A). Prolonged discharge was significantly reduced but not eliminated (58 ± 4% of 116 cells; P < 0.05 vs. control KO), and OD plasticity was maximized by drug treatment (Fig. 3B) as for both GAD65 KO and precritical-period WT mice (16). Similar diazepam application in WT animals near P28 tends to sharpen plasticity but not significantly beyond the normal range (16).
Next we explored the effects of DR to compare the influence of sensory experience across functional response properties. Raising animals in complete darkness from birth degrades orientation selectivity and prolongs the sensitivity to MD in kittens and rats (1, 37). Intriguingly, DR delays NR2A expression and sharpening of NMDA currents as well (6, 10–12, 33). We first determined whether DR also postpones the critical period in mice. Adult WT animals deprived after DR exhibited robust OD shifts at an age when they normally would not (Fig. 3C; ref. 17). The critical period was prolonged similarly in KO mice by DR (Fig. 3C), further indicating normal regulation by sensory input in the absence of NR2A. Although a trend toward weaker shifts in the KO remained, it was no longer significantly different from WT, which is consistent with additional effects of DR on inhibition (45, 46).
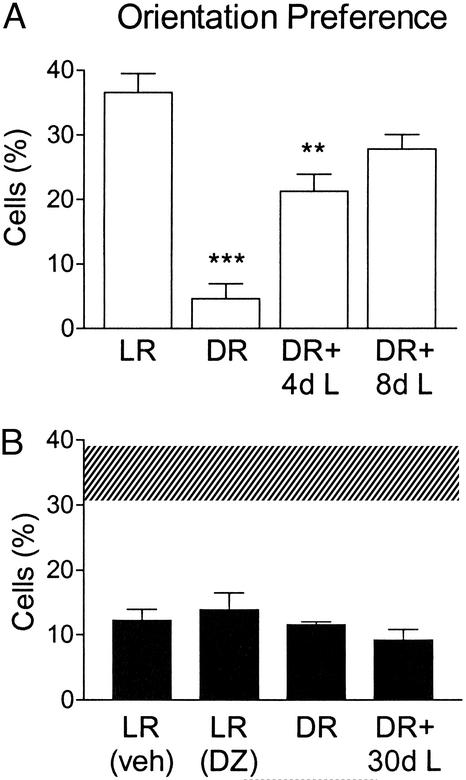
Orientation bias was strongly degraded in dark-reared WT mice but capable of recovery within a few days of exposure to light consistent with a rapid reexpression of NR2A (Fig. 4A; ref. 11). Natural maturation of this property (1, 17, 37) similarly follows onset of NR2A expression after eye-opening (Fig. 1A; refs. 10 and 30–33). In agreement, light-reared (LR) mice lacking NR2A failed to develop orientation preference even when treated with diazepam to restore full OD plasticity (Fig. 4B, LR). Moreover, the orientation impairment occluded any further effects of sensory deprivation from birth and was not rescued by even 1 month of light exposure (Fig. 4B, DR).
Figure 4.
Sensory experience regulates orientation preference through NR2A. (A) The percentage of WT cells (373 cells, 13 mice) responding preferentially to bars of particular orientation is reduced by DR (90 cells, three mice) and recovers with increasing (days) reexposure to light (+4 days light, +8 days light; 156 and 115 cells, five and four mice, respectively). ***, P < 0.0005, and **, P < 0.01 vs. adult LR WT. (B) Normally reared NR2A KO mice (LR; 113 cells, four mice) exhibit poor orientation preference that occludes effects of DR (78 cells, three mice). Neither reexposure to light (DR + 30 days light; 85 cells, three mice) nor brief diazepam at P28 (DZ; 116 cells, four mice) rescues this receptive field property (Veh; 113 cells, four mice). Shaded region, range of adult WT.
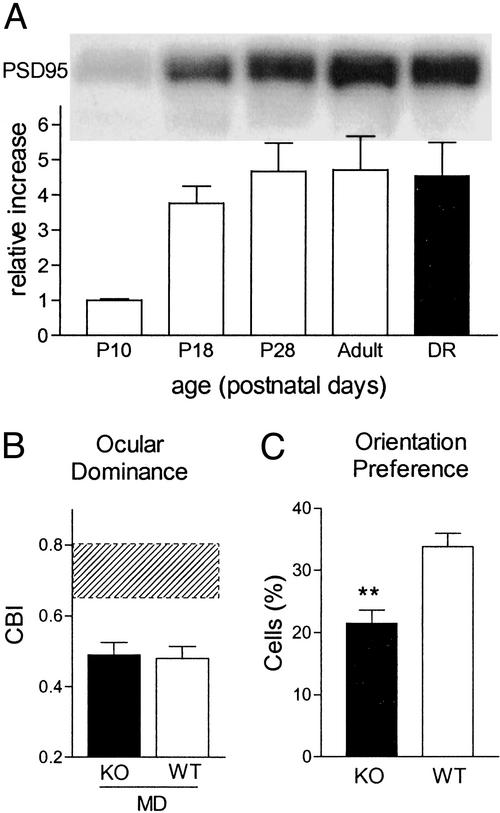
Many of the downstream pathways involved in NR signaling are physically coupled into multiprotein signaling complexes within the postsynaptic density (47). The long C terminus of the NR2 subunit directs delivery of receptors to synapses with different rules controlling NR2A and NR2B (42, 43). In mouse visual cortex, we found the major postsynaptic NR scaffolding protein PSD95 to appear with a late postnatal time course similar to NR2A (Fig. 1A). Yet unlike NR2A (10, 11, 33), PSD95 levels were unaffected by DR (Fig. 5A). In the absence of PSD95 (110 cells, four mice), we observed robust visual responses including linear retinotopy (regression coefficient, 0.99 ± 0.003; P > 0.1), receptive field sizes (6.4 ± 0.4°; P > 0.4), responsiveness (2.91 ± 0.04; P > 0.8), and habituation (1.35 ± 0.14; P > 0.8) compared with WT littermates (76 cells, three mice).
Figure 5.
Full OD plasticity but impaired orientation bias in PSD95 KO mice. (A) Developmental time course of PSD95 in WT mouse visual cortex with no effect of DR (four mice per age normalized to P10 values). (B) Brief MD around P28 is similarly effective in KO and WT mice (five and four mice, respectively; P = 0.8, t test). Shaded region, range of nondeprived mice (four KO and three WT; P = 0.9, t test). (C) Orientation-biased cells remain few in mature PSD95 KO mouse visual cortex compared with WT (246 and 178 cells, nine and seven mice, respectively; **, P < 0.001, t test).
Targeted disruption of PSD95 function enhances LTP by minimizing synaptic depression (36). In the same PSD95 KO mice, we observed an even more robust dissociation of response properties than in NR2A KO mice: impaired orientation bias with no effect at all on OD shift by MD (Fig. 5 B and C). Consistent with the latter, prolonged discharge was moderate in the absence of PSD95 (10 ± 3% vs. 24 ± 5%, 178 WT and 246 KO cells, respectively; P < 0.05, t test), as expected for a molecule downstream of the NR channel itself. Taken together, NR2A and its associated signaling molecules are specifically essential for orientation preference, whereas OD results from a balance of E-I neuronal activity independent of these pathways.
Discussion
In our animal model that can never truncate its NMDA currents due to the absence of NR2A (34), visual cortical plasticity still obeyed a critical period as in WT mice. Candidate plasticity factors have often been linked to a critical-period mechanism by DR (1). Yet simply because a molecule is regulated by activity does not mean that it is involved in plasticity, as shown recently for the immediate early gene zif268 (38). Both DR that is too short to prolong the critical period (12–14) and DR after visual experience has already begun (48) can still regulate expression of NR2A, which is most consistent with plasticity for orientation lasting into adulthood (49, 50). Thus, NR2A is an activity-dependent molecule that is dispensable for establishing a critical period in visual cortex.
Our results suggest unanticipated roles for the NR in vivo during the plasticity process itself. In the standard view, long-duration NMDA-mediated calcium influx could be disruptive to OD plasticity, because during MD strong signals from the normal eye as well as weak inputs from the deprived eye would be interpreted as coincident even when separated by several hundred milliseconds (40). However, enhancing inhibition with diazepam blocks LTP induction by high-frequency stimulation (unpublished observations and refs. 51 and 52) and would not have restored OD shifts in vivo had LTP been required. Moreover, because PSD95 regulates a “sliding” threshold for homosynaptic plasticity (36), this cellular model may be more relevant to the establishment of orientation preference rather than OD, which remains intact in PSD95 KO mice.
Depolarization itself by NMDA currents could instead contribute to a homeostatic balance with inhibition necessary for properly detecting monocular occlusion (16, 53). Specific spike timing-dependent windows for synaptic plasticity have been elucidated recently in developing and neocortical structures (54). Unlike classical models of LTP induced by changes in mean firing rate, spike timing-dependent plasticity relies on physiologically realistic, millisecond-scale changes in the temporal order of pre- and postsynaptic action potentials. Prolonged discharge in both NR2A and GAD65 KO mice would impair OD plasticity by altering the pattern of neural activity encoding visual input (55). Diazepam subtly but significantly improves temporal processing in both animal models to fully restore OD plasticity alone.
General reduction of NR function disrupts both OD plasticity (27) and orientation selectivity in ferret visual cortex (56). Our findings reveal that these effects may be separable through NR2 subunit-specific pathways. Multiple signaling modules in the postsynaptic density complex provide a means to orchestrate the cascade of structural changes unique to either OD (57) or orientation preference by channeling NMDA activity to distinct output cascades. Indeed, PSD95 is involved in regulating spine shape or number and maturation of the presynaptic terminal in culture (58).
Importantly, diazepam selectively rescues OD plasticity without a need for the NR2A receptor subunit per se (Fig. 3B). In contrast, development of orientation preference through sensory experience may be accounted for entirely by NR2A regulation (Fig. 4B). Enhancing inhibition early in development does not accelerate the maturation of orientation nor does prolonged discharge per se alter it in GAD65 KO mice (17). Chronic absence of NR2A signaling may fail to refine intrinsic horizontal connections specifically linked to orientation processing within cortex (59). These scaffolding networks remain to be described in rodents, and transiently diverse receptive fields in the immature thalamus (60) may also contribute to the defect. Both PSD95 and NR2A KO mice may serve as valuable tools to dissect further the substrates generating individual receptive field properties.
Acknowledgments
We thank S. Fujishima, Y. Mizuguchi, Y. Tsuchimoto, N. Mataga, and Y. Iwai for technical assistance. This work was supported in part by Core Research for Evolutional Science and Technology and Special Coordination Funds for Promoting Science and Technology from Japan Science and Technology Corporation.
Abbreviations
- OD
ocular dominance
- NMDA
N-methyl-d-aspartate
- NR
NMDA receptor
- MD
monocular deprivation
- Pn
postnatal day n
- PSD95
postsynaptic density 95-kDa protein
- CBI
contralateral bias index
- DR
dark rearing
- EPSC
excitatory postsynaptic current
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- KO
knockout
- GAD65
glutamic acid decarboxylase 65-kDa isoform
- LTP
long-term potentiation
- E-I
excitatory-inhibitory
- LR
light-reared
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Daw N. Visual Development. New York: Plenum; 1995. [Google Scholar]
- 2.Erwin E, Miller K D. J Neurosci. 1998;18:9870–9895. doi: 10.1523/JNEUROSCI.18-23-09870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D S, Bonhoeffer T. Nature. 1994;370:370–372. doi: 10.1038/370370a0. [DOI] [PubMed] [Google Scholar]
- 4.Nakanishi S, Masu M. Annu Rev Biophys Biomol Struct. 1994;23:319–348. doi: 10.1146/annurev.bb.23.060194.001535. [DOI] [PubMed] [Google Scholar]
- 5.Hestrin S. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- 6.Carmignoto G, Vicini S. Science. 1992;258:1007–1111. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 7.Flint A C, Maisch U S, Weishaupt J H, Kriegstein A R, Monyer H. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stocca G, Vicini S. J Physiol (London) 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tovar K R, Westbrook G L. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nase G, Weishaupt J, Stern P, Singer W, Monyer H. Eur J Neurosci. 1999;11:4320–4326. doi: 10.1046/j.1460-9568.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- 11.Quinlan E M, Philpot B D, Huganir R L, Bear M F. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 12.Philpot B D, Sekhar A K, Shouval H Z, Bear M F. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 13.Williams K, Russell S L, Shen Y M, Molinoff P B. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- 14.Feldman D E, Knudsen E I. Neuron. 1998;20:1067–1071. doi: 10.1016/s0896-6273(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y-P, Shimizu E, Dube G R, Rampon C, Kerchner G A, Zhuo M, Liu G, Tsien J Z. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 16.Hensch T K, Fagiolini M, Mataga N, Stryker M P, Baekkeskov S, Kash S F. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagiolini M, Hensch T K. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- 18.Barth A L, Malenka R C. Nat Neurosci. 2001;4:235–236. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Gonzalez E, Crair M C. Neuron. 2001;32:619–634. doi: 10.1016/s0896-6273(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 20.Iwasato T, Datwani A, Wolf A M, Nishiyama H, Taguchi Y, Tonegawa S, Knöpfel T, Erzurumlu R S, Itohara S. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colonnese M T, Constantine-Paton M. J Neurosci. 2001;21:1557–1568. doi: 10.1523/JNEUROSCI.21-05-01557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smetters D K, Hahm J, Sur M. Brain Res. 1994;658:168–178. doi: 10.1016/s0006-8993(09)90023-3. [DOI] [PubMed] [Google Scholar]
- 23.Stern E A, Maravall M, Svoboda K. Neuron. 2001;31:305–315. doi: 10.1016/s0896-6273(01)00360-9. [DOI] [PubMed] [Google Scholar]
- 24.Bode-Greuel J, Singer W. Dev Brain Res. 1989;46:197–204. doi: 10.1016/0165-3806(89)90283-6. [DOI] [PubMed] [Google Scholar]
- 25.Tsumoto T, Hagihara K, Sato H, Hata Y. Nature. 1987;327:513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- 26.Catalano S M, Chang C K, Shatz C J. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts E B, Meredith M A, Ramoa A S. J Neurophysiol. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 28.Gordon J A, Stryker M P. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrest D, Yuzaki M, Soares H D, Ng L, Luk D C, Sheng M, Steward C L, Morgan J I, Connor J A, Curran T. Neuron. 1994;2:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Inoue Y, Sakimura K, Mishina M. NeuroReport. 1992;3:1138–1140. doi: 10.1097/00001756-199212000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Sheng M, Cummings J, Roldan L A, Jan Y N, Jan L Y. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 32.Cao Z, Lickey M E, Liu L, Kirk E, Gordon B. Brain Res. 2000;859:26–37. doi: 10.1016/s0006-8993(99)02450-6. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Cooper N G, Mower G D. Mol Brain Res. 2000;78:196–200. doi: 10.1016/s0169-328x(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 34.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 35.Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Migaud M, Charlesworth P, Dempster M, Webster L C, Watabe A M, Makhinson M, He Y, Ramsay M F, Morris R G M, Morrison J H, et al. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- 37.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 38.Mataga N, Fujishima S, Condie B G, Hensch T K. J Neurosci. 2001;21:9724–9732. doi: 10.1523/JNEUROSCI.21-24-09724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts E B, Ramoa A S. J Neurophysiol. 1999;81:2587–2591. doi: 10.1152/jn.1999.81.5.2587. [DOI] [PubMed] [Google Scholar]
- 41.Kornau H C, Schenker L T, Kennedy M B, Seeburg P H. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 42.Steigerwald F, Schulz T W, Schenker L T, Kennedy M B, Seeburg P H, Köhr G. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barria A, Malinow R. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 44.Ito I, Sakimura K, Mishina M, Sugiyama H. Neurosci Lett. 1996;203:69–71. doi: 10.1016/0304-3940(95)12258-3. [DOI] [PubMed] [Google Scholar]
- 45.Tsumoto T, Freeman R D. Exp Brain Res. 1987;65:666–672. doi: 10.1007/BF00235990. [DOI] [PubMed] [Google Scholar]
- 46.Morales B, Choi S-Y, Kirkwood A. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant S G, O'Dell T. Curr Opin Neurobiol. 2001;11:363–368. doi: 10.1016/s0959-4388(00)00220-8. [DOI] [PubMed] [Google Scholar]
- 48.Quinlan E M, Olstein D H, Bear M F. Proc Natl Acad Sci USA. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dragoi V, Sharma J, Sur M. Neuron. 2000;28:287–298. doi: 10.1016/s0896-6273(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 50.Yao H, Dan Y. Neuron. 2001;32:315–323. doi: 10.1016/s0896-6273(01)00460-3. [DOI] [PubMed] [Google Scholar]
- 51.del Cerro S, Jung M, Lynch G. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- 52.Trepel C, Racine R J. Synapse. 2000;35:120–128. doi: 10.1002/(SICI)1098-2396(200002)35:2<120::AID-SYN4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 53.Turrigiano G G, Nelson S B. Curr Opin Neurobiol. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 54.Bi G, Poo M. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 55.Feldman D E. Nat Neurosci. 2000;3:303–304. doi: 10.1038/73849. [DOI] [PubMed] [Google Scholar]
- 56.Ramoa A S, Mower A F, Liao D, Jafri S I. J Neurosci. 2001;21:4299–4309. doi: 10.1523/JNEUROSCI.21-12-04299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mataga N, Nagai N, Hensch T K. Proc Natl Acad Sci USA. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Husseini A E, Schnell E, Chetkovich D M, Nicoll R A, Bredt D S. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 59.Katz L C, Callaway E M. Annu Rev Neurosci. 1992;15:31–56. doi: 10.1146/annurev.ne.15.030192.000335. [DOI] [PubMed] [Google Scholar]
- 60.Tavazoie S F, Reid R C. Nat Neurosci. 2000;3:608–616. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]