Abstract
Objective
To determine the frequency and pattern of lymph node metastasis (LNM) from papillary thyroid microcarcinoma (PTMC) and the results of node dissection, and to establish the optimal strategy for neck dissection in these patients.
Summary Background Data
Most PTMCs carry a favorable prognosis, but a few present with palpable lymphadenopathy. Patients with LNM are at risk for nodal recurrence, although they do not have higher mortality. The frequency and pattern of LNM from PTMC and the results of node dissection are not well established.
Methods
The frequency and pattern of LNM from 259 PTMCs were analyzed according to the size and location of the primary tumor. Of the 259, 24 with palpable nodes underwent therapeutic node dissection and the other 235 patients without palpable nodes underwent prophylactic node dissection. The authors compared the results of node dissection between the therapeutic group and the prophylactic group, and between PTMCs 5 mm or smaller and PTMCs larger than 5 mm. The authors also compared nodal recurrence between the prophylactic group and a no-lymph-node-dissection group (155 PTMCs).
Results
Overall, 64.1% (166/259) and 44.5% (93/209) had node involvement of the central and ipsilateral lateral compartment, respectively. Pretracheal (43.2%), ipsilateral central (36.3%), and ipsilateral mid-lower (37.8%) jugular were more commonly involved. LNM was more frequent in the therapeutic group than in the prophylactic group (95.8% vs. 60.9% for central compartment, 83.3% vs. 39.5% for ipsilateral lateral compartment). Nodal recurrence was more common in the therapeutic group than in the prophylactic group (16.7% vs. 0.43%), but did not differ between the prophylactic group and the no-dissection group (0.43% vs. 0.65%). The tumor size did not influence nodal recurrence. Nodal recurrence preferentially occurred in ipsilateral mid-lower jugular nodes.
Conclusions
Patients who have PTMC presenting with palpable lymphadenopathy should have therapeutic node dissection. Prophylactic node dissection is not beneficial in those without palpable lymphadenopathy.
Papillary thyroid microcarcinomas (PTMCs) are small (≤10 mm) thyroid cancers. The majority of these are not palpable and are clinically inapparent. In the past, many PTMCs were found on pathology specimens from thyroid removed for benign diseases, such as multinodular goiter, follicular adenoma, and Graves’ disease. These small cancers were also found commonly on autopsy of patients who had died of non-thyroid-related diseases. 1–4 It is accepted that these small papillary thyroid cancers are common and rarely behave as cancers (with metastasis and invasion). 5–8 A subset of patients with PTMC, however, presents with palpable neck node metastasis, which then leads to the diagnosis of papillary thyroid cancers that were initially not apparent.
With the increasing use of thyroid ultrasound, many nonpalpable thyroid nodules are being found. The management of these clinically inapparent thyroid tumors (thyroid incidentalomas) is controversial. Those that are suspicious usually undergo fine-needle aspiration (FNA) biopsy under ultrasound guidance. 9 If papillary cancer is found on FNA, thyroidectomy is usually performed.
Whether lymph node dissection is indicated for all patients with papillary thyroid cancer is controversial. In Japan, many surgeons perform routine ipsilateral neck node dissection even in the absence of palpable lymph node (prophylactic node dissection) because of the high yield of pathologically involved nodes. In North America and Europe, however, only those patients with palpable lymph nodes undergo a lymph node dissection (therapeutic node dissection), because few patients would develop clinical lymphadenopathy. Thus, although it is agreed that therapeutic neck dissection is almost always indicated, the role of prophylactic neck dissection is unclear.
Since PTMCs are considered a less aggressive disease than papillary thyroid carcinomas of larger size, prophylactic node dissection may be even less useful for patients with PTMC. We therefore undertook this study in patients with PTMC, who underwent either therapeutic or prophylactic neck node dissection, to determine the frequency and pattern of lymph node metastasis and the risk of nodal recurrence after node dissection.
METHODS
Patients
Two hundred fifty-nine patients who underwent thyroidectomy and neck node dissection for PTMC at Ito Hospital between 1988 and 1998 were retrospectively analyzed. There were 29 men and 230 women. The age at initial treatment ranged from 17 to 72 years (mean 48). The mean duration of follow-up was 61.6 (range 13–144) months. PTMC was defined as tumor 10 mm or less in greatest diameter in accordance with the histologic classification of thyroid tumors by the World Health Organization. 10
Of the 259 patients, 235 underwent prophylactic node dissection. In 130 (50.2%) patients, primary thyroid tumors were palpable either during screening or follow-up for other thyroid diseases. In 101 (39.0%) patients, the tumors were discovered by screening with ultrasound. Because the Ito Hospital has a special interest in thyroid diseases, almost all patients seen in our institution undergo a thyroid ultrasound regardless of the presenting diagnosis. Thyroid ultrasound is considered part of the physical examination of the thyroid and is performed by all personnel who care for thyroid patients. Four (1.5%) patients presented with hoarseness due to recurrent nerve palsy.
In 235 patients, lymph nodes were not palpable at initial presentation or during the operation. Twenty-four (9.3%) patients presented with palpable lymphadenopathy and had therapeutic node dissection. Of these 24 patients, only 4 had obvious palpated thyroid nodule.
In 218 (84.2%) patients, preoperative FNA cytology showed papillary carcinoma. In the other 41 (15.8%), the FNA cytology was suspicious but not diagnostic preoperatively. All thyroid tumors and lymph node metastases were confirmed to be papillary thyroid carcinoma on pathologic examination.
To evaluate whether prophylactic node dissection is beneficial, we compared the recurrence rate from the 235 patients who underwent prophylactic node dissection with the recurrence rate from the 155 patients whose PTMCs were found incidentally after thyroidectomy for benign thyroid disease, during the same time period. The 155 patients consisted of 12 men and 143 women. The age at initial treatment ranged from 18 to 81 years (mean 49.4). The mean duration of follow-up was 53.0 (range 12–132) months. At Ito Hospital, we do neck dissection routinely for all patients undergoing thyroidectomy for papillary thyroid carcinoma regardless of stage or size. However, if papillary microcarcinoma is discovered in the thyroid specimen after the operation, we do not reoperate to resect lymph nodes.
Thyroid Tumor Location
The extent of initial thyroid resection was total lobectomy (including the isthmus) in 169 (65.3%), subtotal thyroidectomy (total lobectomy, isthmusectomy, and contralateral subtotal lobectomy) in 62 (23.9%), and total thyroidectomy in 28 (10.8%). Total lobectomy is the standard operation in our institution for routine unifocal papillary thyroid carcinoma smaller than 10 mm. Subtotal thyroidectomy is performed when the carcinoma is located near the contralateral thyroid lobe or another suspicious lesion is present in the lower pole or the middle of the contralateral thyroid lobe. Total thyroidectomy is performed when the carcinoma is multifocal in both thyroid lobes diagnosed by preoperative FNA or if the patient has bilaterally palpable lymph node metastases.
The location of the primary thyroid tumor was categorized as upper third, middle third, lower third, isthmus, multifocal in affected lobe, and multifocal in both lobes.
Node Dissection
Of the 259 patients, 24 with palpable nodes at initial presentation underwent modified neck dissection and were analyzed as the therapeutic node dissection group. Two of the 24 also had upper mediastinum node dissection at initial treatment. The other 235 patients underwent prophylactic node dissection (50 central neck dissection and 185 modified neck dissection) and were analyzed as the prophylactic group.
Definition of Cervical Compartment
Nine node sites in the central and lateral compartments were distinguished to analyze the frequency and pattern of lymph node metastasis, based on the “General Rules for the Description of Thyroid Cancer” by the Japanese Society of Thyroid Cancer. 11 Table 1 shows the correspondence between the node sites in the present study and the levels (II–VI) of cervical lymph nodes by the Head and Neck Service of Memorial Sloan-Kettering Cancer Center. 12 The central compartment was delimited superiorly by the hyoid bone, inferiorly by the innominate vein, laterally by the carotid sheaths, and dorsally by the prevertebral fascia and was divided into three node sites: pretracheal, ipsilateral, and contralateral. The lateral compartment was delimited superiorly by the hypoglossal nerve, inferiorly by the subclavian vein, and laterally by the trapezius muscle. Each lateral compartment (ipsilateral and contralateral) was divided into three node sites: upper lateral (the jugular chain above the bottom of the cricoid cartilage), mid-lower lateral (the jugular chain below the bottom of the cricoid cartilage), and posterior triangle (between the dorsolateral sternocleidomastoid muscle, the trapezius muscle, and the subclavian vein). The internal jugular vein, accessory spinal nerve, and sternocleidomastoid muscle were preserved at cervical node dissection.
Table 1. DEFINITION OF CERVICAL COMPARTMENT
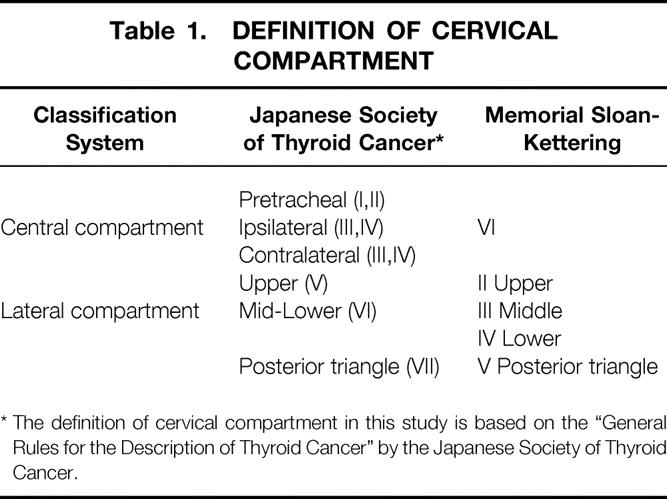
* The definition of cervical compartment in this study is based on the “General Rules for the Description of Thyroid Cancer” by the Japanese Society of Thyroid Cancer.
Frequency and Pattern
The frequency and pattern of lymph node metastasis (LNM) in the central and lateral compartments were analyzed according to the size and location of the primary tumor. For multifocal lesions, the analysis was performed according to the largest dominant tumor. These results were compared between the therapeutic group and the prophylactic group.
Evaluation of Node Dissection
We compared the results of prophylactic node dissection in the 235 patients who underwent thyroidectomy with a diagnosis of carcinoma to 155 patients who underwent thyroidectomy for benign thyroid disease but had incidental findings of PTMC. Because the PTMCs were discovered incidentally, these 155 patients did not have neck node dissection. We also compared the results of lymph node dissection between the therapeutic group and the prophylactic group, and between the 61 patients with PTMC ≤5 mm and the 198 patients with PTMC >5 mm.
Statistics
Results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using the Student t test or the Mann-Whitney test, where appropriate. Frequencies were compared using the chi-square test and Fisher exact probability test. Differences were considered significant when P < .05.
RESULTS
Size Distribution of Thyroid Tumor
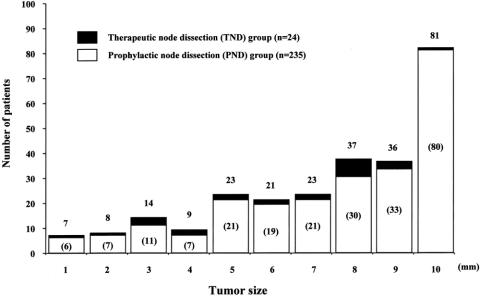
The distribution of the size of primary thyroid tumor in 259 patients with PTMC is shown in Figure 1. The mean ± SD was 7.5 ± 2.6 mm.
Figure 1. Distribution of the size of primary thyroid tumor in 259 patients with papillary thyroid microcarcinoma. In parentheses are the numbers of patients who underwent prophylactic node dissection.
Frequency and Pattern of Node Metastasis
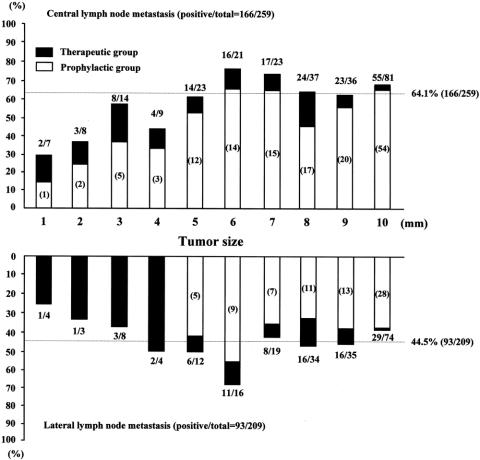
The frequency of LNM in the central and ipsilateral lateral compartments according to the size of the primary thyroid tumor is shown in Figure 2. Overall, 64.1% (166/259) and 44.5% (93/209) had involvement of the central and ipsilateral lateral compartments, respectively, and 69.5% (180/259) had LNM in at least one of the two compartments. The central compartment was involved more than the ipsilateral lateral compartment regardless of the size of the primary tumor.
Figure 2. Frequency of lymph node metastasis in the central (n = 259) and lateral (n = 209) compartment according to the size of primary thyroid tumor in patients with papillary thyroid microcarcinoma. The fraction above the histogram shows the number of patients with the compartment positive for carcinoma as a fraction of the number of patients who underwent dissection of that compartment. In parentheses are the numbers of patients who underwent prophylactic node dissection.
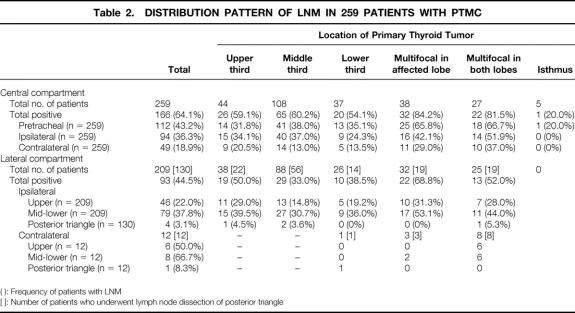
As shown in Table 2, the frequency of LNM in different sites varied. In the central compartment, the pretracheal (43.2%) and ipsilateral (36.3%) sites were more frequently involved than the contralateral (18.9%) site. In the lateral compartment, the ipsilateral mid-lower (37.8%) site was more commonly involved than the ipsilateral upper (22.0%) site. The posterior triangle (3.1%) was rarely involved. The contralateral lateral compartment also showed high frequency, but this is likely due to selection bias because only a few patients had contralateral lateral neck node dissection. Patients with multifocal tumors appear to have more LNM than those with unifocal tumor.
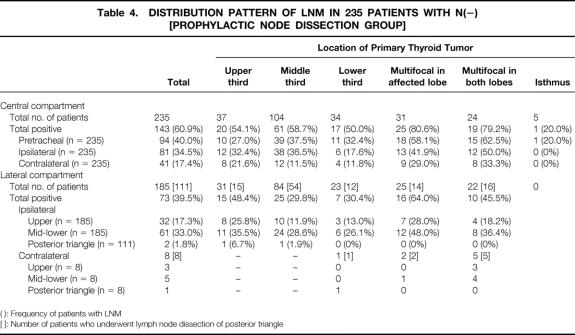
Table 2. DISTRIBUTION PATTERN OF LNM IN 259 PATIENTS WITH PTMC
( ): Frequency of patients with LNM
[ ]: Number of patients who underwent lymph node dissection of posterior triangle
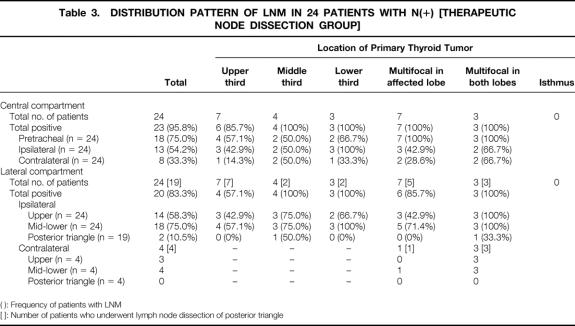
The 24 patients who underwent therapeutic node dissection were more likely to have LNM in most of the sites than the 235 patients who underwent prophylactic node dissection (Tables 3 and 4).
Table 3. DISTRIBUTION PATTERN OF LNM IN 24 PATIENTS WITH N(+) [THERAPEUTIC NODE DISSECTION GROUP]
( ): Frequency of patients with LNM
[ ]: Number of patients who underwent lymph node dissection of posterior triangle
Table 4. DISTRIBUTION PATTERN OF LNM IN 235 PATIENTS WITH N(−) [PROPHYLACTIC NODE DISSECTION GROUP]
( ): Frequency of patients with LNM
[ ]: Number of patients who underwent lymph node dissection of posterior triangle
Clinical Significance of Prophylactic Node Dissection
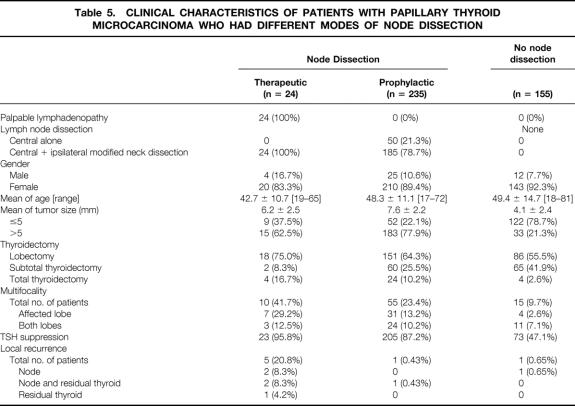
As shown in Table 5, only 1 (0.65%) of the 155 patients with PTMC who did not have lymph node dissection developed nodal recurrence after a follow-up of 12 to 132 (mean 53) months. Only 1 (0.43%) of the 235 patients who had prophylactic dissection developed recurrence in both regional lymph nodes and residual thyroid after a follow-up of 15 to 144 (mean 61.5) months. No statistically significant difference was found between these two groups.
Table 5. CLINICAL CHARACTERISTICS OF PATIENTS WITH PAPILLARY THYROID MICROCARCINOMA WHO HAD DIFFERENT MODES OF NODE DISSECTION
Characteristics of Lymph Node Metastasis
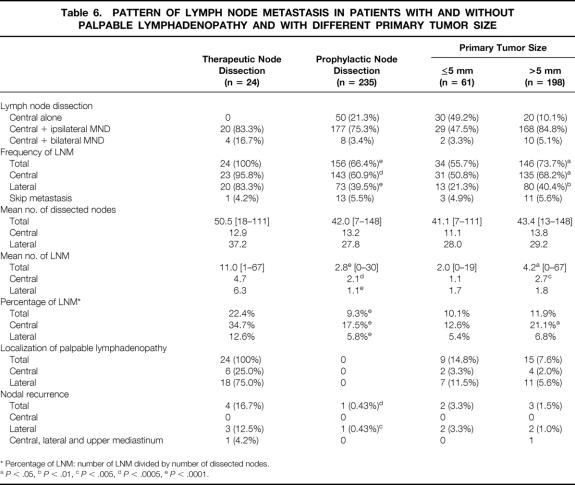
Table 6 compares the differences in the pattern of LNM between the therapeutic group and the prophylactic group, and between the 61 patients with PTMC ≤5 mm and the 198 patients with PTMC >5 mm. LNM was more frequent in the therapeutic group than in the prophylactic group (95.8% vs. 60.9%, P < .0005 for central nodes, 83.3% vs. 39.5%, P < .0001 for lateral nodes, and 100% vs. 66.4% overall). Also, the therapeutic group had more LNM than the prophylactic group both in numbers of nodes involved (11.0 vs. 2.8, P < .0001) and in the percentage of nodes dissected that were involved (22.4% vs. 9.3%, P < .0001). Skip metastasis (defined as lateral LNM in the absence of central LNM) was found in 1 patient (4.2%) in the therapeutic group and in 13 patients (5.5%) in the prophylactic group.
Table 6. PATTERN OF LYMPH NODE METASTASIS IN PATIENTS WITH AND WITHOUT PALPABLE LYMPHADENOPATHY AND WITH DIFFERENT PRIMARY TUMOR SIZE
* Percentage of LNM: number of LNM divided by number of dissected nodes.
aP < .05,
bP < .01,
cP < .005,
dP < .0005,
eP < .0001.
Four (16.7%) of the 24 patients who had therapeutic node dissection developed nodal recurrence, and 2 of these 4 had concurrent recurrence in the residual thyroid. One patient had five reoperations for recurrences in the central and lateral compartments and in the upper mediastinum. Nodal recurrence was rare in the prophylactic group (0.43%, P < .0005). Four of five nodal recurrences occurred in the mid-lower site of the ipsilateral lateral compartment. In the therapeutic group, palpable lymphadenopathy was the first manifestation in the central compartment in 6 (25%) patients and in the lateral compartment in 18 (75%) patients. Interestingly, in 2 of these 24 patients, only the palpable node had metastatic carcinoma (1/29 and 1/32 nodes dissected).
The size of PTMC influenced the frequency of LNM (55.7% for ≤5 mm vs. 73.7% for >5 mm, P < .05) and did not have an effect on nodal recurrence (3.3% vs. 1.5%, P = .3364).
Distant Metastasis and Mortality
None of the 259 patients developed distant metastasis or died from disease after a mean follow-up period of 61.6 months.
DISCUSSION
We analyzed the results of 24 therapeutic neck node dissections and 235 prophylactic neck node dissections in 259 patients with PTMC to determine the frequency and pattern of regional LNM and to establish the optimal strategy for neck dissection in these patients.
Both the central and ipsilateral lateral compartments are frequently affected with LNM, even in patients with very small PTMCs. 7,13 Within the central compartment, the pretracheal and the ipsilateral sites are commonly involved. Within the lateral compartment, the ipsilateral mid-lower site is commonly involved. The posterior triangle, on the other hand, is rarely involved. Skip lesion (involvement of lateral lymph nodes without central lymph nodes) is also rare. This pattern of node involvement is similar to that of the larger papillary thyroid carcinomas. 14–16 Patients with multifocal cancers are more likely to have nodal metastases. Similar to previous studies in papillary thyroid carcinomas and PTMCs, we found that the location of the primary tumor does not predict the pattern of LNM. 16,17 Nodal recurrence preferentially occurred in ipsilateral mid-lower jugular nodes. For therapeutic node dissection, therefore, one should concentrate on the ipsilateral mid-lower site to avoid recurrence, knowing that posterior triangle node metastases and skip metastases are uncommon.
It is not surprising that we found the therapeutic node dissection group to be significantly different than the prophylactic node dissection group, since they were selected based on whether the patient had palpable lymph nodes. Thus, the patients who underwent therapeutic dissection had more lymph node involvement than those who had prophylactic dissection, and they were also more likely to have lymph node recurrences.
In this study there was no patient who received ablation therapy with radioactive iodine at initial therapy. Despite the fact that TSH suppression therapy was performed in 95.8% of the patients in the therapeutic node dissection group, 16.7% of the patients in the therapeutic group had lymph node recurrence (see Tables 5 and 6). This recurrence rate may appear high, but they occurred in patients who had therapeutic neck dissection. This recurrence rate is within the range of those reported after therapeutic neck dissection for patients with larger primary papillary thyroid carcinoma. 18,19 This finding suggests that patients with palpable lymph node metastasis are at high risk of node recurrence regardless of the size of the primary carcinoma.
What may be surprising is the tremendous discrepancy between the high frequency of pathologic lymph node involvement (about 66%) and the rareness of clinical lymph node recurrence (about 0.4%) in the prophylactic dissection group. One may attribute this low rate of lymph node recurrence to the efficacy of the node dissection, but this cannot be, since the nodal recurrence rate was also only 0.65% in those who did not undergo neck node dissection. If prophylactic lymph node dissection were useful in lowering the risk of clinical node recurrence, we would expect to see a higher rate of nodal recurrence in those 155 patients who also had PTMC but did not have lymph node dissection.
The primary tumor size of PTMC influenced the frequency of LNM slightly, 55.7% for tumors 5 mm or less and 73.7% for those 5 to 10 mm (P < .05), but did not have an effect on nodal recurrence (3.3% vs. 1.5%, P = .3364). Kasai and Sakamoto 20 found a more significant difference in the frequency of LNM according to primary tumor size, 13% for those 5 mm or less and 59% for those 5 to 10 mm (P < .01). We found that palpable lymphadenopathy increased significantly the risk of nodal recurrence (16.7% recurred for palpable nodes vs. 0.43% for nonpalpable nodes, P < .0005). This is similar to the series of Sugitani et al., 21 where 20.6% (7/34) of patients with palpable lymph nodes developed nodal recurrence. They considered high-risk patients those with lymph nodes 3 cm or larger.
Prognostic factors or scoring systems have been used for differentiated thyroid carcinoma. 18,22,23 Reviewing 1,628 PTMCs, Yamashita et al. 24 found invasion through the capsule of the metastatic lymph node to be a unfavorable prognostic factor. Capsular invasion correlated with palpable lymphadenopathy (P = .0000); thus, it is more likely to be found in the therapeutic dissection group than in the prophylactic dissection group. In our series, all five patients who developed nodal recurrence had invasion through the capsule of the lymph node metastasis removed in the initial operation.
Other authors have considered familial cases of PTMC to be at high risk for recurrence. 25,26 For example, 5.9% (7/119) of the cases in the series from Lupoli et al. 25 were familial, and the disease recurred in 42.9% (3/7) of the patients with familial PTMC; 2 developed local recurrence and 1 developed lung metastasis and died of disease. Five (1.9%) of our patients had familial disease, but none had developed recurrence after resection. We do not know why our familial cases appeared not to be as aggressive as others have reported. In our series, one patient had a very aggressive disease treated with five reoperations and I131, but this patient did not have familial disease.
Although this was not a randomized controlled study and the results might be influenced by patient selection, a large randomized study for this question is impractical and unlikely. Our findings suggest that cancers from nonpalpable lymph nodes usually remain indolent and rarely become clinically significant; thus, prophylactic neck node dissection for PTMC is not recommended. This is consistent with current practice in North America and Europe for larger papillary thyroid carcinomas.
We conclude that although therapeutic neck dissection for palpable lymphadenopathy is indicated in patients with PTMC, prophylactic neck dissection is not. When performing therapeutic neck dissection for PTMC, one should concentrate on the ipsilateral mid-lower site to avoid recurrence, knowing that posterior triangle node metastases and skip metastases are uncommon.
Footnotes
Correspondence: Kiminori Sugino, MD, Ito Hospital, 4-3-6 Jinguumae, Shibuya-ku, Tokyo 150-8308, Japan.
E-mail: ito-h@kt.rim.or.jp
Accepted for publication July 18, 2002.
References
- 1.Fukunaga FH, Yatani R. Geographic pathology of occult thyroid carcinomas. Cancer. 1975; 36: 1095–1099. [DOI] [PubMed] [Google Scholar]
- 2.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985; 56: 531–538. [DOI] [PubMed] [Google Scholar]
- 3.Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol. 1988; 90: 72–76. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Maeda T, Izumi K, et al. Occult papillary carcinoma of the thyroid. A study of 408 autopsy cases. Cancer. 1990; 65: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 5.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990; 71: 414–424. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, Grant CS, van Heerden JA, et al. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992; 112: 1139–1146. [PubMed] [Google Scholar]
- 7.Noguchi S, Yamashita H, Murakami N, et al. Small carcinomas of the thyroid. A long-term follow-up of 867 patients. Arch Surg. 1996; 131: 187–191. [DOI] [PubMed] [Google Scholar]
- 8.Baudin E, Travagli JP, Ropers J, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998; 83: 553–559. [DOI] [PubMed] [Google Scholar]
- 9.Rosen IB, Azadian A, Walfish PG, et al. Ultrasound-guided fine-needle aspiration biopsy in the management of thyroid disease. Am J Surg. 1993; 166: 346–349. [DOI] [PubMed] [Google Scholar]
- 10.Hedinger C, Williams ED, Sobin LH. Histologic typing of thyroid tumors, 2nd ed, no. 11. In:International Histological Classification of Tumors, World Health Organization. New York: Springer-Verlag, 1988:9–10.
- 11.The Japanese Society of Thyroid Cancer. General rules for the description of thyroid cancer [in Japanese], 5th ed. Tokyo: Kanehara, 1996.
- 12.Shah JP. Cervical lymph node metastases—diagnostic, therapeutic, and prognostic implications. Oncology. 1990; 4: 61–69. [PubMed] [Google Scholar]
- 13.Attie JN, Setzin M, Klein I. Thyroid carcinoma presenting as an enlarged cervical lymph node. Am J Surg. 1993; 166: 428–430. [DOI] [PubMed] [Google Scholar]
- 14.Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg. 1998; 85: 252–254. [DOI] [PubMed] [Google Scholar]
- 15.Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg. 2001; 88: 1241–1244. [DOI] [PubMed] [Google Scholar]
- 16.Mirallie E, Visset J, Sagan C, et al. Localization of cervical node metastasis of papillary thyroid carcinoma. World J Surg. 1999; 23: 970–973. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid. I. Developing pattern of metastasis. Cancer. 1970; 26: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97: 418–428. [DOI] [PubMed] [Google Scholar]
- 19.Sugino K, Kure Y, Iwasaki H, et al. Metastases to the regional lymph nodes, lymph node recurrence, and distant metastases in nonadvanced papillary thyroid carcinoma. Surg Today. 1995; 25: 324–328. [DOI] [PubMed] [Google Scholar]
- 20.Kasai N, Sakamoto A. New subgrouping of small thyroid carcinomas. Cancer. 1987; 60: 1767–1770. [DOI] [PubMed] [Google Scholar]
- 21.Sugitani I, Yanagisawa A, Shimizu A, et al. Clinicopathologic and immunohistochemical studies of papillary thyroid microcarcinoma presenting with cervical lymphadenopathy. World J Surg. 1998; 22: 731–737. [DOI] [PubMed] [Google Scholar]
- 22.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987; 102: 1088–1095. [PubMed] [Google Scholar]
- 23.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988; 104: 947–953. [PubMed] [Google Scholar]
- 24.Yamashita H, Noguchi S, Murakami N, et al. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999; 86: 842–849. [DOI] [PubMed] [Google Scholar]
- 25.Lupoli G, Vitale G, Caraglia M, et al. Familial papillary thyroid microcarcinoma: a new clinical entity. Lancet. 1999; 353: 637–639. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Real JM, Ricart W. Familial papillary thyroid microcarcinoma. Lancet. 1999; 353: 1973–1974. [DOI] [PubMed] [Google Scholar]