Abstract
Objective
To evaluate whether the prognosis of the four categories of patients with hepatocellular carcinoma (HCC) classified as stage IVA in the tumor-node-metastasis (TNM) classification of the International Union Against Cancer (UICC) is homogeneous.
Summary Background Data
Hepatic resection has been proposed as the treatment of choice for patients with TNM stage IVA HCC, which consists of four different categories. It is unknown whether the prognosis of the four categories of patients is homogeneous.
Methods
Clinicopathologic and follow-up data of 106 patients with resection of stage IVA HCC from 1989 to 2000 were prospectively collected. Survival results of the four categories of stage IVA patients were compared.
Results
Among stage IVA patients, survival was significantly worse in those with tumors involving a major branch of the portal or hepatic veins than in those with tumors invading adjacent organs, bilobar multiple tumors, or perforated visceral peritoneum. There were no significant differences in survival among the latter three groups. By Cox regression analysis, invasion of the portal or hepatic veins and presence of cirrhosis were independent adverse prognostic factors of overall survival among stage IVA patients, and invasion of the portal or hepatic veins was the only significant adverse prognostic factor of disease-free survival.
Conclusions
The prognosis of the four categories of patients with stage IVA HCC under the current UICC TNM staging was not homogeneous. A refined classification of stage IV HCC is needed to take into consideration the worse prognosis associated with tumor invasion of a major branch of the portal or hepatic veins.
Hepatocellular carcinoma (HCC) is a common malignancy in Africa and Asia, and its incidence is also rising in Western countries. 1 Better imaging techniques and widespread application of screening programs have resulted in earlier diagnosis of HCC and improved prognosis. 2 However, many patients still present with metastatic or locally advanced HCC. The presence of distant metastasis, which is stage IVB disease according to the tumor-node-metastasis (TNM) classification for primary liver cancer devised by the International Union Against Cancer (UICC), 3 is generally considered a contraindication for hepatic resection. In contrast, surgical resection has been advocated as the treatment of choice for patients with locally advanced HCC that is classified as stage IVA. 4–6 For such patients, other treatment modalities such as local ablative therapy and liver transplantation are usually not indicated because of vascular invasion and large tumor size. 6–8
A few studies have evaluated the prognosis after resection of stage IVA HCC, 5,6,9 but these studies were based on the old UICC TNM classification published in 1987, in which only multiple tumors in more than one lobe and tumors involving a major branch of the portal or hepatic veins were considered stage IVA. 10 The UICC TNM classification was revised in 1997 to include tumor invasion of adjacent organs other than the gallbladder and perforation of visceral peritoneum in stage IVA disease, in addition to the two aforementioned categories. 3 However, it is unknown if these four categories are comparable in terms of prognosis. This study was performed to evaluate whether the long-term survival results were homogeneous among the four categories of HCC patients classified as stage IVA under the current UICC TNM classification.
PATIENTS AND METHODS
Between January 1989 and December 2000, 121 patients with stage IVA HCC defined according to the 1997 UICC pathologic TNM (pTNM) classification 3 underwent hepatic resection with a curative intent, defined as macroscopically complete tumor resection, in the Department of Surgery of the University of Hong Kong. During the same period, 409 patients with stages I to III HCC underwent curative hepatic resection in the same department.
Preoperative imaging studies included chest radiography, abdominal ultrasonography, computed tomography (CT) scan, and in some cases hepatic arteriography. The absolute criteria for resection were absence of extrahepatic metastasis and absence of main portal vein or inferior vena cava tumor thrombus. Tumors with involvement of the ipsilateral branch of the portal or hepatic veins or invasion of adjacent organs were considered resectable provided that all the tumor tissue could be encompassed by an en bloc resection with a clear margin. Multiple tumors in more than one lobe were resected en bloc by extended right or left hepatectomy if the position of the tumors and liver function reserve were favorable; otherwise, separate resections of the right and left lobe tumors were performed. Patients with ruptured HCC were managed conservatively if stable or by transarterial embolization if unstable, and then were offered elective resection if the tumor was resectable. 11
Liver function reserve was assessed by liver biochemistry, Child-Pugh grading, 12 and measurement of indocyanine green retention rate at 15 minutes. 13 Only Child-Pugh class A patients were offered major hepatic resection, defined as resection of three or more segments of the liver according to Couinaud’s classification. 14 Selected Child-Pugh class B patients underwent surgery if the tumor was resectable by a minor hepatic resection, defined as resection of two or fewer segments of the liver.
Details of the operative techniques have been described elsewhere. 15 Since June 1994, laparoscopy and laparoscopic ultrasound have been performed routinely to confirm resectability immediately before laparotomy. 16 In addition to evaluation of the tumor status, laparoscopy allowed assessment of the liver remnant size and the severity of cirrhosis when major hepatic resection was contemplated. Intraoperative ultrasound was routinely performed to detect additional tumors or tumor invasion into a major branch of the portal or hepatic veins, and to mark the line of parenchymal transection for a tumor-free resection margin. Operative mortality was defined as death within 30 days of operation, and hospital mortality was defined as death that occurred within the same admission after surgery. All resected specimens were examined by experienced pathologists for histopathologic features that may have prognostic value. 17
All patients were followed up by the surgical team monthly in the first year and every 3 months thereafter. Serum alpha-fetoprotein (AFP) level was monitored, and abdominal ultrasonography or CT scan was performed every 3 months. Diagnosis of recurrence was based on elevated AFP level and typical imaging findings on the CT scan. Arteriography or percutaneous fine-needle aspiration cytology was performed if necessary. Patients with intrahepatic recurrence and satisfactory liver function reserve underwent re-resection if feasible. Otherwise, they were treated with transarterial chemoembolization (TACE) or percutaneous ethanol injection. 18
All clinicopathologic data were prospectively collected in a computerized database, and follow-up data were regularly updated for each patient. For the purposes of this study, the pTNM stage of each patient in the database was reviewed and reclassified according to the fifth edition of the UICC TNM classification published in 1997. 3 Patients with stage IVA HCC were further stratified according to the following four T4 categories: those with tumor involvement of a major branch of the portal or hepatic veins; those with tumor invasion of adjacent organs other than the gallbladder; those with multiple bilobar tumors; and those with perforated visceral peritoneum (i.e., ruptured tumor). Fifteen stage IVA patients who had more than one of the above features were excluded from analysis in this study. The remaining 106 patients with stage IVA HCC were the subjects of this study.
Continuous data were expressed as mean ± standard deviation and compared using the unpaired t test. Categorical variables were compared using the chi-square test with Yates correction or the Fisher exact test where appropriate. Survival was calculated by the Kaplan-Meier method and compared using the log-rank test. Hospital deaths were included in overall survival analysis but were excluded from disease-free survival analysis. Clinicopathologic variables of potential prognostic value in patients with stage IVA HCC were analyzed for their effects on overall and disease-free survival. Multivariate analysis was performed using the Cox proportional hazards model to identify independent prognostic factors. All statistical analyses were performed using statistical software (SPSS 9.05 for Windows, SPSS Inc., Chicago, IL). Differences were considered significant at P < 0.05.
RESULTS
Of the 106 patients with stage IVA HCC, 20 had tumor involvement of a major branch of the portal or hepatic veins, 27 had tumor invasion of adjacent organs other than the gallbladder, 19 had multiple bilobar tumors, and 40 had perforated visceral peritoneum (i.e., ruptured tumor). Only two patients belonged to Child-Pugh class B; the other 104 were Child-Pugh class A. We did not routinely give preoperative TACE as a neoadjuvant treatment, but eight patients with stage IVA HCC had initial TACE followed by resection after reduction in tumor size. Postoperative adjuvant chemotherapy was not given, except for 10 patients who were either recruited in a randomized trial of adjuvant transarterial and systemic chemotherapy that demonstrated no benefit of the therapy 19 or were given transarterial chemotherapy because of a positive microscopic margin. 20 The follow-up period of this study ended on December 31, 2001. Thus, all patients had at least 1 year of observation after surgery. The median follow-up was 65 months (range 12–146).
Survival Comparisons: Stage IVA Versus Stages I, II, III
Among the 106 patients with stage IVA HCC, hospital mortality after hepatic resection was 5.7% (n = 6). The hospital mortality rates of patients with stage I HCC (n = 38), stage II HCC (n = 192), and stage III HCC (n = 179) were 2.6%, 3.1%, and 8.9%, respectively. The hospital mortality rates of stage III and IVA patients were slightly higher than those of stage I and II patients, but the differences between each stage were not statistically significant. Forty-eight patients with stage IVA HCC had cirrhosis, whereas the other 58 patients had noncirrhotic livers. Among cirrhotic patients with stage IVA HCC, there were four hospital deaths after operation due to intra-abdominal sepsis (n = 1), intra-abdominal bleeding (n = 1), or liver failure (n = 2). There were two hospital deaths due to intra-abdominal sepsis among the noncirrhotic patients with stage IVA HCC. There was no significant difference in the hospital mortality between cirrhotic and noncirrhotic patients with stage IVA HCC (8.3% vs. 3.4%, P = .41).
Overall survival of 106 stage IVA patients was significantly worse than that of stage I, II, or III patients (P < .001). The 1-, 3-, and 5-year survival rates of the 106 stage IVA patients were 59.0%, 28.1%, and 17.8%, respectively. The 5-year survival rates of stage I (n = 38), II (n = 192), and III (n = 179) patients were 78.0%, 64.7%, and 32.2%, respectively. Similarly, the disease-free survival of stage IVA patients was significantly worse than that of stage I, II, or III patients (P < .001). Excluding the hospital deaths, the 1-, 3-, and 5-year disease-free survival rates of 100 stage IVA patients were 29.3%, 12.9%, and 7.2%, respectively. The 5-year disease-free survival rates of stage I (n = 37), II (n = 186), and III (n = 161) patients were 50.9%, 38.0%, and 14.4%, respectively.
Survival Comparisons: Four Categories of Stage IVA HCC
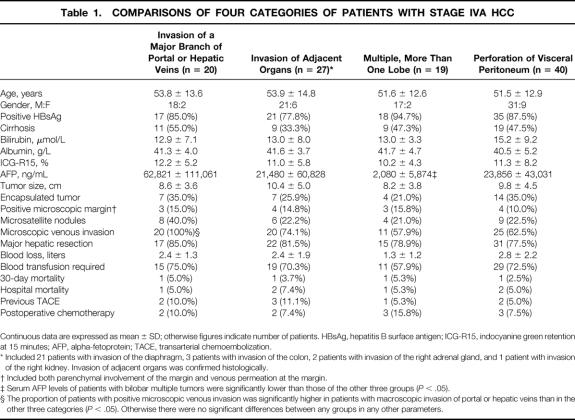
Comparisons of clinical, histologic, and operative data of the four categories of patients with stage IVA HCC are depicted in Table 1. The four groups were not significantly different in liver function parameters and tumor histologic features, except that there was a significantly higher proportion of microscopic venous invasion in tumors involving a major branch of the portal or hepatic veins than the other three categories. Serum AFP levels were significantly lower in patients with bilobar multiple tumors compared with the other three groups. Serum AFP levels were the highest in patients with tumors involving a major branch of the portal or hepatic veins, but the differences from the serum AFP levels of patients with tumor invasion of adjacent organs or ruptured tumors were not significant. The majority of patients in all four groups underwent major hepatic resection. Intraoperative blood loss, the proportion of patients requiring blood transfusion, and operative and hospital mortality rates were all similar.
Table 1. COMPARISONS OF FOUR CATEGORIES OF PATIENTS WITH STAGE IVA HCC
Continuous data are expressed as mean ± SD; otherwise figures indicate number of patients. HBsAg, hepatitis B surface antigen; ICG-R15, indocyanine green retention at 15 minutes; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization.
* Included 21 patients with invasion of the diaphragm, 3 patients with invasion of the colon, 2 patients with invasion of the right adrenal gland, and 1 patient with invasion of the right kidney. Invasion of adjacent organs was confirmed histologically.
† Included both parenchymal involvement of the margin and venous permeation at the margin.
‡ Serum AFP levels of patients with bilobar multiple tumors were significantly lower than those of the other three groups (P < .05).
§ The proportion of patients with positive microscopic venous invasion was significantly higher in patients with macroscopic invasion of portal or hepatic veins than in the other three categories (P < .05). Otherwise there were no significant differences between any groups in any other parameters.
Figure 1 shows the overall survival curves of the four categories of stage IVA patients. The overall survival of patients with tumors involving a major branch of the portal or hepatic veins (median 6.0 months) was significantly worse than that of patients with tumor invasion of adjacent organs (median 15.1 months, P = .04), bilobar multiple tumors (median 25.7 months, P = .02), or perforation of visceral peritoneum (median 19.0 months, P = .04). There were no significant differences among the latter three groups.
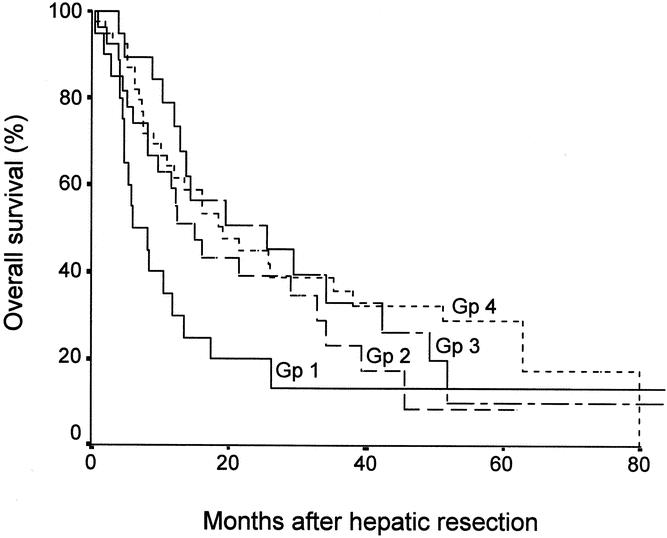
Figure 1. Overall survival curves of four groups of patients with stage IVA HCC. Gp 1, tumors involving a major branch of the portal or hepatic veins; Gp 2, invasion of adjacent organs; Gp 3, multiple tumors in more than one lobe; Gp 4, perforation of visceral peritoneum. P < .05 for comparisons between Gp 1 and Gp 2, 3, or 4; no significant differences among Gp 2, 3, and 4.
Figure 2 shows the disease-free survival curves of the four categories of patients after excluding hospital deaths. There were no significant differences among the four groups, but there was a trend towards earlier recurrence in patients with tumor invasion of a major branch of the portal or hepatic veins compared with the other three groups. The 1-year disease-free survival in patients with invasion of the portal or hepatic veins was 16.7% compared to 34.6% in patients with invasion of adjacent organs, 31.6% in patients with bilobar multiple tumors, and 32.6% in patients with ruptured tumors. By the time of analysis, 18 (94.7%) of 19 patients with tumors involving a major branch of the portal or hepatic veins, 21 (84.0%) of 25 patients with tumor invasion of adjacent organs, 15 (83.3%) of 18 patients with bilobar tumors, and 32 (84.2%) of 38 patients with ruptured tumors had developed recurrent disease. In all four categories, the majority of recurrences occurred in the liver remnant (n = 16 in patients with major venous invasion, n = 17 in patients with tumor invasion of adjacent organs, n = 12 in patients with bilobar tumors, n = 25 in patients with ruptured tumors). Among the patients who developed intrahepatic recurrence, there was a higher proportion of multifocal recurrences in patients with tumor invasion of a major branch of the portal or hepatic veins (n = 12 [75.0%]) than in patients with tumor invasion of adjacent organs (n = 6 [35.3%]), patients with bilobar tumors (n = 5 [41.7%]), or patients with ruptured tumors (n = 10 [40.0%]).
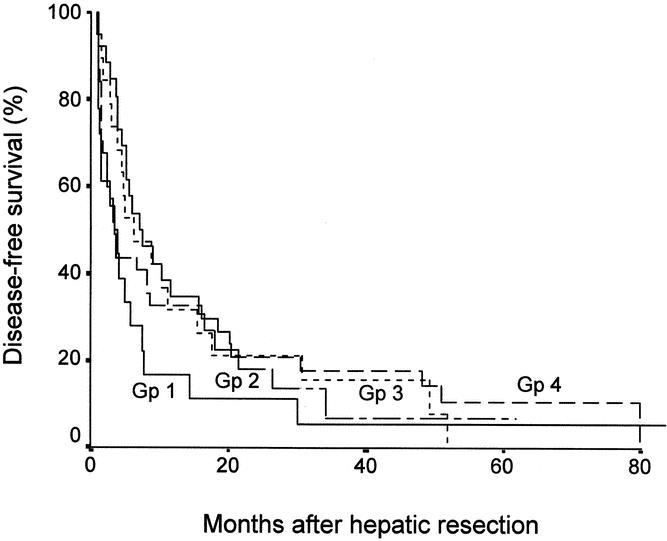
Figure 2. Disease-free survival curves of four groups of patients with stage IVA HCC. Gp 1, tumors involving a major branch of the portal or hepatic veins; Gp 2, invasion of adjacent organs; Gp 3, multiple tumors in more than one lobe; Gp 4, perforation of visceral peritoneum. No significant differences between any groups.
Prognostic Factors of Survival
Tables 2 and 3 summarize the results of univariate analysis of the influence of 12 clinical factors and 10 pathologic factors, respectively, on the overall survival among the 106 patients with stage IVA HCC. The four pathologic features characterizing stage IVA HCC were included in the analysis. Presence of cirrhosis, serum bilirubin level more than 20 μmol/L, and serum AFP level more than 1,000 ng/mL correlated with worse survival. Tumor invasion of a major branch of the portal or hepatic veins was the only pathologic factor that was associated with significantly worse survival.
Table 2. UNIVARIATE ANALYSIS OF INFLUENCE OF CLINICAL FACTORS ON OVERALL SURVIVAL IN PATIENTS WITH STAGE IVA HCC
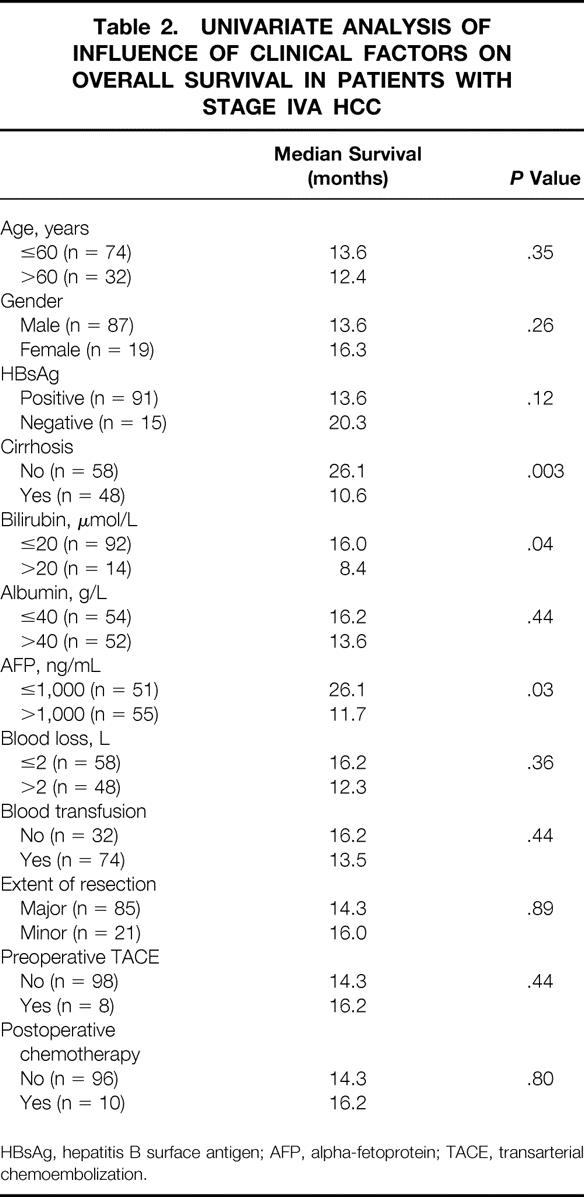
HBsAg, hepatitis B surface antigen; AFP, alpha-fetoprotein; TACE, transarterial chemoembolization.
Table 3. UNIVARIATE ANALYSIS OF INFLUENCE OF PATHOLOGIC FACTORS ON OVERALL SURVIVAL IN PATIENTS WITH STAGE IVA HCC
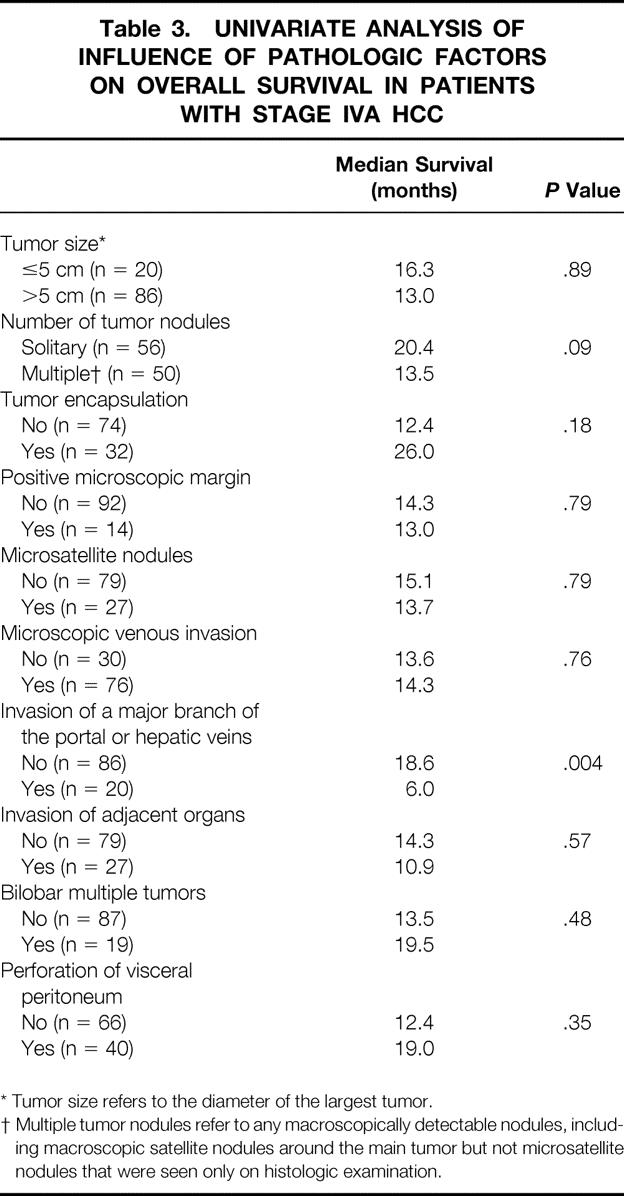
* Tumor size refers to the diameter of the largest tumor.
† Multiple tumor nodules refer to any macroscopically detectable nodules, including macroscopic satellite nodules around the main tumor but not microsatellite nodules that were seen only on histologic examination.
By multivariate analysis, only tumor invasion of a major branch of the portal or hepatic veins (risk ratio [RR] 2.093, 95% confidence interval [CI] 1.412–3.342, P < .001) and presence of cirrhosis (RR 1.814, 95% CI 1.148–2.865, P = .007) were independent predictors of adverse overall survival among patients with stage IVA HCC. The 1-, 3-, and 5-year survival rates of 20 patients with tumor thrombus in a major branch of the portal or hepatic veins were 30.0%, 13.3%, and 13.3%, respectively, whereas the corresponding survival rates of 86 patients without tumor thrombus in a major venous branch were 65.8%, 31.4%, and 19.1%, respectively. The 1-, 3-, and 5-year survival rates of 48 patients with cirrhosis were 44.7%, 16.2%, and 9.7%, respectively, and the corresponding survival rates of 58 patients with noncirrhotic liver were 70.7%, 38.3%, and 25.4%, respectively.
When the same factors in Tables 2 and 3 were analyzed for their prognostic influence on disease-free survival, only serum AFP level (>1,000 vs. ≤1,000 ng/mL: median disease-free survival 2.9 vs. 7.7 months, P < .001) and tumor invasion of a major branch of the portal or hepatic veins (yes vs. no: median disease-free survival 2.9 vs. 6.2 months, P < .001) were significant factors in the univariate analysis. By multivariate analysis, tumor invasion of a major branch of the portal or hepatic veins was the only significant predictive factor of disease-free survival (RR 2.098, 95% CI 1.382–3.188, P < .001). The 1-, 3-, and 5-year disease-free survival rates of 19 patients with tumor thrombus in a major branch of the portal or hepatic veins were 15.0%, 5.0%, and 5.0%, respectively, whereas the corresponding disease-free survival rates of 81 patients without tumor thrombus in a major venous branch were 32.8%, 13.8%, and 7.0%, respectively.
DISCUSSION
The UICC TNM classification is one of the most widely used staging systems for HCC. 5,6,21–23 Several studies using the definition of the fourth edition of the UICC TNM classification 10 have shown that the pTNM stages of HCC correlated well with overall survival after resection. 6,21–23 In contrast, Izumi et al. 24 found that the UICC TNM classification was not of prognostic value in a study of 104 patients with resection of HCC and suggested a modified staging with more emphasis on vascular invasion. The present study, using the definition of the fifth edition of the UICC TNM classification, 3 demonstrated a good correlation between overall and disease-free survival obtained by the Kaplan-Meier method and the pTNM stages of HCC. Our data reaffirmed the value of the TNM staging for prognostic classification in HCC patients undergoing hepatic resection. In another study of actual long-term survivors among a cohort of patients observed for more than 5 years from the time of hepatic resection, the authors showed that among the various clinicopathologic features of HCC, pTNM stage was the most reliable predictor of both 5-year overall and disease-free survivors. 25
The role of hepatic resection for stage IVA HCC has been controversial because of high surgical risk and poor survival results associated with resection of advanced HCC. Shimada et al. 5 reported that the survival of 15 patients who underwent curative resection of stage IVA HCC was not significantly different from patients with stages I to III HCC, but the small sample size in that study should be taken into consideration when interpreting the data. Furthermore, there appeared to be a strict selection of patients for resection in that study, as only 1 of the 15 patients with stage IVA HCC had tumor thrombus in a major branch of the portal or hepatic veins. 5 Ikai et al. 6 reported a 5-year survival rate of 15% after resection of stage IVA HCC and demonstrated that the survival of stage IVA patients was significantly worse than that of stages I to III patients. Nonetheless, the authors proposed hepatic resection as a standard therapy for stage IVA HCC in view of the lack of other effective treatment options. 6 The current study corroborated their findings. The overall 5-year survival rate among the 106 stage IVA patients in this study was 17.8%, which was significantly lower than that of stage I, II, or III patients. Recently, Usatoff et al. 9 reported poor survival after resection of stage IVA HCC in patients with cirrhosis (3-year survival rate 0%), and thus the authors concluded that hepatic resection for stage IVA HCC should be avoided in cirrhotic patients. Our study demonstrated that the presence of cirrhosis was an independent adverse prognostic factor of long-term survival in patients with stage IVA HCC. However, we do not agree that the presence of cirrhosis should be an absolute contraindication for hepatic resection in patients with stage IVA HCC. In cirrhotic patients with preserved liver function, the hospital mortality rate of hepatic resection for stage IVA HCC was not significantly different from that in noncirrhotic patients. The median survival was 10.6 months and the 5-year survival rate was 9.7% among cirrhotic patients with stage IVA HCC. While these survival results cannot be considered satisfactory, they appear to be better than those reported after TACE for advanced HCC. 26,27 In the absence of a more effective treatment, hepatic resection should be offered to cirrhotic patients with stage IVA HCC provided that there is an adequate liver function reserve. It cannot be overemphasized that careful assessment of resectability in terms of the extent of the tumor and the adequacy of the liver remnant is essential to achieve a favorable outcome in patients with stage IVA HCC. We found the use of laparoscopy since 1994 helpful in selecting patients with advanced tumors for resection, and it helped to avoid unnecessary laparotomy in some patients. 16 Laparoscopy and laparoscopic ultrasound may detect extrahepatic metastasis, additional intrahepatic tumor nodules in a critical position, or extension of tumor thrombus from a branch of the portal vein or hepatic vein to the main portal vein or inferior vena cava, all of which would preclude hepatic resection. Furthermore, in cirrhotic patients with a large advanced HCC, laparoscopy allowed assessment of the volume of the liver remnant and severity of cirrhosis that helped us to make decisions on major hepatic resection. To fully justify the role of surgical resection for stage IVA HCC in cirrhotic or noncirrhotic patients, randomized controlled trials comparing resection with other treatments such as TACE have to be performed. It is also important to evaluate quality of life as an outcome in addition to survival when comparing treatments for this group of patients with advanced HCC. 4,28 The few studies that reported aggressive treatment of stage IVA HCC using hepatic resection were almost exclusively from Eastern authors. 4–6 There is a paucity of similar data from Western authors, and this may reflect a more conservative view on the management of this group of patients with advanced HCC in Western countries. Before data on the results of surgical resection for advanced HCC in Western patients are available, the indication of surgery in these cases may remain different in the East and in the West.
To our knowledge, this is the first study that evaluated whether the four categories of patients with stage IVA HCC under the current UICC pTNM classification have a similar prognosis after hepatic resection. This is an important issue not only for the sake of providing more precise prognostic information to patients, but also for the purpose of proper stratification and comparison of survival data among patients with advanced HCC treated by hepatic resection in future studies. Patients with stage IVA HCC are the group most in need of effective adjuvant therapy to improve the prognosis after hepatic resection. Thus far, studies on preoperative neoadjuvant chemoembolization or postoperative adjuvant chemotherapy have failed to demonstrate any benefit. 18,29,30 The high incidence of recurrence in patients with stage IVA HCC after hepatic resection should prompt further clinical trials to evaluate new adjuvant therapies such as transarterial radioactive iodine and adoptive immunotherapy in this group of patients. 31,32 It is crucial that patients recruited into such clinical trials should be properly stratified in terms of prognosis. Interestingly, in this study, histopathologic features such as microscopic venous invasion and positive microscopic margin did not have a significant prognostic influence in this group of patients with macroscopically advanced HCC. Such microscopic pathologic factors have been shown to be of prognostic significance for HCC in some studies. 17,33,34 Our data suggest that in patients with stage IVA HCC, macroscopic venous invasion has an overwhelming adverse prognostic influence. Other authors have also found that macroscopic venous invasion rather than microscopic pathologic features was the most important prognostic factor after resection of HCC. 24,35
The results of this study suggest a need for reconsideration of the classification of stage IV HCC. It seems more reasonable to consider tumor invasion of a major branch of the portal or hepatic veins as a separate entity from the other three categories. Based on our results, the classification of stage IV HCC may be refined by grouping tumor invasion of adjacent organs, bilobar multiple tumors, and perforation of visceral peritoneum as stage IVA, tumor invasion of a major branch of the portal or hepatic veins as stage IVB, and distant metastasis as stage IVC. Of course, the validity of our proposed classification needs to be confirmed by prospective studies.
A previous study using the old UICC TNM classification that included only patients with invasion of a major branch of the portal or hepatic veins and those with multiple tumors in more than one lobe in stage IVA showed that those with venous invasion had significantly worse survival than those with multiple tumors. 6 This is understandable because tumor invasion of the portal or hepatic veins definitely indicates advanced HCC with a high risk of intrahepatic or extrahepatic metastasis. On the other hand, multiple tumors in both lobes may be due to intrahepatic metastasis or multicentric occurrence, which are likely to be associated with a different prognosis. Our study also demonstrated a significantly better overall survival in patients with multiple bilobar tumors than those with tumor invasion of a major branch of the portal or hepatic veins (median survival 25.7 vs. 6.0 months). The finding that patients with multiple bilobar tumors had significantly lower serum AFP levels than the other three groups of stage IVA patients may reflect the possibility that, in some cases, multiple bilobar tumors may represent early multicentric HCCs rather than advanced HCC with intrahepatic metastasis. It has been recently demonstrated by different methods of genetic analysis that multiple HCCs in the liver can be due to intrahepatic metastases or multicentric tumors. 36,37 The differentiation of tumor clonal origin by genetic analysis may provide useful prognostic information for patients with multiple bilobar tumors. However, currently, the technical complexity of genetic analysis precludes its widespread clinical application.
This study also showed that patients with invasion of a major branch of the portal or hepatic veins had a significantly poorer prognosis than those with tumor invasion of adjacent organs or those with perforation of visceral peritoneum. These results suggest that tumors with invasion of a major branch of the portal or hepatic veins may be biologically more aggressive than the latter two categories, which were added to stage IVA HCC in the latest edition of UICC TNM classification in 1997. Although the disease-free survival of patients with tumor invasion of a major branch of the portal or hepatic veins was not significantly different from that of the other three groups, these patients had a higher propensity for early recurrence, as indicated by a lower 1-year disease-free survival rate than the other three groups. A previous study from the authors showed that early recurrences within 1 year after resection of HCC were more likely to arise from metastasis rather than multicentric occurrence and were associated with worse prognosis compared with late recurrences. 38 Furthermore, there was a higher proportion of multifocal recurrences among patients with invasion of the portal or hepatic veins compared with the other three categories. Unlike solitary intrahepatic recurrence, multinodular recurrences were less amenable to effective treatment and were associated with poorer outcome after recurrence. 20 Macroscopic venous tumor thrombus appears to predispose to early multifocal recurrences after resection of HCC, 35 which may account for the particularly poor prognosis of patients with this tumor characteristic. Despite the poorer prognosis of patients with tumor involving a major branch of the portal or hepatic veins compared with the other three categories of stage IVA patients, the 5-year survival rate of 13.3% in this group of patients after hepatic resection appears to be better than that which could be achieved with TACE. 27 Considering that there are no other effective therapeutic alternatives, we recommend surgery for stage IVA HCC patients with tumor involvement of a major branch of the portal or hepatic veins provided that the surgical risk is acceptable. However, it is imperative that active research be pursued to develop an effective adjuvant therapy that can reduce recurrence after hepatic resection in this group of patients.
In conclusion, this study shows that the prognosis of the four categories of stage IVA patients under the current UICC classification is not homogeneous. Patients with HCC involving a major branch of the portal or hepatic veins had significantly worse long-term survival compared with the other three categories. Our data suggest the need for a reclassification of stage IV HCC.
Footnotes
Correspondence: Ronnie Tung-Ping Poon, MS, FRCS (Edin), Associate Professor, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong, China.
E-mail: poontp@hkucc.hku.hk
Accepted for publication August 8, 2002.
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999; 340: 745–750. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001; 234: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobin LH, Whitekind CH, eds. TNM classification of malignant tumours, 5th ed. New York: John Wiley, 1997.
- 4.Tanaka A, Morimoto T, Ozaki N, et al. Extension of surgical indication for advanced hepatocellular carcinoma: is it possible to prolong life span or improve quality of life? Hepato-Gastroenterology. 1996; 43: 1172–1181. [PubMed] [Google Scholar]
- 5.Shimada M, Takenaka K, Kawahara N, et al. Surgical treatment strategy for patients with stage IV hepatocellular carcinoma. Surgery. 1996; 119: 517–522. [DOI] [PubMed] [Google Scholar]
- 6.Ikai I, Yamaoka Y, Yamamoto Y, et al. Surgical intervention for patients with stage IV-A hepatocellular carcinoma without lymph node metastasis. Proposal as a standard therapy. Ann Surg. 1998; 227: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Sala M, Bruix J. Nonsurgical treatment of hepatocellular carcinoma. Liver Transplant. 2000; 6 (6 Suppl 2):S11–15. [DOI] [PubMed] [Google Scholar]
- 8.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999; 19: 311–322. [DOI] [PubMed] [Google Scholar]
- 9.Usatoff V, Isla AM, Habib NA. Liver resection in advanced hepatocellular carcinoma. Hepato-Gastroenterology. 2001; 48: 46–50. [PubMed] [Google Scholar]
- 10.Hermanek P, Sobin LH, eds. TNM classification of malignant tumors, 4th ed. Berlin: Springer-Verlag, 1987.
- 11.Liu CL, Fan ST, Lo CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001; 19: 3725–3732. [DOI] [PubMed] [Google Scholar]
- 12.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 13.Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic functions in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997; 84: 1255–1259. [PubMed] [Google Scholar]
- 14.Couinaud C. Le foie: Etudes anatomiques et chirugicales. Paris: Massori, 1957: 400–409.
- 15.Fan ST, Lo CM, Liu CL, et al. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999; 229: 322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo CM, Lai ECS, Liu CL, et al. Laparoscopy and laparoscopic ultrasonography avoid exploratory laparotomy in patients with hepatocellular carcinoma. Ann Surg. 1998; 227: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng IO, Lai EC, Fan ST, et al. Prognostic significance of pathologic features of hepatocellular carcinoma. A multivariate analysis of 278 patients. Cancer. 1995; 76: 2443–2448. [DOI] [PubMed] [Google Scholar]
- 18.Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999; 229: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998; 133: 183–188. [DOI] [PubMed] [Google Scholar]
- 20.Poon RT, Fan ST, Ng IOL, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma. A critical reappraisal. Ann Surg. 2000; 231: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takenaka K, Kawahara N, Yamamoto K, et al. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg. 1996; 131: 71–76. [DOI] [PubMed] [Google Scholar]
- 22.Nonami T, Harada A, Kurokawa T, et al. Hepatic resection for hepatocellular carcinoma. Am J Surg. 1997; 173: 288–291. [DOI] [PubMed] [Google Scholar]
- 23.Lau H, Fan ST, Ng IOL, et al. Long-term prognosis after hepatectomy for hepatocellular carcinoma. A survival analysis of 204 consecutive patients. Cancer. 1998; 83: 2302–2011. [PubMed] [Google Scholar]
- 24.Izumi R, Shimizu K, Ii T, et al. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994; 106: 720–727. [DOI] [PubMed] [Google Scholar]
- 25.Poon RT, Ng IOL, Fan ST, et al. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001; 19: 3037–3044. [DOI] [PubMed] [Google Scholar]
- 26.Vetter D, Wenger JJ, Bergier JM, et al. Transarterial oily chemoembolization in the management of advanced hepatocellular carcinoma in cirrhosis: results of a Western comparative study in 60 patients. Hepatology. 1991; 13: 427–433. [PubMed] [Google Scholar]
- 27.Chung JW, Park JH, Han JK, et al. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. AJR Am J Roentgenol. 1995; 165: 315–321. [DOI] [PubMed] [Google Scholar]
- 28.Poon RT, Fan ST, Yu WC, et al. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001; 136: 693–699. [DOI] [PubMed] [Google Scholar]
- 29.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995; 82: 122–126. [DOI] [PubMed] [Google Scholar]
- 30.Ono T, Yamanoi A, Nazmy El Assal O, et al. Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: metaanalysis of three randomized controlled trials. Cancer. 2001; 91: 2378–2385. [PubMed] [Google Scholar]
- 31.Lau WY, Leung TW, Ho SK, et al. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999; 353: 797–801. [DOI] [PubMed] [Google Scholar]
- 32.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000; 356: 802–807. [DOI] [PubMed] [Google Scholar]
- 33.Lauwers GY, Vauthey JN. Pathological aspects of hepatocellular carcinoma: a critical review of prognostic factors. Hepato-Gastroenterology. 1998; 45 (Suppl 3): 1197–1202. [PubMed] [Google Scholar]
- 34.Poon RT, Fan ST, Wong J. Risk factors, prevention and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumata T, Kanematsu T, Takenaka K, et al. Patterns of intrahepatic recurrence after curative resection of hepatocellular carcinoma. Hepatology. 1989; 9: 457–460. [DOI] [PubMed] [Google Scholar]
- 36.Wilkens L, Bredt M, Flemming P, et al. Differentiation of multicentric origin from intra-organ metastatic spread of hepatocellular carcinomas by comparative genomic hybridization. J Pathol. 2000; 192: 43–51. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Kajino K, Kudo M, et al. Determination of the clonal origin of multiple human hepatocellular carcinomas by cloning and polymerase chain reaction of the integrated hepatitis B virus DNA. Hepatology. 1999; 29: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 38.Poon RT, Fan ST, Ng IOL, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000; 289: 500–507. [PubMed] [Google Scholar]