Abstract
Objective
To analyze the effect of CO2 pneumoperitoneum on the inflammatory response induced by sepsis during laparoscopy.
Summary Background Data
A growing body of evidence challenges the once generally accepted notion that smaller incisions alone account for the observed benefits of the laparoscopic approach. Furthermore, laparoscopic surgery is now being applied to a broad spectrum of patients, including those in whom the inflammatory response is ignited. Delineation of the effects of CO2 pneumoperitoneum on the inflammatory response induced by sepsis is needed.
Methods
Sepsis was induced in rats by cecal ligation and puncture (CLP) performed either open or laparoscopically using CO2 or helium as insufflation gases. Animals were killed 24 hours postoperatively, at which time whole blood was collected for complete blood cell counts and livers were harvested for analysis of hepatic expression of the rat acute phase genes α2-macroglobulin and β-fibrinogen.
Results
Laparoscopic CLP using CO2 resulted in significantly reduced hepatic expression of the rat acute phase gene α2-macroglobulin compared to both laparoscopic CLP using helium and open CLP. Hepatic expression of another rat acute phase gene, β-fibrinogen, paralleled that of α2-macroglobulin and was significantly reduced following laparoscopic CLP using CO2 compared to laparoscopic CLP using helium. Total white blood cell and neutrophil counts following CLP were both significantly higher when CLP was performed laparoscopically using CO2 than when CLP was performed open or laparoscopically using helium.
Conclusions
Intra-abdominal CO2 present during laparoscopy attenuates the acute phase inflammatory response associated with perioperative sepsis.
Minimally invasive surgical techniques continue to advance in capability and popularity. Shorter hospital stays, decreased postoperative pain, more rapid return to preoperative activity, and decreased postoperative ileus give laparoscopic surgery distinct advantages over conventional surgery for a number of operative procedures. 1–4 While several differences have been described between the physiologic, 5,6 metabolic, 3,6–8 and immune 9–14 responses to conventional and laparoscopic procedures, the molecular basis of the improved results observed following laparoscopic surgery is still unknown.
Refinement of laparoscopic skills and technological advances in the field of laparoscopy now enable surgeons to apply the laparoscopic paradigm to a broader spectrum of patients. Extensive surgical dissections are now being performed in complex laparoscopic operations that can last for hours. Furthermore, exploratory laparoscopy is being used to aid surgeons in the diagnosis and treatment of patients in whom a cause of abdominal sepsis is unclear. 15 Finally, diagnostic laparoscopy is even being used at the bedside to evaluate critically ill patients with physiologic deterioration of suspected intra-abdominal origin. 16–18 Where laparoscopic surgery was once reserved for simple outpatient procedures, it is now being employed in patients with significant associated systemic inflammatory responses. Although the effects of CO2 pneumoperitoneum may be irrelevant for short operations performed in physiologically well-compensated patients, a thorough understanding of the effects of laparoscopy becomes imperative as the effects are magnified during long operations and as they involve patients with little physiologic reserve.
The liver is the central metabolic organ of the body, and hepatocytes are central to the overall response to stress. Hepatocytes are the predominant cellular target for circulating inflammatory molecules (e.g., cytokines). Hepatocytes also produce the metabolic substrates essential for survival during states of increased metabolic demand. Furthermore, because the intra-abdominal gastrointestinal tract is drained by the portal venous system, toxins and responding cytokines generated from sources of enteric infection (e.g., intra-abdominal sepsis) are encountered first by the liver. The liver responds to proinflammatory chemokines by upregulating specific genes, thus increasing the synthesis of proteins collectively termed acute phase reactants. For these reasons, hepatic acute phase response genes are important markers of the body’s physiologic response to stress.
The effects of the combined insults of laparoscopic surgery and postoperative sepsis on the stress response are unknown. The purpose of this study was to analyze the additional influence of laparoscopic procedures, in particular CO2 insufflation, on the response to sepsis in a well-established animal model—cecal ligation and puncture (CLP) in the rat. 19,20
METHODS
Cecal Ligation and Puncture
Female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), 10 to 12 weeks old, were housed in cages where standard chow and water were available ad libitum. The rats were acclimatized to their environment for 3 to 5 days on arrival and then fasted for 16 hours before any procedures. Anesthesia was obtained using inhaled methoxyfluorane. All surgical procedures were performed under aseptic conditions. Pneumoperitoneum was achieved by introducing a Veress needle into the peritoneal cavity and insufflating (Olympus insufflator) the abdomen with 3 mmHg gas. Laparoscopic procedures were performed using 3-mm instruments (Olympus) introduced into the abdomen through ports positioned as shown in Figure 1. Cecal ligation and puncture (CLP) consisted of dissection of the cecum, ligation midway between the ileocecal valve and the terminal cecum using a 2-0 silk tie, and double-puncture of the isolated cecum with a hollow 16-gauge needle introduced through the abdominal wall as shown in Figure 2. Laparotomy, for the sham laparotomy and open CLP groups, consisted of a 5-cm midline abdominal incision. To ensure equal tension on the silk ligatures between groups, the 3-mm laparoscopic graspers were used to tie the cecal ligation knots in the open procedures as well as in the laparoscopic procedures. The duration of the total procedure, and therefore the duration of anesthesia, pneumoperitoneum, and laparotomy, was standardized to 30 minutes for all groups based on the average time required for our group to perform the longest of the procedures, laparoscopic CLP (range 25–35 minutes). Postoperatively, animals were resuscitated with a subcutaneous injection of lactated Ringer’s (30 mL/kg) and were again housed in cages where water was available ad libitum. All procedures were part of an animal protocol reviewed and approved by the Johns Hopkins Medical Institutions Animal Care and Use Committee.
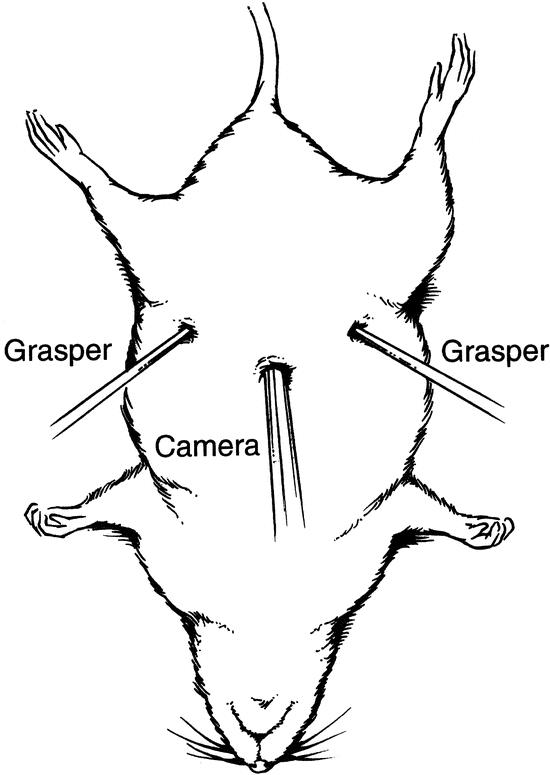
Figure 1. Set-up for laparoscopic cecal ligation and puncture. Placement of the camera port midline in the epigastrium, with the grasper ports placed laterally and slightly inferiorly, allows excellent visualization and manipulation of the rat cecum.
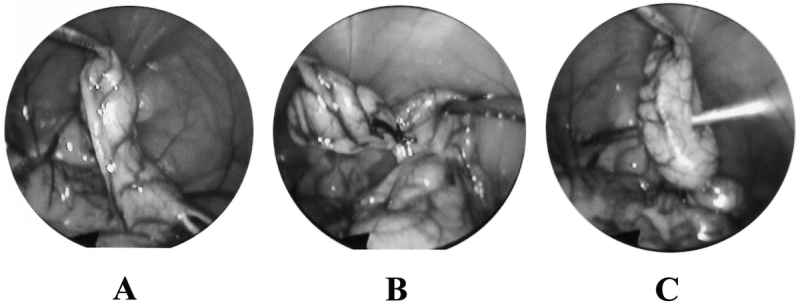
Figure 2. Views of the rat cecum through a 3-mm laparoscope during laparoscopic cecal ligation and puncture: (A) dissection, (B) ligation, (C) puncture.
Inflammatory Response to CLP
Rats (n = 60) were fasted, anesthetized, and then randomized to one of six groups (10 rats each): 1) anesthesia control, 2) CO2 pneumoperitoneum, 3) sham laparotomy, 4) laparoscopic CLP using helium, 5) laparoscopic CLP using CO2, or 6) open CLP. Animals were evaluated 24 hours postoperatively by a blinded observer for clinical signs of CLP-induced sepsis (i.e., piloerection and lethargy), and were then killed via guillotine, immediately after which whole blood was collected from the carotid arteries for determination of complete blood cell counts using a commercially available automated cell counter, and livers were removed for total RNA isolation. At necropsy, the peritoneal cavity was visually inspected for evidence of effective CLP (i.e., cecal necrosis and cloudy peritoneal fluid). The choice to include only a single pneumoperitoneum control group (using CO2) was based on an earlier experiment by our group in which 90 rats had been randomized into five groups: 1) anesthesia (control), 2) Veress needle without insufflation (control), 3) pneumoperitoneum with CO2, 4) pneumoperitoneum with helium, and 5) laparotomy. Within each group, half of the animals had received their intervention (or control procedure) for 40 minutes, and the remaining half for 120 minutes. Hepatic expression of β-fibrinogen was low in all three of the intervention groups, and there were no significant differences between groups. 21 Harvest 24 hours following insult was chosen based on previously published data showing maximal expression of α2-macroglobulin and β-fibrinogen at that time point following similar insult. 22
Hepatic Acute Phase Gene Expression
RNA was isolated from liver samples by the acid guanidinium thiocyanate-phenol-chloroform method. 23 Total RNA (10 μg) was electrophoresed in formaldehyde-agarose gels and visualized by staining of the gel with ethidium bromide to assess the integrity of the preparation. Samples of RNA that appeared degraded were discarded. Total RNA samples (5 μg) were immobilized onto nylon modified membranes (GeneScreen Plus, NEN Research Products, Boston, MA) by slot blotting. Blots were hybridized with radiolabeled cDNA probes for α2-macroglobulin (rat, full-length), β-fibrinogen (pig, fragment), and metallothionein (pig, full-length). To quantitate for differences in RNA loading in each slot, blots were also probed with a radiolabeled probe for 28S rRNA. Radioactive probes were prepared by the random primer method 24 using [a-32P]dATP and [a-32P]dCTP (ICN Pharmaceuticals, Irvine, CA) as previously described. 25 Blots were hybridized in 50% formamide, 75 mmol/L sodium citrate (pH 7.0), 0.75 mol/L NaCl, 1% sodium dodecyl sulfate (SDS), 2.5× Denhardt’s solution, 100 g/mL denatured salmon sperm DNA, 1 mmol/L EDTA, and 20 mmol/L sodium phosphate (pH 6.5) for 16 hours at 42°C. Blots were washed with 50 mmol/L tris (hydroxymethyl) aminomethane (Tris pH 8.6), 1 mol/L NaCl, 2 mmol/L EDTA, and 1% SDS at 42°C for at least 2 hours with a minimum of six changes. Later, blots were washed with 2× saline-sodium citrate (SSC) buffer (0.015 mol/L sodium citrate [pH 7.0] and 0.15 mol/L NaCl) containing 0.1% SDS at 42°C for 30 minutes and 2× SSC with 0.1% SDS at 65°C for 5 minutes. Blots were exposed to X-ray film (Kodak) at −70°C in the presence of intensifying screens. Autoradiograms in the linear range of exposure were quantified by scanning laser densitometry. The signal intensity of individual slots was normalized to the corresponding 28S rRNA. Metallothionein was chosen as a control response gene because it is not an acute phase reactant in the rat.
Statistical Analysis
The one-way analysis of variance (ANOVA) test was used to detect general differences in mRNA expression and hematologic parameters between all groups. To elucidate specific significances in these parameters between groups, multiple pairwise comparison tests were performed using the Student-Newman-Keuls method. Differences between groups were considered significant when P < .05. Analysis was performed using Microsoft Excel (Microsoft Corp.) and SigmaStat (Jandel Scientific) software.
RESULTS
Postoperative Evaluation and Necropsy Findings
Rats in the anesthesia, pneumoperitoneum, and sham laparotomy control groups exhibited normal activity and had no piloerection 24 hours following the control procedure. In contrast, all rats that underwent CLP exhibited decreased activity and significant piloerection. At necropsy, rats from the anesthesia, pneumoperitoneum, and sham laparotomy control groups were found to have only a small amount of clear peritoneal fluid and no cecal necrosis. After CLP, all rats were found to have foul-smelling abdominal cavities and significant cloudy peritoneal fluid, consistent with fecal contamination. Furthermore, the gross appearance of the ligated and punctured cecums was consistent with necrosis (friable and discolored) in all rats that had received CLP.
Hepatic Acute Phase Gene Response
Expression of the rat acute phase genes α2-macroglobulin and β-fibrinogen was analyzed by slot blot/hybridization of RNA isolated from liver samples obtained 24 hours following either CLP or control procedure (Fig. 3). Levels of hepatic α2-macroglobulin and β-fibrinogen mRNA were both low in the absence of CLP-induced sepsis (anesthesia, pneumoperitoneum, and sham laparotomy controls). Open CLP resulted in high hepatic levels of mRNA coding for both α2-macroglobulin and β-fibrinogen. Similar mRNA levels of both these genes were detected following laparoscopic CLP using helium as the insufflation gas. In contrast, levels of α2-macroglobulin mRNA following laparoscopic CLP using CO2 were significantly less than levels following laparoscopic CLP using helium (P < .05) or following open CLP (P < .05). β-fibrinogen expression was significantly reduced following laparoscopic CLP using CO2 compared to expression following laparoscopic CLP using helium (P < .05). While expression of β-fibrinogen following laparoscopic CLP using CO2 was also less than expression of the same gene following open CLP, this difference did not reach statistical significance. None of the groups differed significantly in the hepatic expression of our control response gene, the gene coding for metallothionein (a free radical scavenger protein).
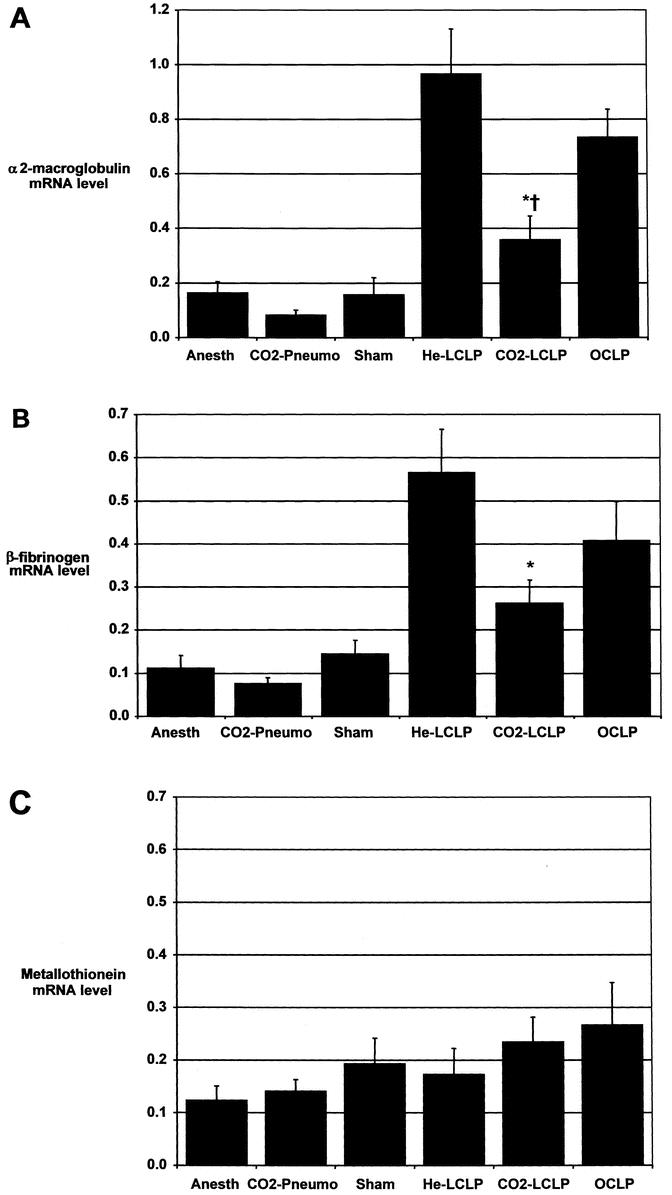
Figure 3. Hepatic gene expression in rats 24 hours following either cecal ligation and puncture (CLP) or control procedure. Anesth, anesthesia alone; CO2-Pneumo, pneumoperitoneum using CO2 as the insufflation gas; Sham, sham laparotomy). Open CLP (OCLP) and laparoscopic CLP using He (He-LCLP) resulted in increased expression of the acute phase genes α2-macroglobulin (A) and β-fibrinogen (B). Laparoscopic CLP using CO2 (CO2-LCLP) yielded significantly less expression of both α2-macroglobulin and β-fibrinogen. No significant differences in the expression of the gene for the free radical scavenger protein metallothionein (C) were found between any of the groups. Livers were harvested from rats immediately following guillotine decapitation 24 hours postprocedure. RNA was isolated from hepatic tissue, separated using slot blotting and radiolabeling techniques, and scanned via laser densitometry to quantify the amount of mRNA for each individual gene. Data are mean ± SEM, expressed in arbitrary mRNA-expression units representing the individual gene densitometry signal normalized to the corresponding 28S rRNA subunit signal (the latter is an indirect measurement of the total amount of RNA in a sample). *P < .05 vs. He-LCLP, †P < .05 vs. OCLP, by one-way ANOVA and Student-Newman-Keuls method.
Hematologic Response
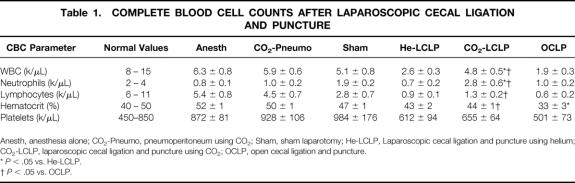
Complete blood cell counts were also analyzed 24 hours following either CLP or control procedure (Table 1). No significant differences in white blood cell count, neutrophil count, lymphocyte count, hematocrit, or platelet count were found between anesthesia and CO2 pneumoperitoneum controls. The sham laparotomy control procedure produced a significant increase in neutrophil count and significant reductions in lymphocyte count and hematocrit compared to anesthesia and pneumoperitoneum controls (P < .05). Laparoscopic CLP using CO2 yielded white cell counts similar to those of controls. Significantly reduced white cell counts were observed following laparoscopic CLP using helium and open CLP compared to laparoscopic CLP using CO2. Laparoscopic CLP using helium and open CLP also yielded neutrophil counts significantly less than laparoscopic CLP using CO2. Significantly reduced lymphocyte counts were detected following all methods of CLP compared to their respective controls (i.e., CO2 and helium laparoscopic CLP compared to pneumoperitoneum control and open CLP compared to sham laparotomy, P < .05 for all three). Open CLP produced a further significant reduction in lymphocyte count compared to laparoscopic CLP using CO2 (P < .05). Laparoscopic LP using helium yielded lower lymphocyte counts than laparoscopic CLP using CO2, but this latter finding did not reach statistical significance. All methods of CLP resulted in significant reductions in hematocrit compared to controls (P < .05), and open CLP yielded a further significant reduction in hematocrit compared to both laparoscopic CLP with helium and laparoscopic CLP with CO2. No significant differences in platelet count were found among any of the CLP groups.
Table 1. COMPLETE BLOOD CELL COUNTS AFTER LAPAROSCOPIC CECAL LIGATION AND PUNCTURE
Anesth, anesthesia alone; CO2-Pneumo, pneumoperitoneum using CO2; Sham, sham laparotomy; He-LCLP, Laparoscopic cecal ligation and puncture using helium; CO2-LCLP, laparoscopic cecal ligation and puncture using CO2; OCLP, open cecal ligation and puncture.
*P < .05 vs. He-LCLP.
†P < .05 vs. OCLP.
DISCUSSION
The mechanism presumed to underlie the advantages of laparoscopy over conventional open surgery has been related primarily to reduced tissue injury secondary to smaller incisions. However, the body of evidence that challenges the once generally accepted notion that smaller incisions alone account for the observed benefits of the laparoscopic approach is growing. The unique role of CO2 pneumoperitoneum in this physiologic process is beginning to receive attention but has not yet been well characterized. As surgeons continue to push the envelope of minimal-access surgery by performing increasingly longer and more complex operations in sicker patients, the degree of stress associated with laparoscopy increases. Furthermore, the tissue trauma associated with access to the surgical site (i.e., the incision) becomes relatively less significant as the degree of operative dissection and internal tissue manipulation increases. Thus, the unique physiology of CO2 pneumoperitoneum may be the predominant difference between the level of insult associated with complex laparoscopic and comparable open surgery.
A few researchers have begun to investigate the unique role of CO2 in the physiology of laparoscopy. Watson et al. demonstrated that air laparoscopy increases peritoneal tissue macrophage release of superoxide and TNF compared to CO2 laparoscopy. 26 West et al. have shown that murine peritoneal macrophages exposed to CO2 in vitro exhibit inhibition of LPS-stimulated IL-1 and TNF cytokine release. 27 Additional work by the same group suggests that this effect is related to the influence of the CO2 environment on the actual release of IL-1 and TNF rather than on regulation at the level of cytokine gene expression. 28 West et al. have also proposed relative intracellular acidosis as the mechanism by which this effect is exerted. 29 Other than animal studies in which peritoneal macrophages have been exposed to CO2 in the intra-abdominal in vivo environment before in vitro stimulation with LPS 29 or other manipulation, 26 all data corroborating these findings are from ex vivo experiments.
Criticisms regarding the importance of the hypothesis that CO2 mediates attenuation of the inflammatory response need to be addressed. There is to date a paucity of in vivo data in support of the hypothesis. Furthermore, while blunting of LPS-stimulated peritoneal macrophage cytokine release following exposure to CO2 is an important finding, it may be less relevant to large laparoscopic procedures associated with a significant inflammatory response but without LPS-laden bacterial contamination (e.g., laparoscopic Nissen, nephrectomy). Finally, understanding altered local host cell physiology does not directly elucidate the ultimate downstream effects of such local changes on the organism’s global physiologic response to injury and inflammation. In other words, alterations in peritoneal macrophage response may or may not be important systemically during laparoscopy.
The utility of the model of laparoscopic CLP in the rat used in these experiments is two-fold. First, the use of a septic animal model magnifies the stress induced by a surgical procedure to more clearly delineate the modifying effects of laparoscopy on the inflammatory response. Second, the combined stressors of bacterial contamination of the peritoneal cavity and bowel ischemia present following laparoscopic CLP provide an environment analogous to clinical situations in which laparoscopy is used to aid in the diagnosis and treatment of critically ill patients with peritonitis. By evaluating the expression of hepatic acute phase genes, we are able to study the clinically relevant “end organ” physiologic response to laparoscopy in vivo.
In the current study, we investigated the influence of different abdominal insufflation gases on the inflammatory response induced by CLP in rats. The validity of our model presupposes that CLP caused sepsis and that the initial degree of insult caused by CLP was equivalent between groups. To validate these assumptions, we clinically evaluated all rats before euthanasia and tissue harvest and performed detailed abdominal necropsies in each rat immediately following completion of the tissue harvest process. We established the presence or absence of sepsis in each rat by asking an observer familiar with typical rat behavior, but blinded to the group assignment of each rat, to evaluate each rat for: 1) the presence or absence of piloerection and 2) normal activity or decreased activity. All 30 rats that had received CLP were identified as having clinical sepsis (piloerection and decreased activity), and all 30 rats that had received a control procedure were identified as not having sepsis (no piloerection and normal activity). Before performing this experiment, our group practiced and refined the technique involved with each procedure. During this phase of our study, ligated cecums appeared different at necropsy if CLP had been performed laparoscopically than if it had been performed open. We attributed this discrepancy to differing tension on the cecal ligation knots (it being easier to tie a knot with greater tension by hand), and resolved the issue by tying all cecal ligation knots in the experiment using the laparoscopic graspers. Indeed, all 30 cecums that were ligated in our experiment appeared similarly friable and discolored at necropsy. Finally, all rats that underwent CLP were found at necropsy to have cloudy, foul-smelling peritoneal fluid consistent with gross fecal contamination of the abdominal cavity.
The inflammatory response was evaluated at the level of acute phase gene expression in the liver. In humans, acute phase reactants include C-reactive protein, prealbumin, transferrin, α1-trypsin inhibitor, retinol binding protein, haptoglobin, and ceruloplasmin. While induction of α2-macroglobulin and β-fibrinogen gene expression is less relevant as a marker of stress in humans, the genes coding for these proteins were chosen in our animal model because they are important acute phase reactants in rats and their behavior parallels that of human hepatic acute phase reactants. 30 The gene coding for the free radical scavenger protein metallothionein was chosen because its expression is not affected by CLP, and thus it provided our experiment with a negative control. In the current study, hepatic expression of α2-macroglobulin and β-fibrinogen in response to CLP was similar when CLP was performed laparoscopically using helium or open using conventional laparotomy but was reduced when CLP was performed laparoscopically using CO2. The reduction of α2-macroglobulin gene expression following laparoscopic CLP with CO2 compared to both laparoscopic CLP with helium and open CLP was statistically significant. For β-fibrinogen, the reduction was statistically significant only when laparoscopic CLP using CO2 was compared to laparoscopic CLP using helium.
Although changes in complete blood cell and differential white blood cell counts are often nonspecific findings, the ubiquitous use of complete blood cell count data in clinical practice led us to evaluate these parameters in our experiment. Total white cell and circulating neutrophil counts were significantly lower following laparoscopic CLP using helium and open CLP than following laparoscopic CLP using CO2. Open CLP also produced a significant reduction in lymphocyte count compared to laparoscopic CLP with CO2, and while laparoscopic CLP with helium did yield lower lymphocyte counts than laparoscopic CLP with CO2, this did not reach statistical significance. The mechanism behind this phenomenon of blunted neutropenia by CO2 cannot be specifically delineated from our data. Perhaps CO2 insufflation inhibits the production of chemotactic signals from peritoneal macrophages, thus effectively reducing leukocyte sequestration in the abdomen. Alternatively, CO2 could have an effect on demargination of leukocytes, thus balancing the loss of leukocytes sequestered in the infected abdomen.
In our study, we observed the attenuation of hepatic acute phase gene expression and preservation of circulating leukocyte volume observed following laparoscopic CLP using CO2 compared to laparoscopic CLP using helium or open CLP. These data suggest that laparoscopy modifies the inflammatory response to abdominal sepsis, and that abdominal insufflation with CO2, rather than a reduction in incision size, is the factor responsible for such an effect. Because CO2 abdominal insufflation is routinely employed during laparoscopic surgery, this finding has potential relevance to a broad spectrum of patients.
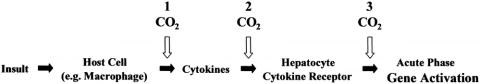
These data demonstrate an important “end organ” (i.e., the liver) effect, but they do not delineate where in the inflammatory response cascade CO2 exerts its blunting effect. The mechanism of hepatic acute phase gene activation via proinflammatory cytokines released from peritoneal macrophages described by Keller et al. may be relevant. 31–33 It is conceivable that, as West et al. 27–29 suggest, intracellular acidification of peritoneal macrophages inhibits the release of cytokines that would otherwise be mobilized by LPS and, perhaps, other stimuli. Such a dam at the headwaters of the inflammatory response cascade would effectively prevent the downstream activation of the hepatic acute phase response (Fig. 4). In future experiments, systemic alkalinization of the host via artificial induction of respiratory or metabolic alkalosis will be performed to test whether reversal of acidosis-mediated attenuation of the inflammatory response occurs. It is also possible that CO2 insufflation physiology influences the clearance of circulating inflammatory mediators or leverages a direct effect on hepatocyte function.
Figure 4. Mechanism of hepatic acute phase gene activation following intra-abdominal insult. CO2 pneumoperitoneum may attenuate this inflammatory response cascade by acting at any of a number of different steps: (1) CO2 may cause local peritoneal cell acidosis, thus blocking the release of cytokines; (2) CO2 may influence the clearance of circulating inflammatory mediators or alter cytokine–cytokine receptor interactions in the liver and elsewhere; or (3) hepatocytes may be affected directly by CO2 via alterations in second messenger system function or transcription factor action.
The implications of this study are far-reaching. If the presence of CO2 in the abdominal cavity blunts the pathologic aspects of the body’s inflammatory response to sepsis and perioperative stress, then perhaps the laparoscopic approach would be preferred in septic patients who require abdominal exploration. Could peritoneal CO2 insufflation alone actually be of benefit in patients with the systemic inflammatory response syndrome? Or perhaps with further delineation of the mechanism whereby CO2 pneumoperitoneum blunts the inflammatory response, a more precise intervention could be developed that targets the critical step in such a mechanism. Our demonstration that the CO2 pneumoperitoneum present during laparoscopic surgery attenuates the acute phase response to abdominal sepsis is an important first step in such endeavors.
In summary, peritoneal insufflation with CO2 blunts the hepatic expression of the rat acute phase genes α2-macroglobulin and β-fibrinogen in response to CLP. Laparoscopic CLP using helium and open CLP also yielded lower total white blood cell, neutrophil, and lymphocyte counts than laparoscopic CLP using CO2. These findings suggest that laparoscopy modifies surgery-associated sepsis and that CO2 insufflation mediates such an effect. CO2-mediated attenuation of the hepatic acute phase response may be an important step in the mechanism of inflammatory response reduction reported after laparoscopic surgery.
Footnotes
Supported by a grant from Tyco/United States Surgical Corporation, Norwalk, Connecticut.
Correspondence: Mark A. Talamini, MD, Department of Surgery, The Johns Hopkins University, School of Medicine, 600 North Wolfe St., Blalock 665, Baltimore, MD 21287-4665.
E-mail: talamini@jhmi.edu
Accepted for publication August 8, 2002.
References
- 1.Barkun JS, Wexler MJ, Hinchey EJ, et al. Laparoscopic versus open inguinal herniorrhaphy: preliminary results of a randomized controlled trial. Surgery. 1995; 118: 703–710. [DOI] [PubMed] [Google Scholar]
- 2.Buanes T, Mjaland O. Complications in laparoscopic and open cholecystectomy: a prospective comparative trial. Surg Laparosc Endosc. 1996; 6: 266–272. [PubMed] [Google Scholar]
- 3.Glaser F, Sannwald GA, Buhr HJ, et al. General stress response to conventional and laparoscopic cholecystectomy. Ann Surg. 1995; 221: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jatzko GR, Lisborg PH, Pertl AM, et al. Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg. 1995; 221: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talamini MA, Mendoza-Sagaon M, Gitzelmann CA, et al. Increased mediastinal pressure and decreased cardiac output during laparoscopic Nissen fundoplication. Surgery. 1997; 122: 345–352. [DOI] [PubMed] [Google Scholar]
- 6.Mealy K, Gallagher H, Barry M, et al. Physiological and metabolic responses to open and laparoscopic cholecystectomy. Br J Surg. 1992; 79: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 7.Jakeways MS, Mitchell V, Hashim IA, et al. Metabolic and inflammatory responses after open or laparoscopic cholecystectomy. Br J Surg. 1994; 81: 127–131. [DOI] [PubMed] [Google Scholar]
- 8.Vittimberga FJ Jr, Foley DP, Meyers WC, et al. Laparoscopic surgery and the systemic immune response. Ann Surg. 1998; 227: 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collet D, Vitale GC, Reynolds M, et al. Peritoneal host defenses are less impaired by laparoscopy than by open operation. Surg Endosc. 1995; 9: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 10.Gitzelmann CA, Mendoza-Sagaon M, Talamini MA, et al. Cell-mediated immune response is better preserved by laparoscopy than laparotomy. Surgery. 2000; 127: 65–71. [DOI] [PubMed] [Google Scholar]
- 11.Kloosterman T, von Blomberg BM, Borgstein P, et al. Unimpaired immune functions after laparoscopic cholecystectomy. Surgery 1994; 115: 424–428. [PubMed] [Google Scholar]
- 12.Mendoza-Sagaon M, Gitzelmann CA, Herreman-Suquet K, et al. Immune response: effects of operative stress in a pediatric model. J Pediatr Surg. 1998; 33: 388–393. [DOI] [PubMed] [Google Scholar]
- 13.Redmond HP, Watson RW, Houghton T, et al. Immune function in patients undergoing open vs laparoscopic cholecystectomy. Arch Surg. 1994; 129: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 14.Trokel MJ, Bessler M, Treat MR, et al. Preservation of immune response after laparoscopy. Surg Endosc. 1994; 8: 1385–1388. [DOI] [PubMed] [Google Scholar]
- 15.Navez B, Tassetti V, Scohy JJ, et al. Laparoscopic management of acute peritonitis. Br J Surg. 1998; 85: 32–36. [DOI] [PubMed] [Google Scholar]
- 16.Iberti TJ, Salky BA, Onofrey D. Use of bedside laparoscopy to identify intestinal ischemia in postoperative cases of aortic reconstruction. Surgery. 1989; 105: 686–689. [PubMed] [Google Scholar]
- 17.Rehm CG. Bedside laparoscopy. Crit Care Clin. 2000; 16: 101–112. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JJ, Puyana JC, Callery MP, et al. The feasibility and accuracy of diagnostic laparoscopy in the septic ICU patient. Surg Endosc. 2000; 14: 617–621. [DOI] [PubMed] [Google Scholar]
- 19.Baker CC, Chaudry IH, Gaines HO, et al. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983; 94: 331–335. [PubMed] [Google Scholar]
- 20.Berguer R, Alarcon A, Feng S, et al. Laparoscopic cecal ligation and puncture in the rat. Surg Endosc. 1997; 11: 1206–1208. [DOI] [PubMed] [Google Scholar]
- 21.Poulose BK, Kutka MF, Mendoza-Sagaon M, et al. Statement of β-fibrinogen is no different between laparoscopic and open approaches. Surg Endosc. 1999; 13: S66. [Google Scholar]
- 22.Steward D, Fulton WB, Wilson C, et al. Genetic contribution to the septic response in a mouse model. Shock (in press). [DOI] [PubMed]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983; 132: 6–13. [DOI] [PubMed] [Google Scholar]
- 25.Beck SC, De Maio A. Stabilization of protein synthesis in thermotolerant cells during heat shock: association of heat shock protein-72 with ribosomal subunits of polysomes. J Biol Chem. 1994; 269: 21803–21811. [PubMed] [Google Scholar]
- 26.Watson RW, Redmond HP, McCarthy J, et al. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in a murine model. Br J Surg. 1995; 82: 1060–1065. [DOI] [PubMed] [Google Scholar]
- 27.West MA, Baker J, Bellingham J. Kinetics of decreased LPS-stimulated cytokine release by macrophages exposed to CO2. J Surg Res. 1996; 63: 269–274. [DOI] [PubMed] [Google Scholar]
- 28.West MA, LeMieur TL, Hackam D, et al. Acetazolamide treatment prevents in vitro endotoxin-stimulated tumor necrosis factor release in mouse macrophages. Shock. 1998; 10: 436–441. [DOI] [PubMed] [Google Scholar]
- 29.West MA, Hackam DJ, Baker J, et al. Mechanism of decreased in vitro murine macrophage cytokine release after exposure to carbon dioxide. Ann Surg. 1997; 226: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hocke GM, Cui MZ, Ripperger JA, et al. Regulation of the rat α2-macroglobulin gene by interleukin-6 and leukemia inhibitor factor. In: Mackiewicz A, Kushner I, Baumann H, eds. Acute phase proteins: molecular biology, biochemistry, and clinical applications. Boca Raton, FL: CRC; 1993.
- 31.Keller GA, West MA, Harty JT, et al. Modulation of hepatocyte protein synthesis during co-cultivation with macrophage-rich peritoneal cells in vitro. Arch Surg. 1985; 120: 180–186. [DOI] [PubMed] [Google Scholar]
- 32.Keller GA, West MA, Wilkes LA, et al. Modulation of hepatocyte protein synthesis by endotoxin-activated Kupffer cells. II. Mediation by soluble transferrable factors. Ann Surg. 1985; 201: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller GA, West MA, Cerra FB, et al. Macrophage-mediated modulation of hepatic function in multiple-system failure. J Surg Res. 1985; 39: 555–563. [DOI] [PubMed] [Google Scholar]