Abstract
Objective
To determine whether subtumoral injection of radiocolloid is useful for lymphoscintigraphic visualization of the internal mammary node and in sentinel lymph node (SLN) biopsy of the axilla in breast cancer patients.
Summary Background Data
The presence of retromammary lymphatics connecting to the axillary and internal mammary basins has been demonstrated by early anatomic studies. Thus, it is hypothesized that some lymph, especially that from the parenchyma under the tumor, may drain into both the axillary and internal mammary basins.
Methods
Patients (n = 196) with T1-2, N0 breast cancer underwent preoperative lymphoscintigraphy with radiocolloid (technetium 99m tin colloid) injection into various sites of the breast, followed by SLN biopsy using the combined method with blue dye. Patients were divided into four groups: group A (n = 41), peritumoral injection of both radiocolloid and blue dye; group B (n = 70), periareolar radiocolloid and peritumoral blue dye; group C (n = 45), intradermal radiocolloid and periareolar blue dye; and group D (n = 40), subtumoral radiocolloid and intradermal blue dye. A retrospective analysis of 1,297 breast cancer patients who underwent extended radical mastectomy with internal mammary node dissection was also conducted to determine the relationship between vertical tumor location (superficial or deep) and frequency of axillary and internal mammary node metastases.
Results
One patient (2%) in group A, 3 (4%) in group B, 0 (0%) in group C, and 15 (38%) in group D exhibited hot spots in the internal mammary region on lymphoscintigraphy (P < .001, group D vs. the other groups). The concordance rate of radiocolloid and blue dye methods in detection of SLNs in the axillary basin was significantly lower in group D than in the other groups. In contrast, the mismatch rate (some SLNs were identified by radiocolloid and other SLNs were identified by blue dye, but no SLN was identified by both in the same patient) was significantly higher in group D than in the other groups. In patients treated with extended radical mastectomy, positivity of axillary and internal mammary metastases was significantly higher in patients (n = 215) with deep tumors than those (n = 368) with superficial tumors.
Conclusions
These results suggest the presence of a retromammary lymphatic pathway from the deep portion of the breast to both axillary and internal mammary basins, which is distinct from the superficial pathway. Therefore, SLN biopsy with a combination of subtumoral and other (peritumoral, dermal, or areolar) injections of radiocolloid will improve both axillary and internal mammary nodal staging.
Since Halsted identified the internal mammary chain as a route of metastasis of breast cancer a century ago, many studies have confirmed that the internal mammary node (IMN) is a second regional basin in breast cancer. Metastases to this basin were studied intensively during the period from 1960 to 1980 when extended radical mastectomy, including IMN dissection, was a standard surgical procedure. This surgical procedure was later abandoned, however, because randomized trials failed to demonstrate the efficacy of IMN dissection in improving prognosis. 1–3 Since then, the IMN has been virtually ignored for two decades. However, recent development of the sentinel lymph node (SLN) biopsy technique has renewed our interest in the IMN, as focal accumulation of radioactivity in the IMN region is occasionally visualized in preoperative lymphoscintigraphy for SLN biopsy, 4,5 and because such information is considered valuable in deciding the indication for biopsy or adjuvant radiotherapy of the IMN. 6
According to earlier studies of lymphatic anatomy, some lymph from the breast gland is transported through the lymphatics penetrating the pectoralis major muscle into the internal mammary basin. 7,8 In our experience of SLN biopsy using peritumoral injection of blue dye, we have sometimes identified several blue-stained lymphatics penetrating the pectoralis major muscle when dissecting the breast parenchyma from the muscle. 9 In addition, Haagensen described another important lymphatic route from the breast comprising a vertical group of lymphatics extending from the dorsal surface of the breast to the axillary basin through the retromammary space. 10 Taken together, these observations lead us to hypothesize that some lymph, especially that from the parenchyma under the tumor, drains into both the IMN and axillary node. To test this hypothesis, we performed preoperative lymphoscintigraphy followed by SLN biopsy with injection of radiocolloid into the parenchyma underneath the tumor in breast cancer patients.
PATIENTS AND METHODS
Patients
After obtaining informed consent, 196 patients with T1-2, N0 breast cancer were enrolled in this study in the Department of Surgical Oncology, Osaka University Hospital, during the period August 1998 to December 2001. The study was approved by the Institutional Review Broad of the Osaka University Medical School. Patients who were pregnant or had previously been treated with radiotherapy or chemotherapy were excluded. This study was composed of four groups: group A (41 patients) received peritumoral injection of both radiocolloid and blue dye, group B (70 patients) received periareolar injection of radiocolloid and peritumoral injection of blue dye, group C (45 patients) received intradermal injection of radiocolloid and periareolar injection of blue dye, and group D (40 patients) received subtumoral injection of radiocolloid and intradermal injection of blue dye. Patient characteristics are summarized in Table 1. The four groups were similar with respect to age, menopausal status, tumor size, tumor location, biopsy type, and histology.
Table 1. CHARACTERISTICS OF PATIENTS WHO UNDERWENT LYMPHOSCINTIGRAPHY AND SENTINEL LYMPH NODE BIOPSY
DCIS, ductal carcinoma in situ.
A retrospective study of 1,297 breast cancer patients who underwent extended radical mastectomy with IMN dissection at Osaka Medical Center for Cancer and Cardiovascular Disease between May 1962 and January 1997 was conducted to determine the relationship between vertical tumor location (superficial or deep) and the frequency of axillary and IMN metastases.
Injection of Radiocolloid
On the day before surgery, 30 to 80 MBq technetium 99m (99mTc) tin colloid (Nihon Medi-Physics Co., Hyogo, Japan) in sterile saline (total volume 1–2 mL) was injected into four different sites (peritumoral, periareolar, intradermal, or subtumoral) after local anesthesia with 0.5 to 2 mL of 1% lidocaine. Peritumoral injection was placed at 3, 6, 9, and 12 o’clock positions into the parenchyma surrounding the tumor, at the same level as the tumor but not underneath the tumor. Periareolar injection was placed at 3, 6, 9, and 12 o’clock positions around the areola, and each injection was composed of a first intradermal injection of 0.25 mL radiocolloid or blue dye followed by a second subdermal injection of the same volume, as previously described. 9 Intradermal injection was placed, raising a wheal, at 3, 6, 9, and 12 o’clock positions into the skin overlying the tumor. Subtumoral injection was placed into one site of the parenchyma underneath the tumor (near the dorsal surface of the breast) using ultrasonographic guidance.
Lymphoscintigraphy
Lymphoscintigraphy was performed 1 to 2 hours after injection of radiocolloid. Anterior-oblique projections were obtained using a large-field stationary scintillation camera. If one or more focal accumulations of radioactivity (hot spots) were visualized, these were assumed to be SLNs.
Biopsy Procedure
With reference to lymphoscintigraphy, a hand-held gamma detection collimated probe (Navigator, US Surgical Co., Norwalk, CT) was used to identify the site of highest radioactivity before marking the skin incision. Just before surgery, 2 mL isosulfan blue dye (1% Lymphazurin, US Surgical Co.) was injected into three different sites (peritumoral, periareolar, or intradermal), and the injection site was massaged manually for about 5 minutes. The SLN was localized with a gamma detection probe, and the radioactivity of the SLN and axillary background (radioactivity of the axillary bed obtained after removal of the SLN) was recorded. SLN (hot) was defined as a lymph node with ex vivo radioactivity (counts per second) measuring at least 400% of that of the axillary background. In vivo radioactivity was not used to define the SLN as it was affected to a varying extent by artifact from the injection site. SLN (blue) was defined as a lymph node partially or completely stained by blue dye, or a lymph node connected to a blue-stained afferent lymphatic tract. After surgical excision of the SLN, the axillary bed was resurveyed by the gamma detection probe to confirm the absence of any residual SLN. When the SLN could not be identified or intraoperative frozen-section analysis of the SLN revealed metastases, complete axillary lymph node dissection was performed. Otherwise, SLN biopsy only was performed (without axillary lymph node dissection.
Internal mammary SLN biopsy was performed when high radioactivity in the internal mammary region was detected by the gamma detection probe, corresponding to a hot spot in the internal mammary region by lymphoscintigraphy, but complete IMN dissection was not performed.
Histopathologic Examination
Frozen-section analysis of the SLN in the axilla was performed intraoperatively. The largest cut surface of approximately 2 mm thickness was subjected to frozen-section analysis. Internal mammary SLNs were not submitted for intraoperative frozen section analysis as they were small in most cases. The remaining parts of the axillary SLN or a whole internal mammary SLN were fixed in 10% buffered formalin, processed overnight, and serially sectioned into slices of approximately 2 mm. All slices were embedded in paraffin and examined by hematoxylin and eosin (H&E) staining. All slices were also studied by immunohistochemistry (avidin-biotin-peroxidase method) using an anticytokeratin antibody AE1/3 (Histofine, Nichirei Co., Tokyo, Japan). Metastases were considered positive when a cluster or clusters of immunohistochemically positive cells were identified. Lymph nodes containing scattered positively stained single cells were not considered metastatic. The SLN was diagnosed as positive when the frozen section, paraffin section (H&E), or immunohistochemistry was positive. For histologic examination of the axillary non-SLNs, one representative paraffin section (H&E) was prepared from each lymph node.
In 1,297 patients treated with extended radical mastectomy, axillary nodes and IMNs were examined by one representative H&E section from each lymph node.
Statistical Analysis
Differences in categorical variables were analyzed by a chi-square test, and differences in the mean values of continuous variables were analyzed by Student t test. P < .05 was considered significant.
RESULTS
Lymphoscintigraphy
A focal accumulation of radioactivity (hot spot) was visualized by lymphoscintigraphy in 21 (51%), 60 (86%), 39 (87%), and 32 (80%) of patients in groups A, B, C, and D, respectively (Table 2). One patient in group A, three patients in group B, and no patients in group C exhibited hot spots in the internal mammary region. In contrast, as many as 15 patients (38%) exhibited hot spots in the internal mammary region in group D (Fig. 1). The incidence of hot spots in the internal mammary region was significantly (P < .001) higher in group D than in the other three groups.
Table 2. LYMPHOSCINTIGRAPHIC VISUALIZATION OF HOT SPOTS IN THE AXILLARY AND INTERNAL MAMMARY REGION
AN, axillary node; IMN, internal mammary node.
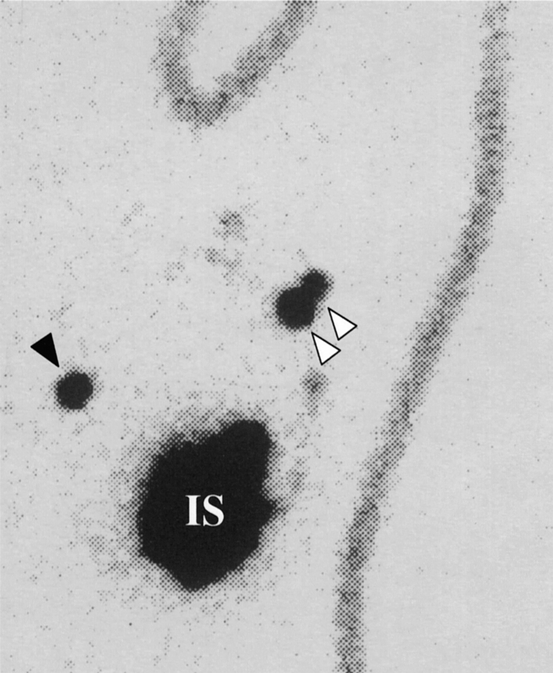
Figure 1. Lymphoscintigram. Focal accumulations of radioactivity (hot spots) are evident in both axillary (white arrowhead) and internal mammary (black arrowhead) basins. This patient with a tumor in the lower outer quadrant received radiocolloid injection into the subtumoral parenchyma. IS, injection site.
Four patients with a hot spot in the internal mammary region in groups A and B were found to have axillary lymph node metastases histologically. Biopsy of the IMN corresponding to the hot spot was performed in one of these four patients, and the biopsied IMN was found to be metastatic by histologic examination. In group D, 5 of 15 patients with a hot spot in the internal mammary region successfully underwent IMN biopsy, but no IMN metastases were detected histologically. All of these biopsied IMNs (SLNs in the internal mammary basin) were identified by radiocolloid but not by blue dye. In the remaining 10 patients with hot spots in the internal mammary region in group D, an SLN in the internal mammary basin could not be identified with an intraoperative gamma probe; thus, IMN biopsy was not performed. In group D, hot spots in the internal mammary region were observed regardless of the location of the primary tumor. Of the 15 patients with a hot spot in the internal mammary region, 5 had a tumor in the upper outer quadrant, 5 in the lower outer quadrant, 2 in the upper inner quadrant, and 3 in the lower inner quadrant. Interestingly, all four patients with hot spots only in the internal mammary region had tumors in the inner quadrants, whereas all 10 patients with tumors in the outer quadrants exhibited hot spots in both the internal mammary region and axilla.
Detection of SLN
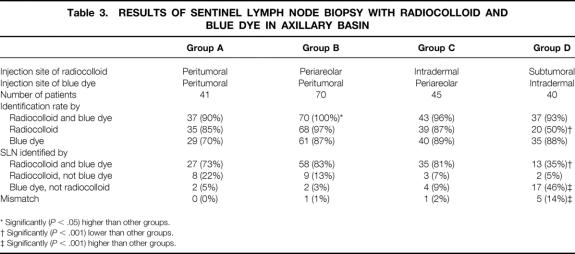
The detection rate of SLNs in the axillary basin was 90%, 100%, 96%, and 93% in groups A, B, C, and D, respectively. Successful SLN localization was significantly (P < .05) greater in group B than in other groups. The mean number of excised SLNs was 2.0 (range 1–4), 1.9 (range 1–5), 2.0 (range 1–6), and 2.5 (range 1–6) in groups A, B, C, and D, respectively. SLNs in the axillary basin were divided into four categories according to the technique by which the SLN was detected on a patient basis: SLNs detected by both radiocolloid and blue dye, radiocolloid but not blue dye, blue dye but not radiocolloid, and mismatch (Table 3). Mismatch means that some SLNs were identified by radiocolloid and other SLNs were identified by blue dye, but no SLN was identified by both in the same patient. There was no significant difference in the frequency of SLNs identified by both radiocolloid and blue dye between groups A, B, and C, but the frequency was significantly (P < .001) lower in group D (35%) than in the other three groups. In contrast, the incidence of mismatch was significantly (P < .001) higher in group D than in the other three groups.
Table 3. RESULTS OF SENTINEL LYMPH NODE BIOPSY WITH RADIOCOLLOID AND BLUE DYE IN AXILLARY BASIN
* Significantly (P < .05) higher than other groups.
† Significantly (P < .001) lower than other groups.
‡ Significantly (P < .001) higher than other groups.
Of the patients in whom SLNs could be detected in the axillary basin, lymph node metastases were identified histologically in 6 (15%), 19 (27%), 9 (20%), and 13 (33%) in groups A, B, C, and D, respectively (lymph node metastases were identified only by immunohistochemistry in 5, 2, and 3 patients in groups B, C, and D, respectively).
Relationship Between Vertical Tumor Location (Superficial or Deep) and Frequency of Axillary and IMN Metastases in Patients Treated With Extended Radical Mastectomy
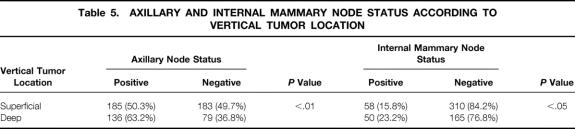
Patients with T1-2 tumors were classified into two groups depending on the vertical tumor location: tumors located in the superficial portion of the breast gland (superficial tumor group, n = 368) and in the deep portion of the breast gland (deep tumor group, n = 215). The superficial tumor group was defined by the presence of tumor invasion to the subcutaneous fat of the breast, and the deep tumor group was defined by the presence of tumor invasion to the retromammary fat. Patients with tumor invasion to both subcutaneous and retromammary fat, and those without tumor invasion to either areas, were excluded from this analysis. Patient characteristics in the superficial and deep tumor groups are summarized in Table 4. The two groups were similar with respect to age, menopausal status, tumor size, tumor location, and histology. Positivity for axillary node metastases and IMN metastases was significantly (P < .01 and P < .05, respectively) higher in the deep tumor group than in the superficial tumor group (Table 5).
Table 4. CHARACTERISTICS OF PATIENTS TREATED WITH EXTENDED RADICAL MASTECTOMY
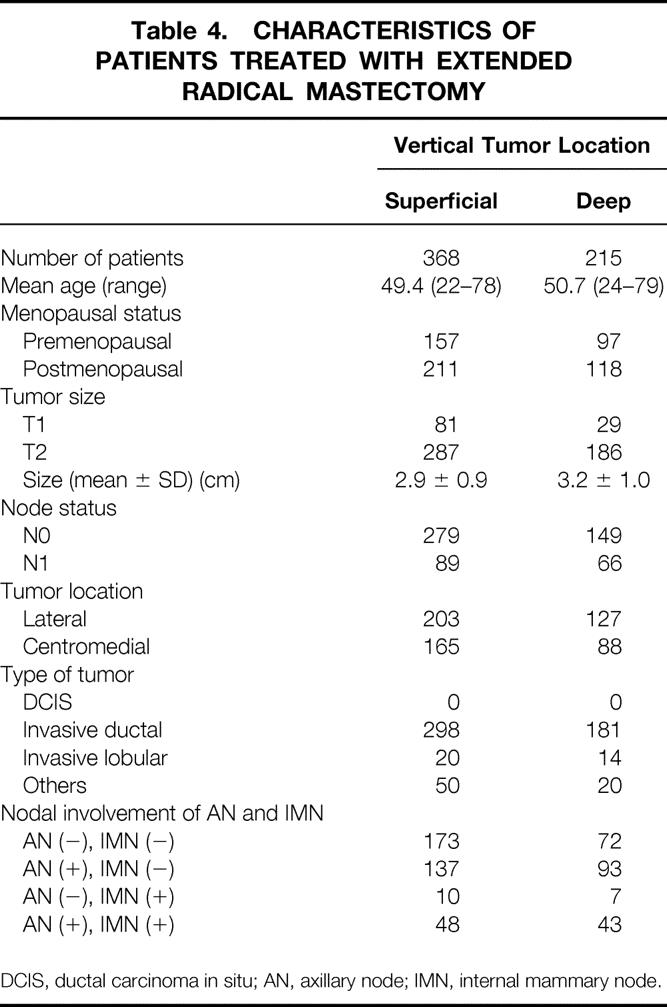
DCIS, ductal carcinoma in situ; AN, axillary node; IMN, internal mammary node.
Table 5. AXILLARY AND INTERNAL MAMMARY NODE STATUS ACCORDING TO VERTICAL TUMOR LOCATION
DISCUSSION
Recently, many studies have focused on the development of an optimal SLN biopsy technique using various sites for radiocolloid injection; that is, peritumoral (intraparenchymal surrounding the tumor), 11,12 intradermal, 13 subdermal, 14 subareolar, 15 periareolar, 9 and intratumoral. 16 These studies appear to indicate that both the identification rate of the SLN and the false-negative rate are essentially similar regardless of the injection site. Thus, it has been considered that radiocolloid, wherever injected, flows to the same SLN in the axilla. 15,17 Borgstein et al. have stated that the breast functions as a single biologic unit and that the skin and parenchyma of the breast share a common lymphatic drainage pathway to the axillary basin. 18
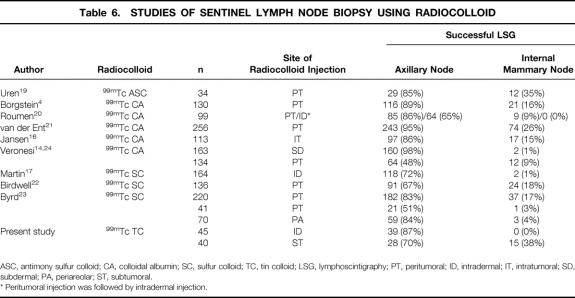
On the other hand, it has been reported that lymphoscintigrams obtained after peritumoral injection of radiocolloid occasionally reveal a hot spot in the internal mammary region, 4,17,19–23 whereas in contrast, lymphoscintigrams obtained by dermal (intradermal or subdermal) injection of radiocolloid rarely exhibit hot spots in the internal mammary region 14,17,20,24 (Table 6). Martin et al. reported that the lymphoscintigraphic visualization rate of hot spots in the internal mammary region was significantly higher after peritumoral injection of radiocolloid than after intradermal injection. 17 Similarly, Veronesi et al. reported that only 1% of patients exhibited hot spots in the internal mammary region after subdermal injection of radiocolloid. 14,24 Moreover, Roumen et al. compared peritumoral with intradermal injection in the same patients and showed that the hot spots in the internal mammary region visualized after peritumoral injection could not be visualized after intradermal injection. 20 These results suggest that the dermal lymphatic flow rarely directs to the internal mammary basin, but that some peritumoral (intraparenchymal) lymphatic flow can travel to it. 25
Table 6. STUDIES OF SENTINEL LYMPH NODE BIOPSY USING RADIOCOLLOID
ASC, antimony sulfur colloid; CA, colloidal albumin; SC, sulfur colloid; TC, tin colloid; LSG, lymphoscintigraphy; PT, peritumoral; ID, intradermal; IT, intratumoral; SD, subdermal; PA, periareolar; ST, subtumoral.
* Peritumoral injection was followed by intradermal injection.
Most breast parenchymal lymph is considered to flow to the axillary basin through the superficial lymphatics (superficial pathway), as there is a rich communication between the dermal and parenchymal lymphatics. It is speculated, however, that some breast parenchymal lymph, especially in the deep portion of the breast, might flow to the internal mammary basin via the retromammary lymphatics (retromammary pathway). 7,8,10 To investigate this hypothesis, we have performed the present study in which radiocolloid was injected into various sites, including the parenchyma underneath the tumor. The detection rate of hot spots in the internal mammary region by lymphoscintigraphy was significantly higher in the subtumoral injection group than in the peritumoral, periareolar, and intradermal injection groups, indicating the presence of lymphatic flow from the deep portion of the breast to the internal mammary basin.
The concordance rate of radiocolloid and blue dye methods in the detection of SLNs was similar between groups A, B, and C (see Table 3), in which radiocolloid and blue dye were injected peritumorally, intradermally, or periareolarly, respectively. Thus, lymph from the peritumoral breast parenchyma, dermis above the tumor, and areola is considered to mostly flow into the same SLN via the superficial pathway. In contrast, the subtumoral injection group showed a significantly lower concordance rate. Since blue dye was injected intradermally in the subtumoral injection group (group D), these results suggest the presence of a lymphatic pathway (retromammary pathway) from the deep portion of the breast to the axillary basin, which is distinct from the superficial pathway. The significantly high incidence of mismatch of radiocolloid and blue dye methods in the detection of SLNs in the subtumoral radiocolloid injection group is noteworthy in consideration of the reason for false-negative results in SLN biopsy. False-negative results are most problematic in SLN biopsy because they lead to incorrect nodal staging and thus to inappropriate decision-making about adjuvant therapies. 26 In the learning phase, most false-negative results are caused by technical failures. 27 As surgeons gain sufficient experience, the false-negative rate usually decreases gradually to a low level of around 5%, but never to 0% even in the hands of skillful surgeons. 28,29 We feel that this phenomenon can be explained, at least in part, by our findings that there are two different lymphatic pathways to the axillary basin; that is, superficial and retromammary pathways. Since radiocolloid and/or blue dye are injected peritumorally, intradermally, or periareolarly in routine SLN biopsy, SLNs receiving lymph via the superficial pathway can be detected, but SLNs receiving lymph via the retromammary pathway cannot. Thus, if tumor cells metastasize to the axillary basin via the retromammary pathway but not the superficial pathway, routine SLN biopsy, which can detect only SLNs receiving lymph via the superficial pathway, is very likely to lead to a false-negative result. However, the fact that the false-negative rate is generally very low (<5%) appears to indicate that, in most cases, tumor cells metastasize to the axillary basin through the superficial pathway, or that SLNs receiving lymph via the superficial pathway and SLNs receiving lymph via the retromammary pathway are identical. Nonetheless, our present findings suggest the possibility that SLN biopsy using subtumoral injection (retromammary pathway) of radiocolloid in combination with peritumoral, dermal, or periareolar injection (superficial pathway) might be clinically useful in eliminating false-negative results.
Tumors located superficially within the breast gland mostly disseminate tumor cells through the superficial pathway to the axillary basin. In contrast, tumors located deep in the breast gland are speculated to disseminate tumor cells through both the superficial and retromammary lymphatic pathways—that is, the superficial pathway carries tumor cells to the axillary basin and the retromammary pathway carries tumor cells to both the axillary and internal mammary basins. To verify this speculation and to elucidate the clinical significance of these two pathways in the establishment of axillary and IMN metastases, we investigated the relationship between vertical tumor location (superficial or deep) and the frequency of axillary and IMN metastases in breast cancer patients who underwent extended radical mastectomy with IMN dissection. We showed first that axillary lymph node positivity was significantly higher in the deep tumor than the superficial tumor group and second that IMN positivity was also significantly higher in the deep tumor than the superficial tumor group (see Table 5). The first observation is consistent with our speculation that deep tumors use both the superficial and retromammary pathways to the axillary basin, whereas superficial tumors use only the superficial pathway. The second observation is also consistent with our speculation that the retromammary pathway is more important in the establishment of IMN metastases than the superficial pathway.
Under physiologic conditions, dermally injected radiocolloid usually drains to the axillary basin but not to the internal mammary basin; however, it can drain outside the axilla when the main flow to the axillary basin is blocked by gross lymph node metastases. In the present study of 1,297 patients treated with extended radical mastectomy, IMN metastasis positivity was consistently significantly (P < .0001) higher in axillary node-positive patients (35.4%) than in axillary node-negative patients (4.4%) (data not shown). Moreover, all three patients who received periareolar radiocolloid injection and exhibited internal mammary region hot spots had massive involvement of the axillary lymph nodes. Halsell et al. also reported that obstruction of lymphatic vessels to the axilla resulted in collateral flow to extra-axillary sites. 30 Under such nonphysiologic conditions, even dermally injected radiocolloid can flow into the internal mammary basin.
Uren et al. reported that the peritumoral injection of 99mTc-antimony sulfur colloid resulted in a high incidence (35%) of IMR hot spots by lymphoscintigraphy (see Table 6). 19 The behavior of radiocolloid is strongly dependent on particle size. 31 A small particle colloid can easily pass into the lymphatics, but the risk of labeling the non-SLN increases over time and the colloid is retained in lymph nodes for only a short time. The particle size of 99mTc-antimony sulfur colloid (ranging from 3–12 nm) is almost identical to that of 198Au colloid, which was used in earlier studies that revealed internal mammary region hot spots in all cases. 8 Uren et al. 19 also reported hot spots in the supraclavicular region in 12% of patients. Therefore, the particle size of 99mTc-antimony sulfur colloid is considered too small for use in SLN biopsy. In the current study, we used 99mTc-tin colloid. The particle size of 99mTc-tin colloid (ranging from 400–5,000 nm) is larger than that of 99mTc-sulfur colloid (ranging from 50–1,000 nm), which is commonly used in the United States but is not commercially available in Japan. A large-particle colloid does not enter the lymphatics easily, but once trapped in the lymph node, it is retained for a relatively long time, and the risk of labeling the non-SLN is low. Studies using injection of 99mTc-sulfur colloid or 99mTc-tin colloid into the peritumoral parenchyma reported that internal mammary region hot spots were detected in no more than 20% of patients. The particle size of 99mTc-colloidal albumin is smaller than that of 99mTc-sulfur colloid and 99mTc-tin colloid but larger than that of 99mTc-antimony sulfur colloid. Accordingly, a few studies using 99mTc-colloidal albumin reported internal mammary region hot spots in more than 20% of the patients (see Table 6). Another technical issue that can affect the frequency of internal mammary region hot spots is how the radiocolloid is injected peritumorally. Radiocolloid is usually injected into the breast parenchyma surrounding the tumor at the same level of the tumor, but not into the parenchyma underneath the tumor. It is, however, possible that the radiocolloid can be accidentally injected into the parenchyma underneath the tumor. Thus, technical differences in radiocolloid injection might partially explain the variation in the frequency of internal mammary region hot spot detection, although the injection technique is not described in detail in most reports.
Four percent to 18% of breast cancer patients with negative axillary nodes are known to have IMN involvement. 32 SLN biopsy in the internal mammary basin would be especially useful for these patients since their prognosis is underestimated if only an axillary SLN biopsy, but not an internal mammary SLN biopsy, is performed. However, before clinical application of internal mammary SLN biopsy, a study (involving SLN biopsy followed by complete IMN dissection) is required to confirm that internal mammary region hot spots actually correspond to the internal mammary SLN that accurately reflects the nodal status of this basin. In addition, our low intraoperative identification rate (33%) of the internal mammary SLN, even in cases where the internal mammary region hot spot was visualized by lymphoscintigraphy, remains a problem, as the axillary SLN can be identified intraoperatively in almost all cases where the axillary hot spot is visualized by lymphoscintigraphy. We speculate that the retention time of radiocolloid differs between the axillary and internal mammary SLNs—that is, radiocolloid is retained for a shorter time in the internal mammary SLN than in the axillary SLN. In the present study, the internal mammary SLN biopsy was performed the day after lymphoscintigraphy, whereas the interval between lymphoscintigraphy and the SLN biopsy would be better shortened so as to increase the identification rate of the internal mammary SLN.
Several clinical trials have failed to demonstrate a survival advantage after IMN dissection. 1–3 However, data derived from these trials may not be readily applicable to current breast cancer patients because of the recent development of effective adjuvant therapies and radiation technology with far lower morbidity than previously. Recently, it has been reported that postmastectomy radiation therapy, including internal mammary field radiation, has significantly improved survival. 33–35 A multicenter randomized trial investigating the efficacy of radiotherapy to the internal mammary basin (European Organization for Research and Treatment of Cancer No. 22922) is ongoing. We feel that, ideally, treatment of the IMN should be planned on the basis of IMN status diagnosed by SLN biopsy in the internal mammary basin, and subtumoral injection of radiocolloid appears to be useful for this purpose.
In conclusion, our results suggest that there are two main lymphatic pathways, superficial and retromammary, in the breast gland, and that the superficial pathway carries tumor cells to the axillary basin and the retromammary pathway carries tumor cells to both the axillary basin and internal mammary basin. Subtumoral injection of radiocolloid appears to be useful in the detection of SLNs in the internal mammary basin; however, due to its low detection rate of SLNs in the axillary basin, subtumoral injection is unlikely to replace the other injections (peritumoral, dermal, or areolar). Rather, subtumoral injection would better be used in combination with another injection site, preferably dermal or areolar because of their high detection rate of SLNs in the axillary basin compared to peritumoral injection. 9,17 We anticipate that SLN biopsy using this combined injection method will facilitate diagnosis of axillary and IMN status with greater accuracy and will reduce the incidence of false-negative results.
Footnotes
Supported in part by a Grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Correspondence: Shinzaburo Noguchi, MD, Department of Surgical Oncology, Osaka University Medical School, 2-2-E10 Yamada-oka, Suita, Osaka 565-0871, Japan.
E-mail: noguchi@onsurg.med.osaka-u.ac.jp
Accepted for publication August 2, 2002.
References
- 1.Veronesi U, Valagussa P. Inefficacy of internal mammary nodes dissection in breast cancer surgery. Cancer. 1981; 47: 170–175. [DOI] [PubMed] [Google Scholar]
- 2.Lacour J, Le M, Caceres E, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection. Ten-year results of an international cooperative trial in breast cancer. Cancer. 1983; 51: 1941–1943. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Marubini E, Mariani L, et al. The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. Eur J Cancer. 1999; 35: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 4.Borgstein PJ, Pijpers R, Comans EF, et al. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998; 186: 275–283. [DOI] [PubMed] [Google Scholar]
- 5.Hill AD, Tran KN, Akhurst T, et al. Lessons learned from 500 cases of lymphatic mapping for breast cancer. Ann Surg. 1999; 229: 528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson N, Soot L, Nelson J, et al. Sentinel node biopsy and internal mammary lymphatic mapping in breast cancer. Am J Surg. 2000; 179: 386–388. [DOI] [PubMed] [Google Scholar]
- 7.Turner-Warwick R. The lymphatics of the breast. Br J Surg. 1959; 46: 574–582. [DOI] [PubMed] [Google Scholar]
- 8.Hultborn KA, Larsson LG, Ragnhult I. The lymph drainage from the breast to the axillary and parasternal lymph nodes, studied with the aid of colloidal AU198. Acta Radiol. 1955; 43: 52–64. [DOI] [PubMed] [Google Scholar]
- 9.Shimazu K, Tamaki Y, Taguchi T, et al. Comparison between periareolar and peritumoral injection of radiotracer for sentinel lymph node biopsy in patients with breast cancer. Surgery. 2002; 131: 277–286. [DOI] [PubMed] [Google Scholar]
- 10.Haagensen CD. Anatomy of the mammary glands. In: Disease of the breast. Philadelphia: WB Saunders; 1986: 1–46.
- 11.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer—a multicenter validation study. N Engl J Med. 1998; 339: 941–946. [DOI] [PubMed] [Google Scholar]
- 12.Cox CE, Pendas S, Cox JM, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998; 227: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan DC, Hill AD, Akhurst T, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimize sentinel node identification in breast cancer patients. Ann Surg Oncol. 1999; 6: 450–454. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997; 349: 1864–1867. [DOI] [PubMed] [Google Scholar]
- 15.Klimberg VS, Rubio IT, Henry R, et al. Subareolar versus peritumoral injection for location of the sentinel lymph node. Ann Surg. 1999; 229: 860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen L, Doting MH, Rutgers EJ, et al. Clinical relevance of sentinel lymph nodes outside the axilla in patients with breast cancer. Br J Surg. 2000; 87: 920–925. [DOI] [PubMed] [Google Scholar]
- 17.Martin RC, Derossis AM, Fey J, et al. Intradermal isotope injection is superior to intramammary in sentinel node biopsy for breast cancer. Surgery. 2001; 130: 432–438. [DOI] [PubMed] [Google Scholar]
- 18.Borgstein PJ, Meijer S, Pijpers RJ, et al. Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg. 2000; 232: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uren RF, Howman-Giles RB, Thompson JF, et al. Mammary lymphoscintigraphy in breast cancer. J Nucl Med. 1995; 36: 1775–1780. [PubMed] [Google Scholar]
- 20.Roumen RM, Geuskens LM, Valkenburg JG. In search of the true sentinel node by different injection techniques in breast cancer patients. Eur J Surg Oncol. 1999; 25: 347–351. [DOI] [PubMed] [Google Scholar]
- 21.van der Ent FW, Kengen RA, van der Pol HA, et al. Halsted revisited: internal mammary sentinel lymph node biopsy in breast cancer. Ann Surg. 2001; 234: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birdwell RL, Smith KL, Betts BJ, et al. Breast cancer: variables affecting sentinel lymph node visualization at preoperative lymphoscintigraphy. Radiology. 2001; 220: 47–53. [DOI] [PubMed] [Google Scholar]
- 23.Byrd DR, Dunnwald LK, Mankoff DA, et al. Internal mammary lymph node drainage patterns in patients with breast cancer documented by breast lymphoscintigraphy. Ann Surg Oncol. 2001; 8: 234–240. [DOI] [PubMed] [Google Scholar]
- 24.Paganelli G, Cicco CD. Sentinel node biopsy. Reply to Bourgeois et al. Eur J Nucl Med. 1998; 25: 1589–1590. [Google Scholar]
- 25.Cserni G, Pap Szekeres J. Internal mammary lymph nodes and sentinel node biopsy in breast cancer. Surg Oncol. 2001; 10: 25–33. [DOI] [PubMed] [Google Scholar]
- 26.McMasters KM, Giuliano AE, Ross MI, et al. Sentinel-lymph-node biopsy for breast cancer—not yet the standard of care. N Engl J Med. 1998; 339: 990–995. [DOI] [PubMed] [Google Scholar]
- 27.Tafra L, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001; 233: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMasters KM, Wong SL, Martin RC 2nd, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001; 233: 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veronesi U, Paganelli G, Viale G, et al. Sentinel lymph node biopsy and axillary dissection in breast cancer: results in a large series. J Natl Cancer Inst. 1999; 91: 368–373. [DOI] [PubMed] [Google Scholar]
- 30.Halsell JT, Smith JR, Bentlage CR, et al. Lymphatic drainage of the breast demonstrated by vital dye staining and radiography. Ann Surg. 1965; 162: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulec SA, Moffat FL, Carroll RG, et al. Gamma probe guided sentinel node biopsy in breast cancer. Q J Nucl Med. 1997; 41: 251–261. [PubMed] [Google Scholar]
- 32.Klauber-DeMore N, Bevilacqua JL, Van Zee KJ, et al. Comprehensive review of the management of internal mammary lymph node metastases in breast cancer. J Am Coll Surg. 2001; 193: 547–555. [DOI] [PubMed] [Google Scholar]
- 33.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;15;353:1641–1648. [DOI] [PubMed]
- 34.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997; 337: 949–955. [DOI] [PubMed] [Google Scholar]
- 35.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997; 337: 956–962. [DOI] [PubMed] [Google Scholar]