Abstract
Developmental dyslexia, characterized by unexplained difficulty in reading, is associated with behavioral deficits in phonological processing. Functional neuroimaging studies have shown a deficit in the neural mechanisms underlying phonological processing in children and adults with dyslexia. The present study examined whether behavioral remediation ameliorates these dysfunctional neural mechanisms in children with dyslexia. Functional MRI was performed on 20 children with dyslexia (8–12 years old) during phonological processing before and after a remediation program focused on auditory processing and oral language training. Behaviorally, training improved oral language and reading performance. Physiologically, children with dyslexia showed increased activity in multiple brain areas. Increases occurred in left temporo-parietal cortex and left inferior frontal gyrus, bringing brain activation in these regions closer to that seen in normal-reading children. Increased activity was observed also in right-hemisphere frontal and temporal regions and in the anterior cingulate gyrus. Children with dyslexia showed a correlation between the magnitude of increased activation in left temporo-parietal cortex and improvement in oral language ability. These results suggest that a partial remediation of language-processing deficits, resulting in improved reading, ameliorates disrupted function in brain regions associated with phonological processing and produces additional compensatory activation in other brain regions.
Developmental dyslexia is a disorder that is defined as a difficulty in reading in people who have the intelligence, motivation, and education necessary for successful reading. It has a prevalence estimated between 5% and 17% (1). An emerging consensus is that developmental dyslexia is characterized by difficulties in language processing. These difficulties are primarily at the level of phonological processing of speech sounds, specifically phonological awareness, which is the ability to recognize and manipulate the sound structure of words (2). Skills like rhyming, syllable counting, and sounding out pseudo-words require phonological awareness and are skills at which individuals with dyslexia are often impaired (3, 4).
In addition to behavioral deficits in phonological processing, studies of brain function have shown a neural deficit in dyslexia during phonological processing (5). Individuals with dyslexia have decreased activity (relative to controls) in left temporo-parietal cortex during phonological processing. This disrupted neural response has been shown in a number of studies (5), across different methodologies [positron emission tomography (PET; refs. 6–10) and functional MRI (fMRI; ref. 11)], with various analysis methods [whole-brain statistical parametric mapping (SPM; refs. 6–8 and 12) and region of interest (ROI; refs. 9–11)] in multiple tasks [letter rhyme (7, 11), pseudo-word processing (6, 8, 10, 11), and explicit and implicit (6, 8) tasks], across ability levels [compensated (6–8) and severely (10) dyslexic], and in different languages (8). The above results were initially reported in adults, making it uncertain whether they reflected an initial impairment or a long-term compensation. This disruption, however, is also evident in children with dyslexia (12, 13), which suggests that it is fundamental to the disorder.
What has not been known is the extent to which this disruption in neural response observed in dyslexia can be changed through remediation. The current study was designed to explore the neural effects of behavioral remediation. The goal was to determine whether remediation in children with dyslexia could alter their disrupted neural response to phonological demands. Twenty children with dyslexia (8–12 years old) underwent fMRI and behavioral testing before and after an 8-week remediation program (Fast ForWord Language; refs. 14 and 15). Twelve normally reading children underwent two fMRI and testing sessions ≈8 weeks apart. These children did not undergo remediation and served as a control for changes in brain function associated with undergoing the imaging process twice, practice effects, normal development over a 2-month period, and any other factors not related to remediation.
The remediation program (see Methods), a commercially available program used in a number of schools and clinics, has as its focus the building of auditory processing and oral language skills important for reading. The program trains subjects by using seven computer-based training exercises that emphasize different aspects of oral language, including auditory attention, discrimination, and memory, as well as phonological processing and listening comprehension.
We hypothesized that the children with dyslexia would both show improvement in language processing and reading ability after this remediation and exhibit changes in brain function. Changes in brain function could be both normalizing and compensatory. The children with dyslexia had previously shown an absence of activation in left temporo-parietal cortex that was present in normal-reading children when they performed a phonological task (12). We hypothesized that after remediation, left temporo-parietal cortex in these children would show increased activity during phonological processing, bringing that region's activity closer to normal functioning. In addition to this “normalizing” effect of remediation, we hypothesized there would be changes in brain function in regions not normally active during phonological processing (compensatory effects), especially in right hemisphere homologues to left hemisphere language areas. Previous research on recovery of function after brain injury has shown that, in the face of left hemisphere damage, the right hemisphere can increase its activity in a compensatory manner (16–18). Because we suspected that the remediation may only partly ameliorate the left hemisphere disruptions, it was hypothesized that right hemisphere compensatory effects would be observed. In addition, we hypothesized increases in the brain regions involved in attention and memory, the anterior cingulate gyrus (19) and hippocampal cortex (20), respectively. We did not have specific hypotheses about possible changes in left frontal language areas. The children with dyslexia had previously shown activation in left frontal cortex during phonological processing (12), but their activation was localized more anteriorly and medially than normal-reading children. However, reports have been varied with regard to left frontal disruptions in dyslexia, so it was not clear whether the differences we observed in left frontal localization were fundamental to dyslexia or whether they would be affected by remediation.
Methods
Subjects.
Subjects, a subset of those described (12), were English monolinguals and informed (with their parents) of their rights before participating in procedures approved by the Stanford Panel on Human Subjects in Medical Research. All children were physically healthy and free of neurological disease, head injury, and psychiatric disorder. Control children and children with dyslexia (Table 1) were between 8–12 years of age and matched for age, gender, handedness, and nonverbal IQ (Block Design subtest of Weschler Intelligence Scale for Children-III). The dyslexic group had a mean scaled score on the Woodcock-Johnson Reading Mastery Test-Revised (WJRMT-R; AGS Publishing, Circle Pines, MN) Word Attack or Word Identification subtests of <85 (standard score = 100, SD = 15). Reading scores also included the Passage Comprehension subtest of WJRMT-R. Language was measured with the Clinical Evaluation of Language Fundamentals-3 (CELF-3; The Psychological Corporation, San Antonio, TX). The Rapid Naming subtest of the Comprehensive Test of Phonological Processing (CTOPP; Pro-Ed, Austin, TX) was also administered.
Table 1.
Subject characteristics
| Dyslexic | Normal | Significance | |
|---|---|---|---|
| Subject characteristics | |||
| Number | 20 | 12 | |
| Age, years | 9.9 (1.5) | 10.7 (9.5) | ns |
| Gender, % male | 75 | 75 | ns |
| Handedness, % left | 17 | 20 | ns |
| Nonverbal IQ-Block Design | 11.75 (2.6) | 13.58 (2.7) | ns |
| Reading: WJ-RMT | |||
| Word ID | 78.2 (9.0) | 109.0 (6.8) | P < 0.0001 |
| Word Attack | 85.5 (7.9) | 112.3 (9.9) | P < 0.0001 |
| Passage Comprehension | 83.3 (7.9) | 112.8 (4.5) | P < 0.0001 |
| Language: CELF | |||
| Receptive Language | 92.5 (12.1) | 118.6 (8.3) | P < 0.0001 |
| Expressive Language | 95 (14.8) | 112.3 (8.3) | P = 0.0009 |
| Rapid Naming–CTOPP | 79.1 (14.5) | 106.8 (7.9) | P < 0.0001 |
WJ-RMT, Woodcock–Johnson Reading Mastery Test; CELF, Clinical Evaluation of Language Fundamentals; CTOPP, Comprehensive Test of Phonological Processing.
Behavioral Remediation.
Fast ForWord Language (Scientific Learning Corporation, Oakland, CA) is a computerized intervention program composed of seven adaptive exercises designed to improve auditory and language processing by using nonlinguistic and acoustically modified linguistic speech (rapid frequency transitions in speech are slowed and amplified). The seven exercises are as follows. Circus Sequence involves discrimination between sequences of two brief successive acoustic frequency sweeps, which are separated by a specified inter-stimulus-interval. Old MacDonald's Flying Farm involves distinguishing between sound changes of individual phonemes. Phoneme Identification involves the identification of specific phonemes from a series of consonant-vowel (CV) and vowel-consonant-vowel (VCV) stimulus pairs. Phonic Match involves matching CV's within simple word structures. Phonic Word involves distinguishing between words that differ only by an initial or final consonant by identifying which of two pictures represents a target word. Block Commander involves following instructions of increasing length and/or grammatical complexity. Language Comprehension Builder, based on Curtiss-Yamada Comprehensive Language Evaluation, involves distinguishing 40 classes of grammatical structures and rules. Children trained on the exercises for 100 min per day, 5 days per week, for an average of 27.9 training days.
FMRI Experimental Procedure and Analysis.
FMRI stimuli, tasks, and imaging procedures were as described (12). Before and after training scans involved identical tasks and imaging acquisition for both dyslexic and control groups. Subjects performed three tasks in a block design. For a phonological processing task, “Rhyme Letters,” the child saw on each trial two letters and was instructed to push a button if the names of the letters rhymed with each other (e.g., “T” and “D” rhyme, “G” and “K” do not). For a nonphonological task that also involved seeing letters, “Match Letters,” children pushed a button if the two letters were identical (e.g., “P” and “P”). For a nonletter baseline task “Match Lines,” children pushed a button if two lines were the same orientation. By comparing “Rhyme Letters” to “Match Letters,” we examined brain activity due to the phonological demands of the rhyme task, rather than orthographic processing of letters or other task demands. Each trial was 3.2 sec; there were five trials plus instruction per block, six blocks for each condition, and a total scan length of 4.5 min.
Imaging was performed on 3T Signa LX (GE Medical Systems). Localizer: gradient echo, 1 echo, echo time (TE) = 9.0, repetition time (TR) = 50, flip = 30, 256 × 128, field of view (FOV) = 24 cm, five coronal slices, 5 mm, 2.5 mm skip, scan = 19 sec. SPGR: 3D fast SPGR, 1 echo, TE = min, flip = 15, 256 × 192, FOV = 24 cm, 128 sagittal slices, 1.5 mm, 1 slab, scan = 2 m 45 sec. Inplane: T1 spin echo, 1 echo, TE MinFull, TR = 500 ms, 256 × 160, FOV = 24 cm, 18 axial slices, 6 mm thick, 0 mm skip, scan = 2 m 16 sec. FMRI: T2* gradient echo spiral (21), 1 interleave, TE = 30 ms, TR = 1.5 sec, flip = 90, FOV = 24 cm, 64 × 64, 180 temporal frames.
Subjects were immobilized with C-spine immobilization tools modified from tools used in emergency response (HeadBed, Cervical Immobilization Device, Laerdal Medical Corp, Wappinger Falls, NY) and a chin strap. Root-mean squared motion correction across all three directions and time points was used to generate an estimate of motion. Motion was minimal, and there were no significant differences between dyslexic (0.4 mm) and control groups (0.3 mm) or between first (0.4 mm) and second (0.3 mm) scans. Preprocessing and analysis were performed primarily with SPM 99 (Wellcome Department of Cognitive Neurology, University College of London) and included motion correction, using AIR V.3.0 (22) and smoothing (Gaussian filter = 8 mm FWHM). Individual analysis was performed by using the general linear model (23) on nonnormalized data (fixed effects model, high-pass filter = 108 sec, low-pass filter = hrf, global scaling). SPGR was normalized to T1 template (MN1305 stereotaxic space) in SPM 99 and then used as a template to normalize the in-plane anatomy (sinc interpolation, 2-mm voxels, using 12 nonlinear iterations). Contrast images for each subject were normalized (tri-linear interpolation, 1 × 1 ×1-mm voxels) by using the parameters from the in-plane normalization. Group analysis for each group (control and dyslexic) for each scan time (pre and post) was performed with a random effects analysis (24). Training effects were analyzed with a random-effects paired t test analysis for controls (12 pairs) and dyslexics (20 pairs) by using each subject's contrast image (rhyme as compared with match letters) pretraining and posttraining. A mask was made that excluded any regions that showed a change in the control group (P < 0.01, 20-voxel threshold) and applied to the dyslexics' paired t test. All reported changes in the dyslexic group, therefore, are in regions that showed no change in the control group.
A left temporo-parietal region of interest was defined by creating a sphere that encompassed left Brodmann area (BA) 39, centered at MNI coordinates −54, 16, 18, radius 10 mm. Other regions of interest were defined functionally by using specified spheres encompassing each of the regions that showed a group effect of training [l inf frontal (r = 6.2 mm at Talairach, −61, −6, 13); r precuneus (r = 13 mm at 12, −64, 11); r mid temporal (r = 55 mm at 47, −61, 13); r auditory cortex (r = 8 mm at 37, −38, 13); r frontal (r = 10 mm at 30, 20, 14); and l hippocampal gyrus (r = 5 mm at −12, −43, −5)]. Normalized contrast images were interrogated to extract parameter estimates from the multiple regression for each subject.
Results
Behavioral Results.
The children with dyslexia improved significantly in reading ability, as measured by tests of real word reading (Word Identification), pseudo-word decoding (a measure of phonological awareness) (Word Attack), and passage comprehension (Table 2). The improvements on these three tests raised the dyslexic group's scores into the normal range (>85). Children with dyslexia also improved in oral language ability and rapid naming. The extent of improvement was significant as measured by paired t tests for each test (Table 2). However, there was individual variability in the extent of improvement. For reading (as measured by a composite Basic Reading Score), one child had <0 and eight children had <5 points improvement. For language ability (as measured by a composite Total Language score), five children had close to or <0 and five children had close to 5 points improvement. We were unable to determine what factors were reliable predictors of extent of improvement. Neither the dyslexic nor control groups showed a change in performance on the letter rhyme task performed during fMRI (percent accuracy: dyslexics: pretraining = 71.2, posttraining = 76.0, P > 0.1; controls: pretraining = 83.1, posttraining = 84.1).
Table 2.
Behavioral measures of reading and language
| Dyslexic-reading children
|
Normal-reading children
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pretraining | Posttraining | T-stat | P | 1st scan | 2nd scan | T-stat | P | |
| Reading: WJ-RMT | ||||||||
| Word ID | 78.2 (56–95) | 86.0 (72–99) | 3.9 | 0.0005 | 109.0 (95–120) | 108.3 (97–126) | 0.6 | 0.6 |
| Word Attack | 85.5 (72–102) | 93.7 (82–109) | 6.8 | 0.0001 | 112.3 (99–132) | 109.4 (99–125) | 1.1 | 0.3 |
| Passage Comp | 83.3 (51–103) | 88.9 (77–107) | 2.9 | 0.005 | 112.8 (104–120) | 110.3 (100–122) | 1.8 | 0.03 |
| Language: CELF-3 | ||||||||
| Receptive | 92.5 (69–120) | 101.3 (75–122) | 3.6 | 0.001 | 118.6 (108–135) | 121.8 (108–139) | 1.5 | 0.2 |
| Expressive | 95.0 (61–125) | 102.2 (80–150) | 2.8 | 0.006 | 112.3 (102–125) | 113.8 (92–139) | 0.5 | 0.6 |
| Rapid Naming | 79.1 (35–97) | 86.5 (67–103) | 2.8 | 0.006 | 106.8 (94–121) | 104.3 (82–124) | 0.9 | 0.4 |
Range is given in parentheses. T-stat for paired t test. P value: one tailed for dyslexics, two tailed for controls. WJ-RMT, Woodcock–Johnson Reading Mastery Test; CELF, Comprehensive Evaluation of Language Fundamentals.
FMRI Results.
Whole-brain analysis, performed to identify brain regions that showed greater activity for rhyming (vs. matching) letters after (vs. before) remediation, showed that children with dyslexia had increased activity in a number of brain regions (Table 3; and see Fig. 1). Increased activity after remediation was observed in left hemisphere temporo-parietal cortex and inferior frontal gyrus, both regions that showed activity in the normal-reading children performing this task. Increases were also seen in brain areas that were not active in the normal-reading children performing this task, including right inferior, middle and superior frontal gyri, and middle temporal gyrus. Other regions showing increased activity after remediation were bilateral cingulate gyrus, left hippocampal gyrus, left inferior temporal gyrus, left lingual gyrus, right precuneus/posterior cingulate, right parieto-occipital sulcus, and bilateral thalamus.
Table 3.
Brain regions showing increased activity in dyslexics after remediation
| Region | X | Y | Z | P | Z score | Brodmann area | Size, voxels |
|---|---|---|---|---|---|---|---|
| Frontal lobe | |||||||
| L inferior frontal/precentral gyrus | −62 | −1 | 8 | 0.001 | 3.15 | 44/6 | 623 |
| R anterior cingulate | 8 | 19 | 33 | 0.001 | 3.06 | 32 | 2264 |
| R middle frontal gyrus | 25 | 23 | 37 | 0.001 | 3.03 | 9 | 3148 |
| R frontal insula/inferior frontal gyrus | 31 | 26 | 8 | 0.003 | 2.79 | 44 | 496 |
| L anterior cingulate gyrus | −12 | 31 | 27 | 0.004 | 2.64 | 23 | 160 |
| R superior frontal gyrus | 10 | 20 | 55 | 0.005 | 2.55 | 8 | 92 |
| Temporal lobe | |||||||
| R middle temporal gyrus | 44 | −58 | 4 | 0.000 | 3.73 | 21 | 8735 |
| L inferior temporal gyrus | −47 | −75 | −6 | 0.000 | 3.72 | 19 | 946 |
| L middle temporal gyrus/angular gyrus | −45 | −80 | 20 | 0.005 | 2.59 | 39 | 68 |
| L hippocampal gyrus | −12 | −42 | −6 | 0.005 | 2.58 | 30 | 103 |
| Parietal lobe | |||||||
| R posterior cingulate gyrus/precuneus gyrus | 7 | −56 | 18 | 0.001 | 3.24 | 23/30/31 | 7880 |
| R parieto-occipital sulcus | 11 | −79 | 32 | 0.003 | 2.73 | 19 | 362 |
| Occipital lobe | |||||||
| L lingual gyrus | −4 | −74 | −5 | 0.002 | 2.96 | 18 | 390 |
| Subcortical | |||||||
| Bi anterior thalamus | −11 | 0 | 8 | 0.001 | 3.08 | n/a | 909 |
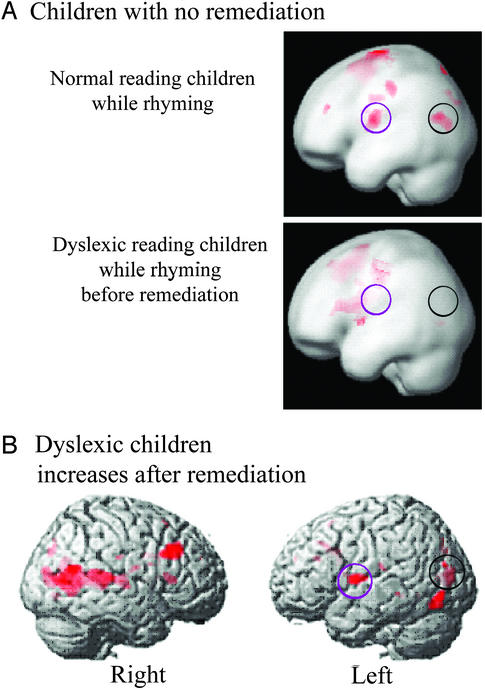
Figure 1.
Neural effects of remediation in children with developmental dyslexia. (A) Left hemisphere activations of control children and children with dyslexia are shown during rhyming (as compared with matching) letters (P < 0.025, 20-voxel threshold; ref. 12). (B) Brain areas that showed increased activity during phonological processing in the dyslexic group after remediation. Shown at P < 0.01, 20-voxel threshold. Black circles highlight left temporo-parietal region, which is disrupted in children with dyslexia and affected by remediation. Purple circles highlight the left frontal region that is active in control children and is affected by remediation in children with dyslexia.
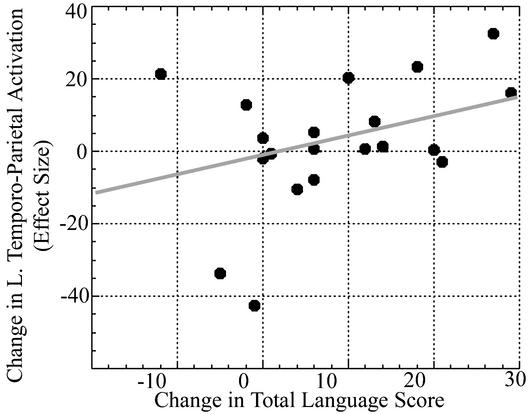
The left temporo-parietal region, which showed increased activity after remediation was near (foci 1.85 cm apart) but not overlapping the temporo-parietal region which had been shown, in these same children, to be under-activated (12) compared with controls. An ROI analysis was performed by using an ROI that encompassed the left temporo-parietal cortex that had shown differences between dyslexic and control groups, and that had shown increased activity in the dyslexic group after training. This analysis showed a positive correlation between increase in oral language ability (CELF-3 total language) and increase in activation in the left temporo-parietal cortex (r = 0.41, P = 0.03) (Fig. 2). Increase in activity in this ROI was also correlated with improvement on the Phonic Word training exercise of Fast ForWord Language (r = 0.58, P = 0.012). Phonic Word requires the child to distinguish between spoken words that differ by only by an initial or final consonant. No correlation was found between increased activity in this ROI and improved scores on reading tests.
Figure 2.
Language improvement and increased brain function. Correlation between magnitude of change in left temporo-parietal ROI (BA 39) and improvement in oral language (r = 0.41, P = 0.03). Left temporo-parietal ROI encompassed brain areas that showed underactivation and increases after training in children with dyslexia. Change in effect size is on the vertical axis; change in total language score (CELF-3) is on the horizontal axis. Effect size is the weighted sum of parameter estimates from the multiple regression for rhyme vs. match contrast pre- and posttraining.
The left inferior frontal region, which showed increased activity after remediation in the children with dyslexia, was entirely overlapping with the left inferior frontal activity seen in the normal-reading control children performing this task. Previously (12), we had observed normal-reading children's left inferior frontal activity to be more posterior than that in the children with dyslexia, encompassing parts of BA 6 and 44 (Fig. 1A). After remediation, the children with dyslexia showed increased activity in this more posterior part of the inferior frontal gyrus (Fig. 1B). There were no significant correlations with behavioral improvements and increased activity in this region.
Functional ROIs were created for five other regions which showed an increase in activity after training. A significant correlation was found between improvement on a phonological processing measure (Blending Words subtest of CTOPP) and increased activity after remediation in right inferior frontal ROI (r = 0.43, P = 0.04). No other correlations between increases in brain activity and reading or language improvement were seen.
Discussion
As far as we are aware, this study is the first fMRI study to show changes in brain function in children with dyslexia after remediation. Remediation resulted in improved language, reading performance, and increased activation in multiple brain regions during phonological processing.
The neural effects of remediation occurred both in brain areas that are normally involved in phonological processing (but dysfunctional in dyslexia) and other regions that are not normally activated during phonological processing. In addition, the children with dyslexia showed a relationship between the amount of improvement in oral language and the extent of increase in left temporo-parietal cortex. Previous research has demonstrated plasticity in reading-impaired children by using electrophysiological (25, 26) or magnetoencephalographic (27) measurements. The current study, by using fMRI, demonstrates the brain location of this plasticity.
Normalizing Effects of Remediation.
Children with dyslexia showed increased activity after remediation in brain regions that had previously been underactive in children (and adults) with dyslexia as compared with controls (5, 12). These increases were seen in left temporo-parietal cortex and the posterior tip of the left inferior frontal gyrus.
Left temporo-parietal cortex has been shown to be involved in phonological processing in children and adults and to be disrupted in children and adults with dyslexia during phonological processing (5, 12, 28–32). We hypothesized that remediation would ameliorate this disrupted response. In support of this hypothesis, there was an increase in activity in left temporo-parietal cortex after training (Fig. 1). The increased temporo-parietal activity was near but not the same as the focus of activation seen in normal-reading control children doing the same task. This increase in left temporo-parietal activity, seemed, therefore, to reflect a partial but not a complete amelioration of the disrupted temporo-parietal response.
The left inferior frontal gyrus has been shown to be involved in phonological processing in children and adults (28, 31, 32). Before remediation, the children with dyslexia had shown activity in left inferior frontal gyrus, but in a different location than normal-reading children doing the same task (12). After remediation, these children showed increased activity in the portion of the left inferior frontal gyrus activated by control children doing this task. We had not made a specific hypothesis about the effects of remediation in this region because of conflicting previous results regarding left frontal dysfunction in dyslexia [increased activity in dyslexics (6, 11, 12), mislocalized (12), and no difference in dyslexics (7, 10)]. These results may reflect another normalizing effect of remediation; however, the role of left frontal language areas in dyslexia will need to be clarified to interpret more fully the changes in this region after remediation.
“Compensating” Effects of Remediation.
In addition to the above changes in brain activity seen in regions that are normally involved in phonological processing, children with dyslexia showed increases after remediation in several brain areas that are not normally active during this task. A number of right hemisphere regions showed increases in activity after remediation, including right inferior and superior frontal gyri, and middle temporal gyrus. These results are consistent with studies of recovery of function after left hemisphere damage that have shown increased activity in right hemisphere homologues of left hemisphere language areas associated with improved language ability (16–18).
A number of other regions showed increased activity in the children with dyslexia after remediation. Bilateral anterior cingulate gyrus, a region associated with increased attention (19) and deficient in attention disorders (33), showed increased activity after remediation. Attentional control was specifically trained, and this may reflect improvement in attention ability after remediation. Changes also were seen in left hippocampal gyrus, a region associated with memory and the ability to form associations (20), skills that are also emphasized in the program. The left inferior temporal gyrus, within an area some researchers have suggested is sensitive to visual processing of words (34), showed increases after remediation. An increase in this area may reflect changes in the way children with dyslexia process visually presented letters. This possibility is an interesting one because word-form analysis is not trained by the program, but hearing the sounds within words is trained. Right parieto-occipital sulcus, left lingual gyrus, precuneus, and bilateral thalamus also showed significant changes. It is plausible that many of these compensatory activations in brain regions not normally engaged in phonological processing will diminish as children learn to read better, and that many of the final changes in successful remediation will manifest in continued normalization of left temporo-parietal and frontal areas typically engaged in phonological processing. The present study examined the immediate effects of training on brain function, and future studies will need to examine the enduring effects of such training.
Correlations with Behavioral Improvements.
The left temporo-parietal cortex showed a relationship between increased activity after remediation and improvement in oral language ability and word blending, a measure of phonological awareness (Fig. 2). This program does not provide training in reading per se, but does provide training in oral language skills. Improvements in oral language ability and phonological processing have been associated with improvements in reading ability and are thought to subserve learning to read (2–4). Several sources of evidence indicate that visual orthography is mapped onto phonological knowledge in left temporo-parietal cortex, and that this mapping is impoverished in many children with dyslexia. Thus, it is plausible that the major direct initial benefit of this training lies in enhanced phonological awareness, with benefits to reading as a secondary consequence. This explanation may account for the fact that no significant correlation with reading improvement was found. These results suggest the possibility that this remediation program changed brain areas related to the sound structure of language, which in turn led to improved language and reading.
Limitations and Future Directions.
This study included one control group, a group of normal-reading children who underwent fMRI scanning twice. The use of this control group rules out any possible brain changes that may be caused by undergoing fMRI scanning twice, practice effects associated with undergoing the tasks a second time, and normal development that occurs in children over an 8-week period.
Another control that would be important to include in future research is an untreated dyslexic control group. This group would more closely match the dyslexic experimental group and would ensure that any test-retest, practice, or developmental effects that were controlled for here with normal-reading children are not different in children with dyslexia. In this initial study, we did not include this group so we could offer the remediation program to all of the children with dyslexia. Future research using a delayed entry dyslexic group could scan the children twice before remediation to obtain this important control, while still providing the remediation program to all children.
Another future experimental manipulation that would serve to clarify further these results would be a dyslexic experimental group that undergoes a different training program (placebo or another remediation). This step will be an important one to determine which of the changes in brain function we observed may be caused by remediation in general, and which are caused by this remediation program specifically. The current study showed increased neural response after the specific remediation program, Fast ForWord Language, which focuses on auditory processing and oral language. It is not known if the increases in brain activity seen here are unique to this remediation program. It may be that some changes in brain function seen here would be evident from any remediation program that improves reading ability, whereas other changes would prove specific to the type of remediation the subject receives. This research, by showing both improvement in reading ability and increased activity in brain areas associated with reading and language, supports the view that auditory processing is important for reading disorders. However, without a direct comparison with phonologically-based training programs, this research cannot make direct claims about the specificity of the changes in brain function we observed. Future work exploring these issues will be important in determining which brain changes are associated with remediation in general. and which are associated with specific remediation programs.
Finally, this remediation program, as with most programs designed to address language and literacy, has multiple components, including auditory processing training, phonological processing training, attention and memory training, and many other specific cognitive and social processes that are manipulated by the program. It is not clear from this study alone which specific components of the remediation program may be driving which specific changes in brain activity. It is likely that different components of the remediation program were driving changes in different brain regions (for example, changes in anterior cingulate may reflect training in attentional mechanisms). Future research that breaks down this and other training programs into their specific components will address these important questions.
Conclusions
This study demonstrates several important findings. These results demonstrate, first, that it is possible to visualize changes in brain function after reading remediation. Second, these results showed that the specific remediation program, Fast ForWord Language, resulted in changes in brain function that include left hemisphere language regions, right hemisphere homologues, and a number of other brain areas. Some of the changes brought the brain function of children with dyslexia closer to that seen in normal-reading children, whereas other changes seemed to be compensatory in nature. Finally, these results demonstrated that the commonly observed dysfunction in dyslexics' left temporo-parietal brain function (5) can be at least partly ameliorated through behavioral remediation. Children between 8 and 12 years old with dyslexia can show increased activity in this region after training, and the extent of the increases seen in this region correlated with the extent of improvement in language ability.
Acknowledgments
We thank the Haan Foundation for Children, which helped fund this research, the Charles Armstrong School, from which many of the dyslexic-reading children came, and the children and their families who participated in the project. E.T. was funded in part by the Howard Hughes Medical Institute. S.L.M., P.T., and M.M.M. are cofounders of Scientific Learning Corporation, the company that developed the Fast ForWord family of training programs.
Abbreviations
- fMRI
functional MRI
- ROI
region of interest
References
- 1.Shaywitz S E. N Engl J Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- 2.Snow C E, Burns M S, Griffin P. Preventing Reading Difficulties in Young Children. Washington, DC: Natl. Acad. Press; 1998. [Google Scholar]
- 3.Wagner R K, Torgenson J K. Psychol Bull. 1987;101:192–212. [Google Scholar]
- 4.Wagner R K, Torgesen J K, Rashotte C A, Hecht S A, Barker T A, Burgess S R, Donahue J, Garon T. Develop Psychol. 1997;33:468–479. doi: 10.1037//0012-1649.33.3.468. [DOI] [PubMed] [Google Scholar]
- 5.Temple E. Curr Opin Neurobiol. 2002;12:178–183. doi: 10.1016/s0959-4388(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 6.Brunswick N, McCrory E, Price C J, Frith C D, Frith U. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- 7.Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak R S, Frith C D. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- 8.Paulesu E, Demonet J F, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa S F, Cossu G, Habib M, Frith C D, Frith U. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- 9.Rumsey J M, Andreason P, Zametkin A J, Aquino T, King A C, Hamburger S D, Pikus A, Rapoport J L, Cohen R M. Arch Neurol. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- 10.Rumsey J M, Nace K, Donohue B, Wise D, Maisog J M, Andreason P. Arch Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- 11.Shaywitz S E, Shaywitz B A, Pugh K R, Fulbright R K, Constable R T, Mencl W E, Shankweiler D P, Liberman A M, Skudlarski P, Fletcher J M, et al. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Temple E, Poldrack R A, Salidis J, Deutsch G K, Tallal P, Merzenich M M, Gabrieli J D. NeuroReport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- 13.Shaywitz B A, Shaywitz S E, Pugh K R, Mencl W E, Fulbright R K, Skudlarski P, Constable R T, Marchione K E, Fletcher J M, Lyon G R, Gore J C. Biol Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- 14.Merzenich M M, Jenkins W M, Johnston P, Schreiner C, Miller S L, Tallal P. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 15.Tallal P, Miller S L, Bedi G, Byma G, Wang X, Nagarajan S S, Schreiner C, Jenkins W M, Merzenich M M. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- 16.Vikingstad E M, Cao Y, Thomas A J, Johnson A F, Malik G M, Welch K M. Neurosurgery. 2000;47:562–570. doi: 10.1097/00006123-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Cao Y, Vikingstad E M, George K P, Johnson A F, Welch K M. Stroke. 1999;30:2331–2340. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- 18.Thulborn K R, Carpenter P A, Just M A. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 19.Bush G, Luu P, Posner M I. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 20.Brewer J B, Moghekar A. Trends Cogn Sci. 2002;6:217–223. doi: 10.1016/s1364-6613(02)01881-8. [DOI] [PubMed] [Google Scholar]
- 21.Glover G H, Lai S. Magn Reson Med. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- 22.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomog. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Friston K J, Holmes A P, Worsley K J, Poline J P, Frith C D, Frackowiak R S J. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 24.Friston K J, Holmes A P, Price C J, Buchel C, Worsley K J. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 25.Kraus N, McGee T, Carrell T, Sharma A, Nicol T. Electroencephalogr Clin Neurophysiol Suppl. 1995;44:211–217. [PubMed] [Google Scholar]
- 26.Kujala T, Karma K, Ceponiene R, Belitz S, Turkkila P, Tervaniemi M, Naatanen R. Proc Natl Acad Sci USA. 2001;98:10509–10514. doi: 10.1073/pnas.181589198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simos P G, Fletcher J M, Bergman E, Breier J I, Foorman B R, Castillo E M, Davis R N, Fitzgerald M, Papanicolaou A C. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- 28.Poldrack R A, Wagner A D, Prull M W, Desmond J E, Glover G H, Gabrieli J D. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 29.Demb J B, Poldrack R A, Gabrieli J D E. In: Converging Methods for Understanding Reading and Dyslexia. Klein R M, McMullen P A, editors. Cambridge, MA: MIT Press; 1999. pp. 245–304. [Google Scholar]
- 30.Fiez J A, Petersen S E. Proc Natl Acad Sci USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pugh K R, Shaywitz B A, Shaywitz S E, Constable R T, Skudlarski P, Fulbright R K, Bronen R A, Shankweiler D P, Katz L, Fletcher J M, Gore J C. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- 32.Cabeza R, Nyberg L. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 33.Bush G, Frazier J A, Rauch S L, Seidman L J, Whalen P J, Jenike M A, Rosen B R, Biederman J. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 34.Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]