Abstract
Objective
To investigate the state of activation of the ATP-ubiquitin-dependent proteolytic system in the skeletal muscle of gastric cancer patients.
Summary Background Data
Muscle wasting in experimental cancer cachexia is frequently associated with hyperactivation of the ATP-dependent ubiquitin-proteasome proteolytic system. Increased muscle ubiquitin mRNA levels have been previously shown in gastric cancer patients, suggesting that this proteolytic system might be modulated also in human cancer.
Methods
Biopsies of the rectus abdominis muscle were obtained intraoperatively from 23 gastric cancer patients and 14 subjects undergoing surgery for benign abdominal diseases, and muscle ubiquitin mRNA expression and proteasome proteolytic activities were assessed.
Results
Muscle ubiquitin mRNA was hyperexpressed in gastric cancer patients compared to controls. In parallel, three proteasome proteolytic activities (CTL, chymotrypsin-like; TL, trypsin-like; PGP, peptidyl-glutamyl-peptidase) significantly increased in gastric cancer patients with respect to controls. Advanced tumor stage, poor nutritional status, and age more than 50 years were associated with significantly higher CTL activity but had no influence on TL and PGP activity.
Conclusions
These results confirm the involvement of the ubiquitin-proteasome proteolytic system in the pathogenesis of muscle protein hypercatabolism in cancer cachexia. The observation that perturbations of this pathway in gastric cancer patients occur even before clinical evidence of body wasting supports the thinking that specific pharmacologic and metabolic approaches aimed at counteracting the upregulation of this pathway should be undertaken as early as cancer is diagnosed.
Loss of lean body mass is commonly observed in cancer as well in other catabolic conditions such as sepsis, burns, severe injury, and renal failure. The progressive muscle wasting may have a negative impact on both the quality of life and the tolerance to antineoplastic therapies, accounting for one to two thirds of deaths in cancer patients. 1,2
Muscle depletion reflects an imbalance between the rates of protein synthesis and breakdown. Studies performed in experimental models as well as in human cancer have shown that muscle atrophy may result from increased degradation, reduced synthesis, or both. However, hypercatabolism of muscle protein is a frequent feature, while changes in protein synthesis seem to occur less frequently. 3
Intracellular protein degradation in the skeletal muscle depends on several proteolytic systems: the acidic lysosomal pathway, the calcium-dependent pathway, and the ATP-ubiquitin-dependent pathway. 4 The role played by the lysosomal and the calcium-dependent systems in the onset of muscle protein hypercatabolism does not seem to be crucial. Recently, however, calcium-dependent proteases have been proposed to be required for the initial degradation of myofibrillar proteins 5 and have been found to be activated in the skeletal muscle of tumor-bearing rats. 6 Upregulation of components of the ATP-ubiquitin-dependent pathway has been reported in experimental models of sepsis, trauma, burns, renal failure, acidosis, and cancer. 7–14 Overexpression of ubiquitin mRNA closely parallels the enhancement of protein degradation in the skeletal muscle of rats bearing the Yoshida AH-130 ascites hepatoma. 11 Moreover, treatment of the AH-130 hosts with agents able to block the onset of tissue protein hypercatabolism, such as anti-TNF antibodies or clenbuterol, also reduces muscle ubiquitin mRNA levels. 12,14 Modulations of the ubiquitin-proteasome pathway have been also documented in patients suffering from sepsis, trauma, AIDS, and cancer. 14–17
The ATP-ubiquitin-dependent proteolysis involves an enzymatic cascade by which multiple ubiquitin molecules are covalently attached to the protein substrate, which is then degraded by the 26S proteasome complex. The catalytic core of the 26S proteasome is the 20S proteasome, characterized by five peptidase activities: the trypsin-like (TL), chymotrypsin-like (CTL), peptidyl-glutamyl peptidase (PGP), branched-chain amino acid- preferring, and small neutral amino acid-preferring activities. 18
The aim of the present study was to investigate the state of activation of the ATP-ubiquitin-dependent proteolytic system in the skeletal muscle of gastric cancer patients. The results show that the cleavage of specific fluorogenic substrates is increased in the muscle of cancer patients with respect to controls, indicating that proteasome proteolytic activity is significantly enhanced in cancer hosts.
PATIENTS AND METHODS
The study was approved by the local ethic committees. Twenty-three consecutive patients with gastric cancer admitted to the Istituto di Clinica Chirurgica of the Università Cattolica del Sacro Cuore and to the Dipartimento di Medicina Clinica of the University ‘La Sapienza’ of Rome, Italy, between January 2000 and January 2001 were included in the study protocol. Diagnosis of gastric cancer was made by endoscopic biopsy.
Fourteen patients undergoing surgery for benign abdominal diseases served as a control group. Exclusion criteria for both groups were as follows: acute or chronic renal failure (serum creatinine > 1.2 mg/dL), liver failure, diabetes, metabolic acidosis, sepsis, AIDS, inflammatory bowel disease, autoimmune disorders, chronic heart failure, acute and chronic hepatitis, hyperthyroidism, and chronic obstructive pulmonary disease.
Protocol
Written informed consent for the study procedures was obtained from the patients. All subjects were studied at 8 am, after overnight fasting. Blood samples for subsequent biochemical analyses were obtained from an antecubital vein immediately before entering the operating room.
Nutritional Assessment
The nutritional assessment included anthropometric (height, actual body weight, body mass index [BMI]), usual body weight, percent weight loss, and immunologic (total lymphocyte count) and biochemical (serum albumin) indices.
Muscle Biopsy
A biopsy specimen was obtained from the rectus abdominis muscle during the initial phase of the operation. After skin incision and dissection through the subcutaneous fat, the anterior sheet of the rectus abdominis muscle was opened with scissors and a muscle biopsy specimen weighing ∼0.5 g was obtained. The biopsy specimen was immediately frozen in liquid nitrogen and then stored at −70°C until analyzed. After the muscle biopsy had been obtained, small bleeding vessels were carefully controlled with ligatures and cautery and the operation continued in a routine fashion. No complications occurred from the biopsy procedure.
Ubiquitin mRNA Expression
Total RNA from the rectus abdominis muscle was extracted using the guanidinium isothiocyanate/phenol/chloroform method as described by Chomczynski and Sacchi. 19 RNA samples (40 μg/mL) were denatured, subjected to 1.2% agarose gel electrophoresis, and transferred to Hybond H membranes (Amersham International, Buckinghamshire, UK). RNA was fixed to membrane by UV illumination for 4 minutes.
Prehybridization was done in 50% formamide/5× SSC (1× is 0.3 mol/L NaCl, 65 mmol/L sodium citrate)/5× Denhart’s solution (1× Denhart’s solution is 0.1% polyvinylpyrrolidone, 0.1% Ficoll, 0.1% BSA)/20 mmol/L sodium phosphate pH 6.8/0.1% SDS μg/mL denatured salmon sperm DNA overnight at 42°C. Membranes were hybridized with appropriate probes (106–107 cpm/mL) at 42°C for 18 hours. Nonspecifically bound probe was removed by successive washes in 2× SSC (15 minutes at 55°C, twice), 2× SSC + 0.1% SDS (30 minutes at 55°C), and 0.1× SSC + 0.1% SDS (15 minutes at 55°C, twice). Specific hybridization was then detected by autoradiography. 13 Radiolabeled probes were prepared by the random priming method (Boehringer-Mannheim, Barcelona, Spain). The ubiquitin probe used was a cDNA clone containing 12 bp of the second ubiquitin coding sequence plus a complete third and fourth ubiquitin coding sequence and 120 bp of the 3′-untranslated region of the chicken polyubiquitin gene UBI. 16 A probe for the 18S ribosomal subunit was used as a correction factor to quantitate ubiquitin mRNA units. Filters were exposed to X-Omat AR-5 films (Eastman Kodak Co., Rochester, NY) at −70°C for 2 to 4 days.
Proteasome Activities
Muscle proteasome activity was determined by evaluating the cleavage of specific fluorogenic substrates. 20 The muscle was homogenized in 20 mmol/L TRIS-HCl pH 7.2 containing 0.1 mmol/L EDTA, 1 mmol/L 2-mercaptoethanol, 5 mmol/L ATP, 20% glycerol, and 0.04% (v/v) Nonidet P-40. Muscle homogenates were then centrifuged at 13,000 g for 15 minutes at 4°C. The supernatant was collected and protein concentration determined by the method of Lowry et al. 21 Aliquots of 50 μg protein were then incubated for 60 minutes at 37°C in the presence of fluorogenic substrates (succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin [LLVY-mca], benzyloxycarbonyl-Leu-Leu-Glu-7-naphtylamide [Z-LLE-nap], t - butoxycarbonyl - Leu - Ser - Thr - Arg - 7 - amidocoumarin [LSTR- mca]; Sigma, St. Louis, MO). The incubation buffer for the evaluation of proteasome activity was 50 mmol/L HEPES pH 8.0 containing 5 mmol/L EGTA. Fluorescence was read with a spectrofluorometer (380 nm excitation/460 nm emission; Perkin-Elmer, Norwalk, CT). The activity, expressed as nkatal/mg protein, was calculated by using free amidocoumarin as working standard.
Data Presentation and Statistics
Data are presented as means ± SD. For each parameter, patients and controls were compared by the Student t test for unpaired data and the Mann-Whitney test, as appropriate. The one-way ANOVA test was used when indicated. P < 0.05 was considered statistically significant.
RESULTS
Subject characteristics are shown in Tables 1 and 2. Cancer patients were divided into three groups according to the UICC classification of tumor stage: group I-II includes stages 1a, 1b, and 2; group II includes stages 3a and 3b; group III includes stage 4. 17 Mean weight loss with respect to usual body weight was significantly higher (P = .001) in cancer patients than in controls. When patients were stratified according to the degree of weight loss, 15 of 23 (65%) were non-weight-losing (NWL; weight loss <10% usual body weight), while 8 of 23 (35%) were weight-losing (WL; weight loss ≥10% usual body weight).
Table 1. CHARACTERISTICS OF SUBJECTS
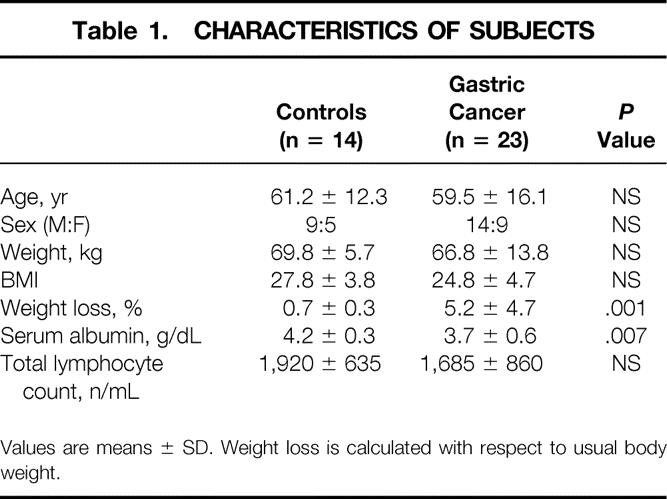
Values are means ± SD. Weight loss is calculated with respect to usual body weight.
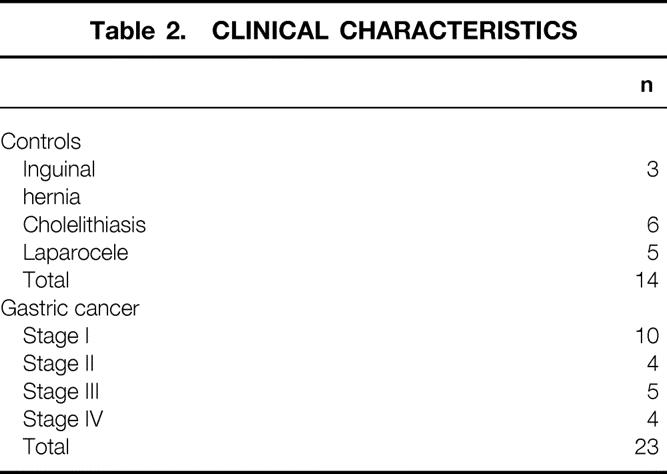
Table 2. CLINICAL CHARACTERISTICS
The expression of ubiquitin mRNA was analyzed in muscle samples by Northern blotting. Confirming our previous results, muscle ubiquitin mRNA levels were significantly higher in gastric cancer patients than in controls (P = .0005;Table 3).
Table 3. UBIQUITIN mRNA LEVELS

Values are arbitrary units, means ± SD.
Figure 1 shows that the fluorogenic substrates specific for three different proteasome proteolytic activities (CTL, TL, and PGP) were cleaved more efficiently in preparations from gastric cancer patients than from controls, demonstrating that proteasome proteolytic activity is significantly increased in the former (fivefold for CTL, twofold for both TL and PGP).
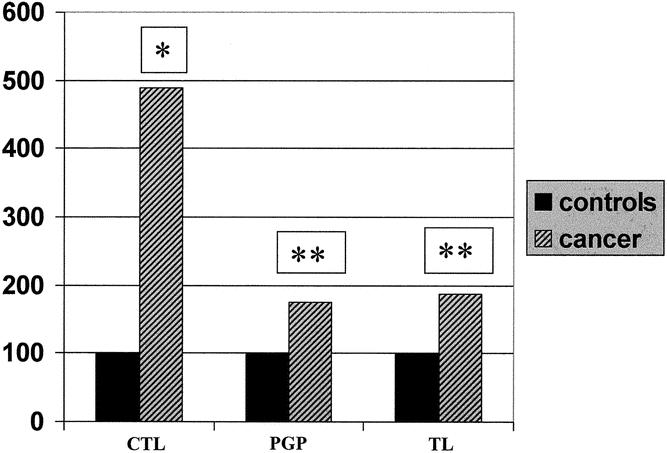
Figure 1. Proteasome activity in rectus abdominis muscle biopsies. The activity has been measured against the fluorogenic substrates succinyl-leu-leu-val-tyr-7-amido-4-methylcoumarin (LLVY), N-carbenzoxy-leu-leu-glu-7-amido-4-methylcoumarin (LLE), and t-butoxycarbonyl-Leu-Ser-Thr-Arg-7-amido-4-methylcoumarine (LSTR-mca) in gastric cancer patients, expressed as percentage of controls. *P = .01, **P < .05. CTL, PGP, and TL activities (nkatal × 10−3/mg protein) in controls were 67.5 ± 37.4, 18.39 ± 13.6, and 8.7 ± 8.4, respectively
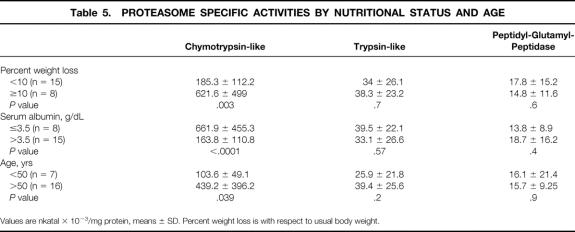
Among the three proteasome activities, CTL was affected by tumor stage, nutritional status (percent body weight loss and albuminemia), and age (Tables 4 and 5). Indeed, CTL activity was significantly higher in stage IV than in stage III and stage I-II patients (P = .032) as well as in WL than in NWL subjects (P = .003). CTL activity was also increased in NWL cancer patients compared to controls (185.3 ± 112.2 vs. 67.5 ± 37.4 nkatal × 10−3/mg protein, P < .0001). A huge increase in CTL activity was observed in patients with low serum albumin concentrations (≤3.5 g/dL) with respect to normoalbuminemic patients (P < .0001). The presence of both weight loss ≥ 10% and low serum albumin (in 5/23 patients) was associated with a further, although not significant, increase in CTL activity (860 ± 472 nkatal × 10−3/mg protein). Finally, when patients were stratified according to age, CTL activity was higher in older (>50 years) than in younger subjects (<50 years;P = .039). TL and PGP activities, although increased with respect to controls, were unaffected by tumor stage, nutritional status, and age.
Table 4. PROTEASOME SPECIFIC ACTIVITIES BY DISEASE STAGE
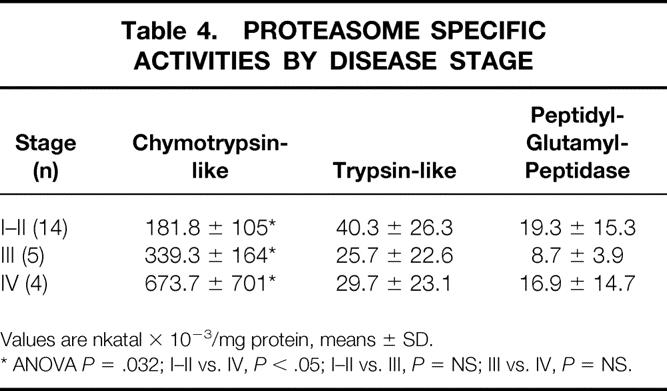
Values are nkatal × 10−3/mg protein, means ± SD.
* ANOVA P = .032; I–II vs. IV, P < .05; I–II vs. III, P = NS; III vs. IV, P = NS.
Table 5. PROTEASOME SPECIFIC ACTIVITIES BY NUTRITIONAL STATUS AND AGE
Values are nkatal × 10−3/mg protein, means ± SD. Percent weight loss is with respect to usual body weight.
DISCUSSION
Muscle wasting in experimental cancer cachexia has been shown to result mainly from enhanced protein degradation rates, which are associated with hyperexpression of components of the ATP-ubiquitin-dependent proteolytic pathway. 22–25 Consistent with these observations, increased levels of ubiquitin mRNA have been observed as well in human pathologies such as AIDS and cancer. 8,17,26
The ATP-ubiquitin-dependent proteolysis relies on ubiquitylation of target proteins, which are subsequently degraded by the 26S proteasome, a multicatalytic complex that contains a core (20S proteasome) endowed with multiple proteolytic activities. 18 Enhanced proteasome activity has been shown in rats with sepsis, burns, and cancer or after acute starvation, 27–30 while Dahlmann et al. 31 reported that neither the total amount of 20S proteasome nor its peptidase activity increases during starvation.
To the best of our knowledge, this is the first clinical study demonstrating that muscle proteasome activity is increased in human cancer. Indeed, a fivefold increase in CTL activity and a two-fold increase in both the TL and PGP ones were detected in cancer patients with respect to controls. These changes are paralleled by concomitant hyperexpression of muscle ubiquitin mRNA and appear to be of particular interest since 65% of the patients enrolled in the present investigation were NWL. The observation that the three activities are differently modulated, although intriguing, remains to be elucidated. The individual role of each proteasome catalytic subunit in muscle protein degradation is still unclear.
Advanced disease stage, weight loss, and hypoalbuminemia were associated with high CTL activity, in keeping with the observation that muscle ubiquitin mRNA levels are influenced by tumor stage, being higher in stages III and IV. 17 Low serum albumin and severe weight loss significantly correlate with CTL activity but not with ubiquitin mRNA levels, suggesting that modulations in the proteasome catalytic activity may precede changes in the ubiquitin mRNA pool and thus may be indicative of protein metabolic perturbations more accurately than ubiquitin mRNA levels. This possibility is particularly supported by the observation that CTL activity was significantly higher than in controls even in NWL and normoalbuminemic cancer patients.
Finally, CTL activity is influenced by age in cancer patients, being higher in subjects over 50 years old, though not in controls. This difference suggests that aging may substantially alter the response to the catabolic stimuli evoked by the tumor. The observation in the present study that the majority of older patients (10/16 [62.5%]) were affected by stage I-II tumors rules out the possibility that increased activity in older patients may be consequent to more advanced neoplastic disease.
In the light of the data reported in the present study, the proteasome may be suggested as an obvious target for anticatabolic therapy. Specific inhibitors such as lactacystin, peptide aldehydes, vinyl sulfones, and dipeptide boronic acid analogs have been recently described and tested. 32–35 In addition, anticytokine treatments may be useful to interfere with the activity of the ATP-ubiquitin-dependent proteolytic pathway. Indeed, several pathologic states associated with muscle wasting are characterized by detectable circulating cytokines, which are known to modulate the expression of molecules pertaining to the ATP-ubiquitin-dependent system. 36–41 Consistently, it has been shown that the levels of soluble TNFα-receptor are increased in gastric cancer patients. 17 In this regard, the hyperexpression of ubiquitin and proteasome mRNA in the skeletal muscle of tumor-bearing rats can be prevented by treatment with pentoxifylline, an inhibitor of TNFα synthesis, or with anti-TNFα antibodies. 16,42 In addition, pentoxifylline, alone or combined with suramin, a drug known to inhibit the interaction of several cytokines with their receptors, partially prevents the increase of proteasome CTL activity observed in the muscle of rats transplanted with the Yoshida AH-130 hepatoma. 30
In conclusion, our results demonstrate that muscle ATP-ubiquitin-dependent proteolysis is activated in human cancer, and this activation is associated with weight loss, hypoalbuminemia, and aging. These data strengthen the view that the ubiquitin-proteasome pathway is indeed involved in the progressive loss of muscle mass characteristic of cancer cachexia. The observation that ubiquitin mRNA hyperexpression and enhanced CTL activity are detectable even before clinical evidence of body wasting and cachexia supports the concept that early interventions aimed at counteracting the upregulation of this proteolytic pathway may represent a rational approach to cancer patient management.
Footnotes
Supported by the Società Italiana di Nutrizione Parenterale ed Enterale (SINPE) Research Plan Grant, Ministero per la Ricerca Scientifica e Tecnologica (MURST, Rome, Italy), Associazione Italiana per la Ricerca sul Cancro (Milan, Italy).
Correspondence: Maurizio Bossola, MD, Istituto di Clinica Chirurgica, Università Cattolica del Sacro Cuore, Roma, Largo Agostino Gemelli 8, 00168 Roma, Italy.
E-mail: maubosso@tin.it
Accepted for publication July 31, 2002.
References
- 1.Costelli P, Baccino FM. Cancer cachexia: from experimental models to patient management. Curr Opin Clin Nutrition Metab. 2000; 3: 177–181. [DOI] [PubMed] [Google Scholar]
- 2.DeWys W. Management of cancer cachexia. Semin Oncol. 1985; 12: 452–460. [PubMed] [Google Scholar]
- 3.Argilés JM, Costelli P, Carbó; N, et al. Tumour growth and nitrogen metabolism in the host. Int J Oncol. 1999; 14: 479–486. [PubMed] [Google Scholar]
- 4.Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001; 233: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams AB, Decourten-Myers GM, Fischer JE, et al. Sepsis stimulates release of myofilaments in skeletal muscle by a calcium-dependent mechanism. FASEB J. 1999; 13: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 6.Costelli P, Tullio RD, Baccino FM, et al. Activation of Ca(2+)-dependent proteolysis in skeletal muscle and heart in cancer cachexia. Br J Cancer. 2001; 84: 946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pallares-Trujillo J, Agell N, Garcia-Martinez C, et al. The ubiquitin system: a role in disease? Med Res Rev. 1998; 17: 139–161. [DOI] [PubMed] [Google Scholar]
- 8.Llovera M, Garcia-Martinez C, Agell N, et al. Ubiquitin and proteasome gene expression is increased in skeletal muscle of slim AIDS patients. Int J Mol Med. 1998; 2: 69–73. [PubMed] [Google Scholar]
- 9.Bailey JL, Wang X, England BK, et al. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996; 97: 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May RC, Kelly RA, Mitch WE. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J Clin Invest. 1986; 77: 614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costelli P, Garcia-Martinez C, Llovera M, et al. Muscle protein wasting in tumor-bearing rats is effectively antagonized by a beta 2-adrenergic agonist (clenbuterol). Role of the ATP-ubiquitin-dependent proteolytic pathway. J Clin Invest. 1995; 95: 2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costelli P, Carbó; N, Tessitore L, et al. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J Clin Invest. 1993; 92: 2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovera M, Garcia-Martinez C, Agell N, et al. Ubiquitin gene expression is increased in skeletal muscle of tumour-bearing rats. FEBS Lett. 1994; 338: 311–318. [DOI] [PubMed] [Google Scholar]
- 14.Mansoor O, Beaufrere B, Boirie Y, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA. 1996; 93: 2714–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiao G, Hobler S, Wang JJ, et al. Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J Clin Invest. 1997; 99: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llovera M, Carbo N, Garcia-Martinez C, et al Anti-TNF treatment reverts increased muscle ubiquitin gene expression in tumour-bearing rats. Biochem Biophys Res Commun. 1996; 221: 653–655. [DOI] [PubMed] [Google Scholar]
- 17.Bossola M, Muscaritoli M, Costelli P, et al. Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol. 2001; 280: R1518–R1523. [DOI] [PubMed] [Google Scholar]
- 18.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin- proteasome pathway in muscle atrophy. Curr Opin Clin Nutr Metab Care. 2001; 4: 183–190. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem. 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 20.Beyette J, Mason GGF, Murray RZ, et al. Proteasome activities decrease during dexamethasone-induced apoptosis of thymocytes. Biochem J. 1998; 332: 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry O, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193: 265–275. [PubMed] [Google Scholar]
- 22.Temparis S, Asensi M, Taillandier D, et al. Increased ATP-ubiquitin-dependent proteolysis in skeletal muscles of tumor-bearing rats. Cancer Res. 1994; 54: 5568–5573. [PubMed] [Google Scholar]
- 23.Baracos VE, DeVivo C, Hoyle DH, et al. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am J Physiol. 1995; 268: E996–E1006. [DOI] [PubMed] [Google Scholar]
- 24.Llovera M, Garcia-Martinez C, Agell N, et al. Muscle wasting associated with cancer cachexia is linked to an important activation of the ATP-dependent ubiquitin-mediated proteolysis. Int J Cancer. 1995; 61: 138–141. [DOI] [PubMed] [Google Scholar]
- 25.Lorite MJ, Smith HJ, Arnold JE, et al. Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer. 2001; 85: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams A, Sun X, Fischer JE, et al. The expression of genes in the ubiquitin-proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery. 1999; 126: 744–749. [PubMed] [Google Scholar]
- 27.Hobler SC, Williams AB, Fischer D, et al. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am J Physiol. 1999; 277: R434–R440. [DOI] [PubMed] [Google Scholar]
- 28.Fang CH, Li BG, Fischer DR, et al. Burn injury upregulates the activity and gene expression of the 20S proteasome in rat skeletal muscle. Clin Sci. 2000; 99: 181–187. [PubMed] [Google Scholar]
- 29.Whitehouse AS, Tisdale MJ. Downregulation of ubiquitin-dependent proteolysis by eicosapentaenoic acid in acute starvation. Biochem Biophys Res Commun. 2001; 285: 598–602. [DOI] [PubMed] [Google Scholar]
- 30.Costelli P, Bossola M, Muscaritoli M, et al. Effects of pentoxyphilline (PTX) and suramin (SUR) on cancer cachexia (CC) in rats bearing the AH-130 hepatoma [abstract]. Nutrition. 2000; 19; 30. [Google Scholar]
- 31.Dahlmann B, Kuehn L, Reinauer H, et al. Multicatalityc proteinase activity in skeletal muscle from starving rat. Biochem Soc Trans. 1987; 15: 963–964. [Google Scholar]
- 32.Adams J, Palombella AJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999; 59: 2615–2622. [PubMed] [Google Scholar]
- 33.Hobler SC, Tiao G, Fischer JE, et al. Sepsis-induced increase in muscle proteolysis is blocked by specific proteasome inhibitors. Am J Physiol. 1998; 274: R30–R37. [DOI] [PubMed] [Google Scholar]
- 34.Fang CH, Wang JJ, Hobler S, et al. Proteasome blockers inhibit protein breakdown in skeletal muscle after burn injury in rats. Clin Sci. 1998; 95: 225–233. [PubMed] [Google Scholar]
- 35.Tawa NE, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997; 100: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bossola M, Muscaritoli M, Bellantone R, et al. Serum tumor necrosis factor-α levels in cancer patients are discontinuous and correlate with weight loss. Eur J Clin Invest. 2000; 30: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 37.Shibata M, Takekawa M, Amano S. Increased serum concentrations of soluble tumor necrosis factor receptor I in noncachectic and cachectic patients with advanced gastric and colorectal cancer. Surg Today. 1998; 28: 884–888. [DOI] [PubMed] [Google Scholar]
- 38.Tessitore L, Costelli P, Baccino FM. Humoral mediation for cancer cachexia. Br J Cancer. 1993; 67: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Llovera M, Carbo N, Lopez-Soriano J, et al. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett. 1998; 133: 83–87. [DOI] [PubMed] [Google Scholar]
- 40.Saarinen UM, Koskelo EK, Teppo AM, et al. Tumor necrosis factor in children with malignancies. Cancer Res. 1990; 50: 592–595. [PubMed] [Google Scholar]
- 41.Balkwill F, Osborne R, Burke F, et al. Evidence for tumour necrosis factor/cachectin production in cancer. Lancet. 1987; 2: 1229–1232. [DOI] [PubMed] [Google Scholar]
- 42.Combaret L, Ralliere C, Taillandier D, et al. Manipulation of the ubiquitin-proteasome pathway in cachexia: pentoxifylline suppresses the activation of the 20S and 26S proteasomes in muscles from tumor bearing rats. Mol Biol Rep. 1999; 26: 95–101. [DOI] [PubMed] [Google Scholar]