Abstract
Objective
To evaluate the clinical significance of modulating the recipient portal inflow (rPVF) through perioperative ligation of the splenic artery in adult living-donor liver transplantation (ALDLTx) by focusing on vascular complications, intractable ascites production, and the prevention of small-for-size syndrome (SFSS).
Summary Background Data
In ALDLTx, portal graft flow is enhanced to at least twice the donor value, raising the total liver inflow. Recipient hepatic arterial flow (rHAF) is lower than expected. Portal hyperperfusion of small grafts in larger recipients is thought to be one of the main causes of posttransplant graft dysfunction/SFSS.
Methods
Seventeen ALDLTx were reviewed for a minimum of 2 months. Patients were divided retrospectively into two groups: G1 (n = 7), without modulation of rPVF, and G2 (n = 10), with splenic artery ligation to decrease rPVF perioperatively. Donor and recipient hepatic hemodynamics were evaluated against graft function and outcome, including correlations between rPVF, graft weight, graft:recipient body weight ratio, and recipient weight.
Results
Following portal and arterial reperfusion, mean rPVF and rPVF/graft weight were much higher than in the donors, whereas mean rHAF and rHAF/graft weight were much lower. No differences were found between groups, except for rPVF and rHAF, which were much more higher and lower, respectively, before splenic artery ligation. In G1 patients, SFSS was seen in two patients and vascular complications occurred in two others. In G2 patients, splenic artery ligation permitted a significant decrease in rPVF, an improvement in rHAF, and the resolution of refractory ascites. Neither SFSS nor vascular complications were seen in G2 patients.
Conclusions
When a suboptimal graft:recipient body weight ratio is accompanied by high rPVF in ALDLTx, the portal flow should be modulated perioperatively; splenic artery ligation is a simple and safe method that is sufficient to allow this modulation in most patients.
An increase in hepatic blood flow following reperfusion has been shown in whole-organ liver transplantation. In this setting, the graft inflow is predominantly portal (PVF) and is mostly associated with high cardiac output, low peripheral vascular resistance, and reduced hepatic arterial flow (HAF). 1–3 Adult living-donor liver transplantation (ALDLTx) has become an established procedure worldwide, allowing a welcome enlargement of the donor organ pool. 4–6 However, specific problems (i.e., prolonged cholestasis, ascites) have been encountered when adults are transplanted with partial grafts from living donors, presumably because of the size mismatch between the graft and the recipient. In small-for-size grafts, liver dysfunction has been described when the graft represents less than 40% to 50% of the volume expected for the recipient. 7 It has been speculated that this dysfunction is principally associated with graft exposure to excessive portal perfusion (small-for-size syndrome [SFSS]). In fact, an extreme imbalance in donor–recipient PVF has been recorded successively in ALDLTx, characterized by increased portal venous inflow and a relatively reduced supply from the hepatic artery. 8 In the present study we investigated the clinical significance of modulating portal inflow through perioperative ligation of the splenic artery in a series of 17 consecutive ALDLTx, focusing on vascular complications, intractable ascites production, and the prevention of SFSS. Hemodynamic features in donors and recipients were analyzed, including the variations induced by splenic artery ligation.
PATIENTS AND METHODS
Patients
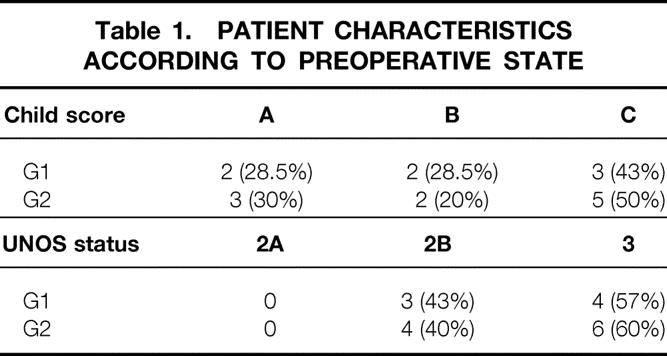
Between September 1999 to June 2001, 17 right lobe ALDLTx were performed on 12 men and 5 women, mean age 50 ± 19 years, at Ghent University Hospital. Indications for ALDLTx were postnecrotic HCV-related end-stage liver cirrhosis in all the patients, complicated by multifocal hepatocellular carcinoma in five. Patients were divided retrospectively into two groups: G1 (n = 7), with a mean age of 56 ± 4 years, transplanted without modulation of portal flow, and G2 (n = 10), with a mean age of 58 ± 4 years, transplanted with a modified technique using splenic artery ligation to decrease temporarily the portal graft inflow. Most of the recipients in G1 and G2 were scored as Child-Pugh class C or UNOS status 2B and were comparable in terms of cardiopulmonary status and preoperative serum creatinine values (Table 1). Severe portal hypertension with esophageal varices and ascites characterized the clinical state of Child C and UNOS 2B patients. None of the recipients had had previous abdominal operations or pretransplant septic episodes.
Table 1. PATIENT CHARACTERISTICS ACCORDING TO PREOPERATIVE STATE
Seventeen individuals (10 male and 7 female), aged 19 to 45 years (mean 30 ± 17 years), with a compatible or identical blood group and free from medical illness, donated their right lobes. Family donor-to-recipient relationships were 10 sons, 5 daughters, 1 wife, and 1 son-in-law. The right lobe volume was calculated by computed tomography or magnetic resonance imaging. Further evaluation of the biliary tree and vascular anatomy was done with magnetic resonance imaging. A graft:recipient body weight ratio (GRBWR) of at least 0.8 was required, according to the Kyoto experience. 4
Surgical Technique
The technique for donor hepatectomy has been described previously. 9 In brief, the liver was dissected from the vena cava, and all accessory hepatic veins with a significant outflow on back-table perfusion were preserved to be reimplanted in the recipient. The donor hepatectomy was done without vascular clamping, starting at the right side of the middle hepatic vein. The glissonian sheath around the biliary duct was left undisturbed to avoid ischemia until beginning the graft flushing and retrieval. The harvested grafts were weighed routinely before ex situ flushing with either histidine-tryptophan-ketoglutarate (HTK; Custodiol) or University of Wisconsin (UW; Viaspan) solution. Total venovenous bypass was not used. During the recipient hepatectomy the vena cava was preserved and a temporary hemiportocaval shunt was employed using the left or right portal vein. Venous drainage of the anteromedial sector through the middle hepatic vein of the donor liver was reconstructed in the recipients with an autologous saphena conduit in all the grafts showing one to three more veins on the perioperative ultrasound Doppler evaluation, allowing homogeneous perfusion and decoloration during the back-table flushing. Portal, arterial, and biliary anastomoses were performed under ×3.5 (R.T.) or ×5.5 (B. de H.) loupe magnification; dissolvable suture (6-0 PDS) was used for the portal and biliary anastomoses and interrupted suture 8-0 prolene was used for the arterial anastomosis. PVF was modulated intraoperatively by splenic artery ligation in seven patients presenting with enhanced PVF (4–7 times more than that of donors) associated with a low intraoperative HAF (<100 mL/min). In one patient, the hemiportocaval shunt was not taken down due to difficulty in decreasing the PVF below 500 mL/100 g graft weight by splenic artery ligation alone. For the same reason, portal vein banding was combined with splenic artery ligation in another recipient, in whom a spontaneous portosystemic shunt was not ligated following graft reperfusion. Three other patients exhibited intractable ascites (mean 7 L/day) refractory to medical therapy during the first 3 postoperative days. Their PVF was enhanced and HAF signal became undetectable on Doppler ultrasound. During a second-look laparotomy in these cases, a mean rPVF of 350 mL/min per 100 g graft weight was found, combined with a very low rHAF (<50 mL/min). Splenic artery ligation was then carried out, assuming that a reduced PVF could ameliorate the splanchnic congestion and, subsequently, the volume of ascites. The biliary tree was reconstructed either with a Roux-en-Y loop or hepaticocoledocho-anastomosis according to the anatomic variations encountered in the right liver. Basic immunosuppression consisted of tacrolimus (Prograft, Fujisawa Belgium) and a short course of steroids. Mofetil mycophenolate (Cell Cept, Roche Belgium) was administered in cases of renal insufficiency to decrease the toxicity associated with the calcineurin inhibitor.
Hemodynamic Studies
Systemic hemodynamic parameters were measured continuously only in the recipients. Intraoperative flowmetry was done with electromagnetic probes (Transonic System, Ithaca, NY) both in donors and recipients, and expressed as total mL/min and as mL/min per 100 g graft. Donor right PVF and donor right HAF were measured before starting the intraparenchymal dissection, following the cholecystectomy and cholangiography. Serial readings of the recipient portal and arterial flows (rPVF and rHAF) were obtained after reperfusion and before skin closure. In G2 patients, measurements were made before and after splenic artery ligation for both portal and arterial flows (rPVF1 and rPVF2, rHAF1 and rHAF2).
Statistical Analysis
Data are expressed as mean ± SD. Correlations between donor/recipient graft inflows were evaluated using the Mann-Whitney test. Correlations between rPVF or rHAF and weight of the implanted graft, the GRBWR, and the recipient weight were tested by the Spearman rank order correlation. Differences at P < .05 were considered significant.
RESULTS
Graft Characteristics
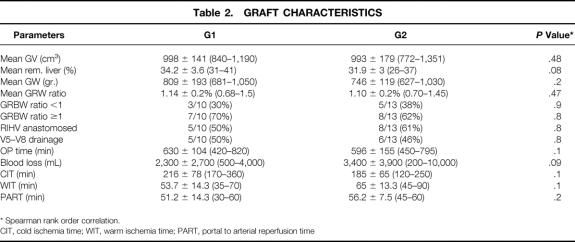
Anatomic variations in the right lobe occurred in seven (47%) patients: a separate origin for the portal branches in segments 5 to 8 and 6 to 7 was encountered in four cases; a biliary trifurcation was seen in three; confluence of the posterolateral segment into the left hepaticus was noted in one. No double arterial feed for the right lobe was found. Looking at venous anatomy, grafts in both groups were similar for requiring additional anastomoses of accessory veins (Table 2).
Table 2. GRAFT CHARACTERISTICS
* Spearman rank order correlation.
CIT, cold ischemia time; WIT, warm ischemia time; PART, portal to arterial reperfusion time
Overall mean graft volume measured in the donors was 995 ± 160 cm3 (range 772–1,351), with a remnant left liver of 34 ± 7% (range 26–41%). Mean right lobe weight was 744 ± 191 g (range 627–1,050). However, all the grafts were smaller than expected, with a mean discrepancy of 22% (range 0.2–36%) between estimated graft volume and actual graft weight. The predicted mean GRBWR was 1.3% but the real value was only 1.10%; among these, four had an actual GRBWR lower than 0.8% (two in G1 and two in G2).
Except for their rPVF and rHAF, both groups were comparable for several graft-related and operation-related variables including cold ischemia time, warm ischemia time, and the time between portal and arterial reperfusion.
Postoperative graft regeneration was assessed by computed tomography on days 10 and 30. Regeneration as measured by computed tomographic volumetry was not affected by the modulation of the portal flow and was similar in both groups (unpublished data), even in patients who had SFSS.
Looking at the early graft function according to the preservation solution used, ALT values on day 1 and 7 were 131 ± 123 and 99 ± 79 U/L, 370 ± 260 and 162 ± 53 U/L for G1 UW (n = 3) and G1 HTK (n = 4), respectively (P = NS) and 165 ± 150 and 109 ± 46 U/L, 215 ± 170 and 180 ± 130 U/L for G2 UW (n = 4) and G2 HTK (n = 6), respectively (P = NS).
PT time values on day 1 and 7 were 38 ± 4 and 58 ± 21%, and 44 ± 2 and 66 ± 4% for G1 UW and G1 HTK, respectively (P = NS) and 43 ± 21 and 83 ± 8%, and 50 ± 16 and 87 ± 9% for G2 UW and G2 HTK, respectively (P = NS). Normal PT time was achieved overall from the fourth postoperative day, whereas slight hypertransaminasemia was recorded until the third postoperative week. Severe impairment of liver function (coagulopathy) was observed a few days following transplantation, and increasing cholestasis was seen at the beginning of the second postoperative week in patients with SFSS.
Donor Hemodynamics
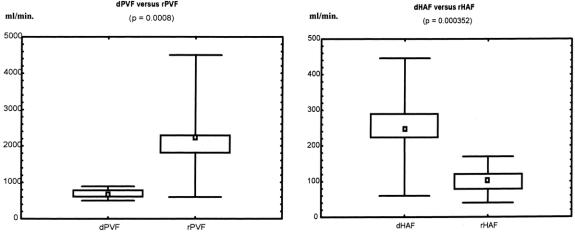
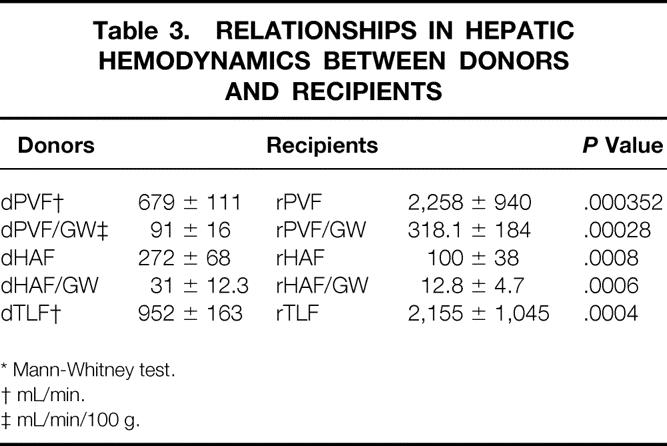
Data on the perioperative management of living donors and outcome have been described elsewhere. 10 In the 17 donors who underwent right hepatectomy, intraoperative right lobe flowmetry showed a mean dPVF of 679 ± 111 mL/min (range 546–855), equivalent to 91 ± 16 mL/100 g (range 63–121), and a mean dHAF of 272 ± 68 mL/min (range 60–446), equivalent to 31 ± 12 mL/100 g (range 8.8–53.8) (Table 3;Fig. 1). The portal contribution to the right lobe was 70% (range 62.6–83.5%), with a ratio of portal to arterial flow of 2.5 (range 1.67–3.5).
Table 3. RELATIONSHIPS IN HEPATIC HEMODYNAMICS BETWEEN DONORS AND RECIPIENTS
* Mann-Whitney test.
† mL/min.
‡ mL/min/100 g.
Figure 1. Donor’s and recipient’s relationships in PVF and HAF.
Recipient Systemic and Hepatic Hemodynamics
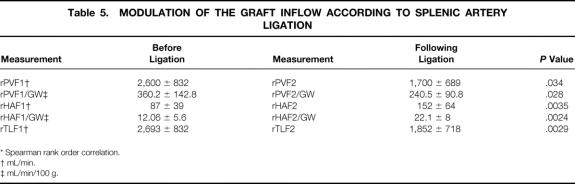
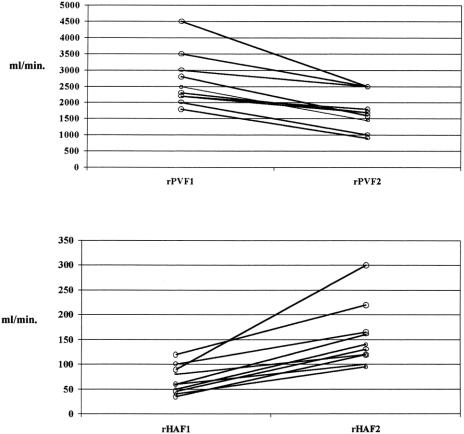
Mean cardiac output after reperfusion was 9.2 ± 1.5 L/min (range 7.5–12.2), remaining stable until the end of the procedure. Overall mean rPVF and rPVF/graft weight following portal and arterial reperfusion were much higher than in the donors, whereas mean rHAF and rHAF/GW were much lower (see Table 3;Fig. 1). The mean portal contribution to the graft was 94% (range 83.3–98.7%), with a mean portal to arterial ratio of 29 (range 5–77.7). However, before splenic artery ligation, statistically significantly higher rPVF and lower rHAF values were recorded with respect to G1 (Table 4). Performing splenic artery ligation at the artery’s origin from the celiac trunk allowed us to increase the rHAF by decreasing the rPVF in G2 patients from 360.2 ± 142.8 to 240.5 ± 90.8 mL/min per g graft weight (Table 5;Fig. 2). An inverse statistically significant, Spearman correlation was found only between rPVF, graft weight, and GRBWR (P = .045 and .038, respectively).
Table 4. HEMODYNAMIC FEATURES IN G1 AND G2 GRAFTS
* Mann-Whitney test.
† mL/min.
‡ mL/min/100 g.
Table 5. MODULATION OF THE GRAFT INFLOW ACCORDING TO SPLENIC ARTERY LIGATION
* Spearman rank order correlation.
† mL/min.
‡ mL/min/100 g.
Figure 2. Relationships before and following splenic artery ligation in rPVF and rHAF.
Outcome
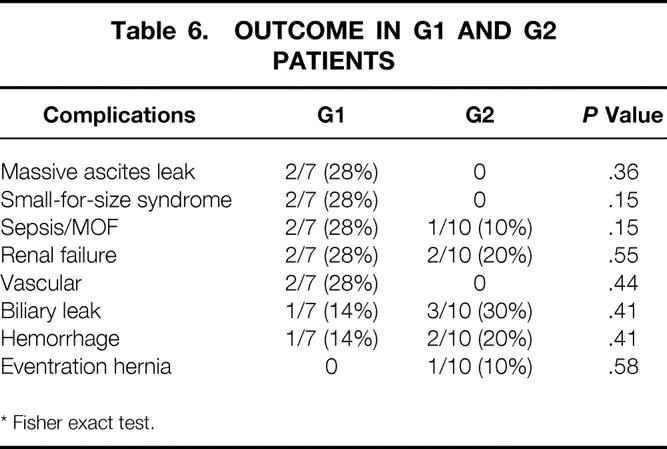
Primary nonfunction did not occur, and some complications were related to SFSS (Table 6). Early graft dysfunction, characterized by prolonged postoperative hyperbilirubinemia, ascites, and renal insufficiency, occurred in two patients in G1 with severe portal hypertension during the first postoperative weeks following ALDLTx. Their GRBWRs were 0.78 and 0.68. Both patients had exhibited high perioperative rPVF and rPVF/graft weight (2,200 and 3,500 mL/min with 364 and 754 mL/100 g liver, respectively). The mean rHAF recorded was 85 ± 7 mL/min (80 and 90 mL/min, respectively). Total hepatectomy specimens from the two failed grafts showed, on macroscopic evaluation, increased graft weight (627 to 1,360 g and 681 to 1,422 g [+217% and 208%, respectively]). On microscopy, enhanced cholestasis, especially in the centrolobular zone, was seen in both. Hepatocytes exhibited balloon and vacuolar degeneration. Focal necrosis was also present. These patients were retransplanted (the first with an auxiliary graft). Only the small-for-size grafts did poorly in this group. One bleeding episode due to poor graft function complicated the outcome of the second small-for-size graft. Vascular complications (28%) occurred in two patients in G1: early arterial thrombosis (12 hours postoperatively) was recorded in one patient, but graft salvage was achieved by local thrombolysis (100,000 U Actosolv); arterial hypoperfusion (intimal dissection) and a caval thrombosis occurred in the other, leading to massive pulmonary embolism 1 month after ALDLTx. Massive postoperative ascites leak was related to graft dysfunction due to SFSS in G1 and successfully resolved in G2 after splenic artery ligation.
Table 6. OUTCOME IN G1 AND G2 PATIENTS
* Fisher exact test.
No cases of SFSS or vascular complications occurred in G2 patients. However, two had a GRBWR lower than 0.8%, combined with rPVF of 684 and 522/mL/min per g and a mean rHAF of 95 ± 35 mL/min (70 and 120 mL/min, respectively). In these grafts, neither drainage of the anteromedial sector nor separate anastomoses of accessory right hepatic veins was needed. Biliary leak occurred in one (14%) case in G1 and in three (30%) patients in G2. Treatment consisted of revision laparotomy in the first case and a percutaneous drainage in the others. Thrombocytopenia following ALDLTx was not influenced by splenic artery ligation (mean platelet counts before and following splenic artery ligation: 70,000 ± 37,825 and 55,250 ± 31,792 mm3/L). In a total median follow-up of 6 ± 5 months, neither pancreatitis nor splenic infarction followed splenic artery ligation.
DISCUSSION
Organ shortage is the greatest limit to transplantation of patients with end-stage liver failure. Liver transplantation from living donors into adult patients is an attractive procedure, permitting enlargement of the donor organ pool. However, the disparity between the size of the graft and the recipient weight is the main factor limiting wider application of this procedure in adults. The hemodynamic features of standard liver transplantation have already been characterized; increased portal flow is commonly observed. Factors such as the presence of a hyperdynamic splanchnic state or portocollateral circulation, or the effects of the loss of sympathetic hepatic innervation, could be responsible for these phenomena. 3,11,12 Increase in portal pressure following resection of more than 30% of the liver, regardless of the presence or absence of cirrhosis, has been reported, and like the liver remnant after extensive hepatic resection, small-for-size grafts are exposed to excessive portal flow. 13 In cadaveric liver transplants with a whole liver graft, mean rPVF values around 130/mL/100 g graft have been reported 14 and, more recently, 8,12 hemodynamic changes in donors and recipients have been evaluated in living-donor liver transplantation.
This study has shown that following reperfusion of a partial graft, the hemodynamic changes are much more pronounced than those occurring in cadaveric liver transplants, with an inverse correlation to the GRBWR. Overall mean rPVF values are increased at least twice the donor values (2–7 times more according to our data) and the portal contribution may raise the total graft inflow. Since a right lobe graft represents 60% to 70% of the total liver mass, the size of the sinusoidal vascular bed is reduced, and one may predict the postoperative portal hypertension. However, the hemodynamic changes to the right lobe are even greater than the one third as expected in a right lobe graft from a theoretical point of view. Enhanced recipient portal hypertension with hyperperfusion of small grafts is thought to be one of the main causes of posttransplant graft dysfunction (SFSS), and poor graft outcome has been reported in patients with PVF values of more than 260 mL/min/100 g graft and small grafts. 7,12 The detrimental role of portal hyperperfusion has been studied in experimental liver transplantation in dogs, resulting in sinusoidal disruption, patchy hemorrhage, and degenerative changes in hepatocytes. 15 In humans, exposure of the graft to excessive portal perfusion has resulted in hepatocyte ballooning, centrolobular necrosis, and parenchymal cholestasis. Graft regeneration is, however, not affected, and the histologic changes are reversible with time. Intestinal endotoxins, pre-existing recipient hyperbilirubinemia, and ascites may also affect the postoperative period. 7,16,17 Nevertheless, even if small grafts can regenerate, they remain vulnerable to other insults and at risk for complications during the recovery period (e.g., sepsis). The reduction in HAF subsequent to portal hyperperfusion may also increase the risk of thrombosis, which is reported for small grafts and contributes to bad outcome. 18,19 In this setting, modification of the portal flow has been suggested in patients with enhanced portal hypertension. 20
To avoid graft failure by portal hyperperfusion, the diversion of the superior mesenteric flow by a mesocaval shunt has been described in ALDLTx using a small-for-size graft. 21 Except for portal hyperperfusion contributing to the phenomenon of SFSS, the outcome with small grafts will be further worsened by other factors such as the severity of illness (UNOS status 2A, Child C patients), graft size mismatching, the functional graft mass, and certain postoperative variables (septic episodes, bile leak, renal insufficiency). 22,23 Recipients with a GRBWR lower than 0.8% have a significantly lower chance of survival, and most ALDLTx programs do not offer this procedure to the sickest patients with chronic disease. 7,20,24–27
Although an accurate determination of right lobe volume can be obtained by computed tomography, 28 we recorded a mean discrepancy of 22% between estimated graft volume and actual graft weight, so all the grafts were smaller than expected. In our experience, the reduced accuracy of volumetric measurements could be partially explained by the use of three different imaging modalities (nuclear magnetic resonance, single-slice and multidetector multiphase computed tomography) and the intersubject variability of four independent radiologists. Thus, because graft weight may be overestimated in about 20% with respect to volumetric measurements, this must be anticipated when calculating GRBWR. 29
Another variable leading to severe graft dysfunction is the postreperfusion graft congestion for anatomic reasons (i.e., insufficient drainage of the anteromedial sector) or technical problem (i.e., outflow obstruction). In this condition, the real graft size does not correspond to the functional graft mass, so its relationship may be affected by the presence of portal hyperperfusion or disease severity. 20 In our experience, two small-for-size grafts with a GRBWR lower than 0.8% and enhanced rPVF of over 250 mL/min per g graft weight had a poor outcome, in contrast to two others that did not exhibit SFSS after manipulating the rPVF.
Preoperative patient state (Child-Pugh C patients), graft characteristics, and mean rHAF and rPVF values were similar in both groups as well as the graft’s venous anatomy without the requirement for additional drainage. Other complications accounting for graft dysfunction were not recorded in the early postoperative period. However, in the two small-for-size grafts in G2, the portal flow was manipulated intraoperatively to values below 250 mL/min per g liver. Our data and the pathologic findings strongly suggest that portal hyperperfusion has been a factor leading to severe graft dysfunction in G1, and that SFSS might be avoided by lowering the rPVF. Splenic artery ligation is a technique permitting adequate HAF in standard liver transplantation, and it is claimed to prevent thrombocytopenia in ALDLTx. 30,31 We recently proposed the use of splenic artery ligation to resolve ascites and to increase HAF in ALDLTx. 9,32 The rPVF was safely modulated in our G2 patients, permitting a significant lowering of the flow to values of less than 250 mL/min per g (mean values). However, in two cases with a flow over 500 mL/min per g liver, we were unable to decrease significantly the rPVF by means of splenic artery ligation without banding the portal vein or leaving the hemiportocaval shunt (used during the recipient hepatectomy to relieve splanchnic congestion in the anhepatic phase) patent. A benefit of splenic artery ligation in reducing posttransplant thrombocytopenia 31 was not confirmed in our series, but the procedure did allow us to resolve refractory ascites, a common feature of ALDLTx. 33,34 Careful intraoperative procedures (temporary portocaval shunt) and postoperative infusion of fresh-frozen plasma have been proposed to support small-for-size grafts until regeneration. 34 The unrelieved portal graft hypertension may play a primary role, as intractable ascites is the main complication in patients transplanted with very small grafts. However, it is unclear how the effects of a portocaval shunt may prevent graft injury when the shunt has been taken off following reperfusion. Spontaneous portosystemic shunts are commonly taken off during standard liver transplantation, since the escape of portal inflow through collaterals (“steal phenomenon”) may lead to ischemic graft damage. 35 The takedown of spontaneous portosystemic shunts has also been proposed in pediatric living-related liver transplantation, unless there were other causes responsible for the decrease in portal blood flow. 36 Therefore, we share with others the concept that spontaneous portosystemic shunts should be left in place when small grafts with hyperkinetic portal flow are used in ALDLTx, since their ligation will potentially increase sinusoidal pressure, worsening graft hyperperfusion. 22 However, when small-for-size grafts exhibit intraoperatively a sufficient portal inflow, careful postoperative ultrasound Doppler monitoring is needed because of the enhanced risk of reversed hepatopetal flow just after liver transplantation. 37 According to our experience, we believe that splenic artery ligation is a simple and useful method for decreasing portal graft inflow when rPVF does not exceed 500 mL/min per g. Other techniques, such as portocaval shunting or banding of the portal vein or portomesenteric disconnection, should be considered in cases with more enhanced flows or when splenic artery ligation appears insufficient to relieve portal hyperperfusion. Due to the gradual organization of an arterial splenopancreatic collateral network, splenic artery ligation is only a temporary method to lower the rPVF. Nevertheless, we can speculate that it is probably sufficient to limit or avoid the damage to the sinusoidal bed during the early postoperative days when graft regeneration is occurring.
In conclusion, when small grafts (GRBWR < 0.8%) are accompanied by an excessive portal inflow (>250 mL/min/100 g graft weight), any effort to modulate rPVF should be attempted, since this combination is at risk for SFSS and vascular complications. Although our findings and observations are limited, rPVF of 250 mL/min per 100 g liver appears to be a suitable target level for the prevention of SFSS when small grafts are reperfused by a hyperkinetic splanchnic circulation. Splenic artery ligation is usually sufficient to allow temporarily the modulation of portal graft inflow. However, this study and its limitations suggest that a prospective randomized trial proving or disapproving the value of this treatment to avoid SFSS might be designed.
Footnotes
Presented at the Annual Meeting of the American Society of Transplant Surgeons, May 11–16, 2001, Chicago, Illinois.
Correspondence: Roberto Troisi, MD, Associated Clinic-Head, Dept. of General Surgery, Division of Hepato-Biliary and Liver Transplantation Surgery, Ghent University Hospital, De Pintelaan 185, 2K12 IC, Gent 9000 Belgium.
E-mail: roberto.troisi@rug.ac.be
Accepted for publication August 9, 2002.
References
- 1.Navasa M, Feu F, Garcia-Pagan JC, et al. Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology. 1993; 17: 355–360. [PubMed] [Google Scholar]
- 2.Henderson JM, Gilmore GT, Mackay GJ, et al. Hemodynamics during liver transplantation: the interactions between cardiac output and portal venous and hepatic arterial flows. Hepatology. 1992; 16: 715–718. [DOI] [PubMed] [Google Scholar]
- 3.Hadengue A, Lebrec D, Moreau R, et al. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993; 17: 175–178. [PubMed] [Google Scholar]
- 4.Inomata Y, Uemoto S, Asonuma K, et al. Right lobe graft in living donor liver transplantation. Transplantation. 2000; 69: 258–264. [DOI] [PubMed] [Google Scholar]
- 5.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transplant. 2000; 6: 296–301. [DOI] [PubMed] [Google Scholar]
- 6.Testa G, Malago M, Broelsch CE. Living-donor liver transplantation in adults. Langenbeck’s Arch Surg. 1999; 384: 536–543. [DOI] [PubMed] [Google Scholar]
- 7.Emond J, Renz JF, Ferrel LD, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996; 224: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcos A, Olzinski AT, Ham JM, et al. The interrelationship between portal and arterial blood flow after adult-to-adult living donor liver transplantation. Transplantation. 2000; 70: 1697–1703. [DOI] [PubMed] [Google Scholar]
- 9.Troisi R, Cuomo O, de Hemptinne B. Adult-to-adult living-related liver transplantation using the right lobe. Case report. Digest Liver Dis. 2000; 32: 238–242. [DOI] [PubMed] [Google Scholar]
- 10.Cammu G, Troisi R, Cuomo O, et al. Anaesthetic management and outcome in right-lobe living liver-donor surgery. Eur J Anaest. 2002; 19: 93–98. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JM, Millikan WJ, Hooks M, et al. Increased galactose clearance after liver transplantation: a measure of increased blood flow through the denervated liver? Hepatology. 1989; 10: 288–291. [DOI] [PubMed] [Google Scholar]
- 12.Shimamura T, Tanigichi M, Jin MB, et al. Excessive portal venous inflow as a cause of allograft dysfunction in small-for-size living donor liver transplantation. Transplant Proc. 2001; 33: 1331. [DOI] [PubMed] [Google Scholar]
- 13.Kanematsu T, Takenaka K, Furuta T, et al. Acute portal hypertension associated with liver resection. Analysis of early postoperative death. Arch Surg. 1985; 120: 1303–1305. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen AW, Klintmalm GBG. Direct measurement of hepatic blood flow in native and transplanted organs, with accompanying systemic hemodynamics. Hepatology. 1992; 16: 100–111. [DOI] [PubMed] [Google Scholar]
- 15.Ku Y, Fukumoto T, Nishida T, et al. Evidence that portal vein decompression improves survival of canine quarter orthotopic liver transplantation. Transplantation. 1995; 59: 1388. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y, Shimazu M, Wakabayashi G, et al. Significance of portal venous flow in graft regeneration after living related liver transplantation. Transplant Proc. 2001; 33: 1484–1485. [DOI] [PubMed] [Google Scholar]
- 17.Kita Y, Harihara Y, Hirata M, et al. Factors influencing persistent hyperbilirubinemia following adult-to-adult living-related liver transplantation. Transplant Proc. 2000; 32: 2193–2194. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara Y, Makuuchi M, Takayama T, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001; 192: 510–513. [DOI] [PubMed] [Google Scholar]
- 19.Habib N, Tanaka K. Living related liver transplantation in adult recipients: a hypothesis. Clin Transplant. 1995; 9: 31–34. [PubMed] [Google Scholar]
- 20.Ben-Haim M, Emre S, Fishbein TM, et al. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transplant. 2001; 11: 948–953. [DOI] [PubMed] [Google Scholar]
- 21.Boillot O, Delafosse B, Méchet I, et al. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002; 359: 406–407. [DOI] [PubMed] [Google Scholar]
- 22.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transplant. 2000; 3: 296–301. [DOI] [PubMed] [Google Scholar]
- 23.Schiano TD, Kim-Schluger L, Gondolesi G, et al. Adult living donor liver transplantation: the hepatologist’s perspective. Hepatology. 2001; 33: 3–9. [DOI] [PubMed] [Google Scholar]
- 24.Furukawa H, Shimamura T, Ishikawa H, et al. What is the limit of graft size for successful living donor liver transplantation in adults? Transplant Proc. 2001; 33: 1322. [DOI] [PubMed] [Google Scholar]
- 25.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999; 68: 1112–1116. [DOI] [PubMed] [Google Scholar]
- 26.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999; 67: 321–327. [DOI] [PubMed] [Google Scholar]
- 27.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplants in adults and children: a single-center experience. Ann Surg. 2001; 234: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamel I, Kruskal JB, Warmbrand G, et al. Accuracy of volumetric measurements after virtual right hepatectomy in potential donors undergoing living adult liver transplantation. AJR Am J Roentgenol. 2001; 176: 483–487. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto S, Uemoto S, Uryuhara K, et al. Graft size assessment and analysis of donors for living donor liver transplantation using the right lobe. Transplantation. 2001; 71: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 30.Settmacher U, Haase R, Heise M, et al. Variations of surgical reconstruction in liver transplantation depending on vasculature. Langenbeck’s Arch Surg. 1999; 384: 378–383. [DOI] [PubMed] [Google Scholar]
- 31.Matsukura A, Kita Y, Harihara Y, et al. Is splenic artery ligation effective for thrombocytopenia early after liver transplantation? Transplant Proc. 1999; 31: 2906–2907. [DOI] [PubMed] [Google Scholar]
- 32.Troisi R, Hoste E, Van Langenhove P, et al. Modulation of liver graft hemodynamics by partial ablation of the splenic circuit: a way to increase hepatic artery flow? Transplant Proc. 2001; 33: 1445–1446. [DOI] [PubMed] [Google Scholar]
- 33.Taniai N, Harihara Y, Kita Y, et al. Persistent pleural and peritoneal fluid discharge after adult-to-adult living related liver transplantation. Transplant Proc. 2000; 32: 2213–2214. [DOI] [PubMed] [Google Scholar]
- 34.Nishizaki T, Ikegami T, Hiroshige S, et al. Small graft for living donor liver transplantation. Ann Surg. 2001; 233: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Carlis L, Del Favero E, Rondinara G, et al. The role of spontaneous portosystemic shunts in the course of orthotopic liver transplantation. Transplant Int. 1992; 5: 9–14. [DOI] [PubMed] [Google Scholar]
- 36.Fujimoto M, Moriyasu F, Someda H, et al. Evaluation of portal hemodynamics with doppler ultrasound in living related donor liver transplantation in children: implications for ligation of spontaneous portosystemic collaterals pathways. Transplant Proc. 1995; 27: 1174–1176. [PubMed] [Google Scholar]
- 37.Kita Y, Harihara Y, Sano K, et al. Reversible hepatofugal portal flow after liver transplantation using a small-for-size graft from a living donor. Transplant Int. 2001; 14: 217–222. [DOI] [PubMed] [Google Scholar]