Abstract
Objective
To analyze gene expression patterns in skeletal muscle from burned children.
Summary Background Data
Analysis of gene expression patterns in skeletal muscle from burned children can help provide a fundamental understanding of muscle wasting at the molecular level. This study is the first to use such an approach in burned children receiving anabolic treatment.
Methods
Children who received 0.1 mg/kg oxandrolone twice a day (n = 7) were compared to placebo (n = 7). Net protein balance was determined before and after treatment with oxandrolone. Total RNA, extracted from muscle biopsies obtained from burned children age 3 to 18 years, was purified, reverse transcribed, and biotinylated cRNA hybridized to the human high-density oligonucleotide array (U95Av2). Western blot analysis verified the mRNA changes at their protein level.
Results
DNA microarray analysis showed two genes significantly changed in muscle from burned children receiving placebo, while the expression of 21 genes was altered with oxandrolone. Muscle net protein balance increased with oxandrolone treatment compared to placebo.
Conclusions
DNA microarray technology will help identify molecular changes that can serve as targets for new therapies to attenuate muscle wasting in severely burned children and thus improve recovery and early rehabilitation.
The hypermetabolic stress response to severe burn is characterized by persistent muscle weakness, tachycardia, early fatigue with normal activity, growth arrest, loss of lean body mass, and muscle wasting. 1–6 A prolonged hypermetabolic response keeps burn patients debilitated and severely limits their rehabilitation. It has been shown that protein synthesis and breakdown are stimulated in human muscle after severe burn, with protein breakdown exceeding synthesis. 7–9 Adequate nutritional support, with additional protein and amino acid intake increased several times higher than normal requirements, has failed to compensate for the protein breakdown in muscle after a severe thermal injury. 10 Increased activity of several protein breakdown pathways has been identified, the most prominent being an increase in expression and activity of the ubiquitin-proteasome pathways. 11,12
Pharmacologic agents have been used after trauma to stimulate growth and increase muscle mass and strength. We have used several anabolic agents to diminish muscle catabolism in burn patients. Insulin has been shown to be beneficial in protein metabolism, primarily by stimulating protein synthesis. 13,14 Recombinant human growth hormone and IGF-1-BP3 have also shown efficacy in improving muscle protein kinetics and wound healing in severely burned children. 15–17 Testosterone can increase protein synthesis, but there are risks of virilism and hepatotoxicity. 18
Anabolic steroids have been used to restore lean body mass and muscle loss as a consequence of starvation and more recently severe thermal injury, trauma, or HIV and chronic infections. 19–21 Oxandrolone, a synthetic analog of testosterone, is being studied as an adjunctive therapy to facilitate weight gain after surgery, chronic infections, and severe trauma. Oxandrolone has been used to enhance protein synthesis and lean muscle mass in burned children. 22 It is currently used in the treatment of delayed growth and puberty in patients with Turner syndrome, in patients with AIDS wasting myopathy, 20,23,24 and in acute treatment and the rehabilitation of adult burn patients. 17,25
The purpose of this study was to investigate the effect of oxandrolone on gene expression changes in skeletal muscle of burned children using high-density oligonucleotide arrays and to determine muscle protein kinetics. Patients selected for this study were admitted to our institution at least 7 days after injury and demonstrated signs of acute starvation and had documented weight loss. Oxandrolone was used to blunt weight loss as the treatment of choice.
METHODS
Patients
Fourteen burned children were enrolled in the study. All were between 3 and 18 years of age and admitted to our hospital at least 1 week after burn. The average age was 7.9 ± 1.4 years and the average burn size was 42 ± 5% total body surface area.
Standard Treatment
Within 48 hours after admission each patient underwent total burn wound excision and grafting with 4:1 meshed autograft skin with human homograft overlay, or 2:1 autograft skin. All remaining open areas were covered temporarily with homograft skin. Patients were returned to the operating room after healing of autograft donor sites. Sequential staged grafting procedures were performed until wound closure was completed. During the study period, all patients received total enteral nutrition (Vivonex TEN, Novartis Nutrition, Minneapolis, MN) through a nasoduodenal tube, containing 82.3% carbohydrate, 3% fat (linoleic acid), and 14.7% protein. Daily caloric intake delivered 1,500 kcal/m2 of total body surface area burned plus 1,500 kcal/m2 of total body surface area. Enteral nutrition began with admission and continued until wound healing.
Study Design
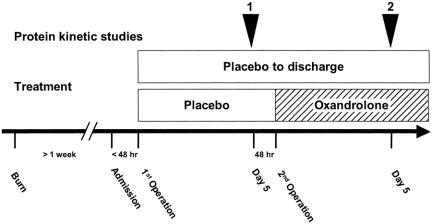
Burn children were randomized to receive oxandrolone (n = 7) or placebo (n = 7). All underwent stable isotope studies. Figure 1 depicts the schedule protocol. Beginning in the morning, children in the continuously fed state were infused with a prime-constant L-[ring-2H5]phenylalanine (Cambridge Isotopes, Andover, MA). The initial priming does was 2 μmol/kg, followed by a dose of 0.08 μmol/kg/min given intravenously. Phenylalanine is not synthesized or degraded in the peripheral tissues; thus, measurements across the leg reflect net balance of protein synthesis and breakdown. Indocyanine green dye concentration was measured between hours 3 and 4 to determine leg blood flow. Femoral arteriovenous sampling during the fifth hour measured cross-leg phenylalanine balance. Biopsy of the vastus lateralis muscle was taken 2 and 5 hours after the start of infusion. Muscle biopsies for DNA microarray analysis, Western blot analysis, and protein kinetic studies were snap-frozen and stored at −70°C. Cross-leg amino acid kinetics were calculated according to the three-compartment model described by Biolo et al. 26,27 Immediately after the second operation, the oxandrolone group received oral oxandrolone at 0.1 mg/kg twice daily. Five days later, a second series of protein kinetic studies were performed. Arterial and venous amino acid concentrations were determined in heparinized plasma at 4 and 5 hours of the isotope infusion study by high-performance liquid chromatography.
Figure 1. Study protocol for burned children admitted at least 7 days after burn who were nutritionally depleted. Initial baseline studies were conducted 5 days after the first operation and muscle biopsies were taken. Patients were treated with placebo or oxandrolone until discharge. Five days after the second operation, protein kinetic studies and muscle biopsies were repeated.
Total RNA Extraction
Total RNA was isolated from muscle biopsies by acid guanidinium thiocyanate-phenol-chloroform extraction using TRI Reagent (Molecular Research Center Inc., Cincinnati, OH). This method was based on the single-step method of RNA isolation described by Chomcyzynski and Sacchi. 28 Samples were homogenized in TRI Reagent on ice and total RNA was extracted following the manufacturers’ instructions. Purified RNA was quantified by UV absorbance at 260 and 280 nm and stored in 25-μg aliquots at −70°C for DNA microarray hybridization and analyses. The adequacy and integrity of the extracted RNA were determined by gel electrophoresis. Three oxandrolone-treated and one placebo muscle biopsy showed an insufficient quantity of RNA for microarray analysis. These patients were omitted from any further analysis.
High-Density Oligonucleotide Array Analysis
Probe labeling, hybridization, and image acquisition were done according to the standard Affymetrix protocol. Briefly, 25 μg purified, total RNA was transcribed into cRNA, purified, and used as templates for in vitro transcription of biotin-labeled antisense RNA. Biotinylated antisense RNA preparation was fragmented and placed in a hybridization mixture containing four biotinylated hybridization controls (BioB, BioC, BioD, and Cre). Samples were then hybridized to an identical lot of Affymetrix Gene Chip arrays (HG-U95 Av2) for 16 hours. The arrays were washed and stained using the instrument’s standard Eukaryotic GE Wash 2′ protocol and antibody-mediated signal amplification. The images were scanned and analyzed with Affymetrix GeneChip Analysis Suite 3.2. Images from each Gene Chip were scaled and adjusted to an average intensity value for all arrays of 1,500. Scaled average difference values and absolute call data from each Gene Chip were exported to data files and used for statistical analysis. 29 In vitro transcription and chip hybridization were performed in collaboration with the University of Texas Medical Branch Genomic Core Facility.
Data Analysis
Data analysis of genomic data included chip validation, cluster analysis of transcription profiles, identification of genes expressed only in one group, temporal analysis of gene expression at different time points after burn or treatment, and analysis of interaction among groups. The first step in the analysis was to cluster the data to detect gross discrepancies among array data. The degree of similarity or dissimilarity among sample transcription profiles was tested using model-based expression analysis of oligonucleotide arrays. 29 The next step was the elimination of genes that showed little variation across the samples or that were absent in the majority of the samples. The first criterion was that the ratio of standard deviation and the mean of a gene’s expression values across all samples was greater than the threshold (of 0.85 and the upper limit of 8). Data were discarded if there was a large deviation in the number of present calls or if the correlation coefficient among samples within the group was less than 0.85. The second criterion required a gene to be called present in more than 80% of arrays at all times. We determined the presence or absence of each probe within the group (according to the Affymetrix algorithm). A probe is present if its absolute call was P for at least two members of the group containing three samples; otherwise, the gene was considered not expressed in the group. The primary goal was to identify genes with significant differences in expression between the test and control group. The within-group average of expression was calculated and comparisons were made between groups. Comparisons were done by computing the expression fold difference for each gene and listing those that showed larger than twofold increase or decreases in activity. An entry was discarded as an outlier when its value was outside three standard deviations. Only the statistically significant differences at P < .05 were retained. 30 Considering the number of samples per group and its influence on the validity of the analysis, the power of the t test was computed. If it was less than 0.8, results were discarded even when significant. The expression profiles of the skeletal muscle biopsies taken from oxandrolone- and placebo-treated burned children were analyzed. Samples were taken at time point 1 (baseline), before oxandrolone treatment, and at time point 2, after treatment began. By using HG-U95A Affymetrix arrays, about 4,000 genes of 12,000 genes present in the array were expressed. This was in agreement with Affymetrix DNA array analysis of mouse skeletal muscle. 31
Western Blot Analysis
Total protein from the muscle tissue was extracted and 20 μg was separated on a 4% to 20% SDS-polyacrylamide gel under reducing conditions and transferred to nitrocellulose membranes (Hybond-C; Amersham Pharmacia Biotech, USA) in a semidry blotting chamber. After blocking nonspecific binding sites with 5% nonfat milk in TBS containing 0.1% Tween-20 (Sigma, St. Louis, MO), blots were incubated in a 1:1,000 dilution of anti-Myf-6 rabbit polyclonal antibody, anti-GADD45 mouse monoclonal antibody (Santa Cruz Biotechnology), antimyosin goat polyclonal antibody (ventricular light chain) (Cortex Biochem, CA), and anti-actin rabbit polyclonal antibody (Sigma) as an internal control, for 2 hours at room temperature. After three to five washings, the blots were incubated with HRP conjugated anti-rabbit IgG, anti-mouse IgG, and anti-goat IgG, respectively (final concentration 1:2,000), for 90 minutes at room temperature. Bound antibodies were detected with ECL Western blotting detection reagents (Amersham Pharmacia Biotech) according to the manufacturer’s instructions.
This study meets all requirements for exemption from the IND regulation and was conducted in compliance with the requirements for institutional review and informed consent at the University of Texas Medical Branch, Galveston, Texas.
RESULTS
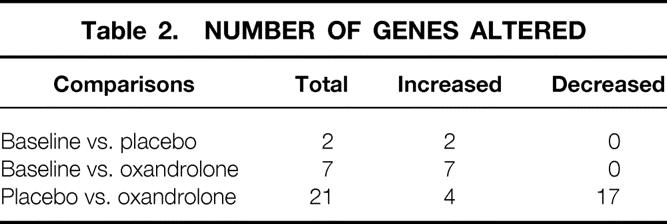
Age, gender distribution, weight, burn size, admission time from injury, and delay to first study were not significantly different between groups (Table 1). Comparison of 12,000 genes in burned children receiving placebo or oxandrolone showed increased expression of 11 genes compared to placebo, while the expression of 17 genes decreased (Table 2). Thus, 0.18% of the genes were altered in muscle from burned children treated with oxandrolone when compared to placebo.
Table 1. DEMOGRAPHICS OF STUDY PATIENTS
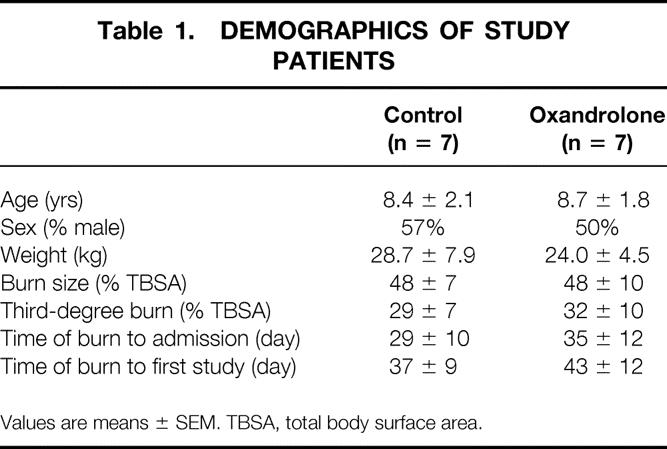
Values are means ± SEM. TBSA, total body surface area.
Table 2. NUMBER OF GENES ALTERED
Gene Expression Patterns
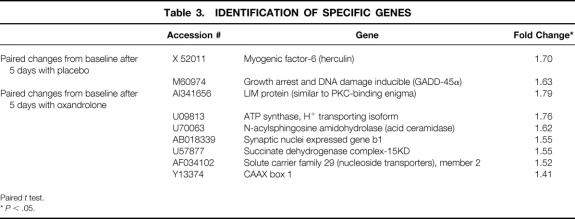
There were 30 genes affected by oxandrolone treatment as determined by DNA microarray analysis. Categories of genes affected were transcription factors, growth factors, and stress response modulators and muscle-associated proteins. We further grouped genes whose expression increased or decreased according to their function and involvement in metabolic pathways. Exemplary genes whose expression was altered in the muscle of burned children treated with oxandrolone are shown in Tables 3 and 4.
Table 3. IDENTIFICATION OF SPECIFIC GENES
Paired t test.
*P < .05.
Table 4. PLACEBO GROUP VS. OXANDROLONE GROUP AFTER 5 DAYS

Unpaired t test.
*P < .05.
Negative signs indicate downregulation.
Verification of mRNA Changes
We verified mRNA changes through expression of related proteins by Western blot analyses. Myogenic factor-6 and GADD-45, whose transcription was inhibited by oxandrolone treatment, showed a significant decrease in their protein expression (Fig. 2). The stimulatory effect of oxandrolone on myosin light chain was verified by a significant increase in protein expression after treatment.
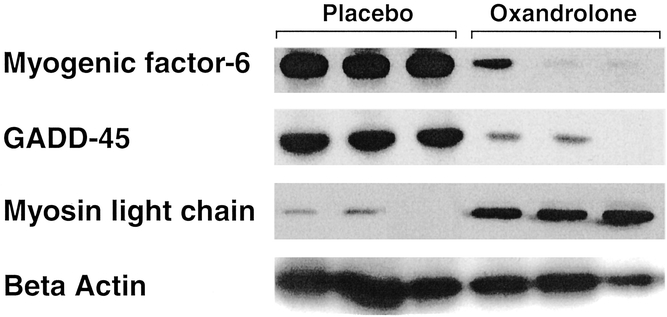
Figure 2. Western blot analysis using antibodies against myogenic factor-6, GADD-45, and myosin light chain in muscle from burned children treated with oxandrolone. Beta-actin is depicted here to show any loading differences. Each lane represents total protein extracted from one muscle biopsy.
Protein Kinetics
Muscle protein synthesis, presented as nmol phenylalanine min−1 per 100 mL leg volume, was calculated from a three-pool model of amino acid kinetics. 26,27 Oxandrolone increased muscle protein synthesis, calculated from the incorporation of labeled phenylalanine into skeletal muscle protein, from a baseline value of 125 to 384 nmol phenylalanine min−1 per 100 mL leg volume (n = 4) compared to a decrease in muscle protein synthesis from 258 to 189 nmol phenylalanine min−1 per 100 mL leg volume for placebo (n = 6). The change in net protein balance for placebo-treated burned children was −50 nmol phenylalanine min−1 per 100 mL leg volume compared to +146 nmol phenylalanine min−1 per 100 mL leg volume for oxandrolone treatment.
DISCUSSION
The bulk of muscle protein is made up of myofibrillar components, specifically actin and myosin. Animal studies have shown a decrease in net myofibrillar protein synthesis in response to burn and sepsis. Myofibrillar protein constituted the majority of protein undergoing breakdown, which resulted in net protein loss. Adequate nutritional support with additional protein and amino acid intake several times higher than the normal requirement has failed to completely reverse the net protein catabolism. Increased activity of several protein breakdown pathways has been shown, with the most prominent being an increase in expression and activity of the ubiquitin-proteasome pathways.
In our study, oxandrolone did not change the inward transport of protein but did increase the efficiency of protein synthesis in muscle. With an increase in protein synthesis while protein breakdown remained unchanged, the net balance of protein increased. This supports the hypothesis that the mechanism for improved net protein balance across the leg was the stimulation of muscle protein synthesis. We found no adverse side effects with oxandrolone treatment in children. While the number of patients reported in this study is relatively small, the comparison of gene expression patterns in a paired study design provides evidence that the results are not outliers associated with the large number of gene probes used (12,000-Affymetrix).
We found gene expression changed for 32 genes in males treated with oxandrolone compared to 12 genes for females treated with oxandrolone. In no instance did a gene whose expression changed in males show a change in females. A further dramatic difference is that the expression of only 6 of 32 affected genes in the male pool increased. The majority of the oxandrolone-induced changes here represent repression of transcription. Just the opposite was true of the female pool, where 11 of 12 genes affected by oxandrolone showed activated transcription. A larger number of subjects are needed to validate these differences between males and females; thus, these variations are not presented in the results.
For oxandrolone-treated versus untreated burned children, there was a strong decrease in the expression of a multitude of transcription factors and signaling molecules consistent with a “quenching” effect of the treatment along with stimulation of myosin actin gene expression, which is consistent with the beneficial effects associated with oxandrolone. This would suggest that a component of the benefit provided by oxandrolone treatment is due to its attenuation of the inflammatory response to burns.
GADD45 has been shown to play an important role in stress response processes, typically believed to be robust and prompt but transient. GADD45 is known to stimulate p38- and JNK-mediated cellular commitment to apoptosis. 32 The fact that there was a continued or delayed increase in GADD45 expression would suggest that there are delayed or long-term responses to burn trauma that correlate well with the known consequences on developmental growth following burns. Specifically, this would be in agreement with the hypothesis that GADD45 may play a key role both in early trauma-induced cell death and in tissue remodeling events that occur later in wound healing that may involve apoptosis of activated cells (fibroblasts and macrophages) involved in the inflammatory response. Thus, GADD45 could trigger inflammation as well as be involved in its quenching.
Comparison of the gene expression profile in muscle biopsies before treatment and 10 days after treatment with oxandrolone showed a general stimulatory effect significant in the expression of seven genes with a variety of roles. As an example, after 10 days, there was an increase in the expression of acid ceramidase, which plays an important role in NF-κB transcription factor activation. The NF-κB transcription factor is known to play an important role in stress responses to trauma and the regulation of cytokine-mediated expression mediating inflammation and cell death processes. There were also significant changes in the expression of several zinc finger proteins and genes known to be regulated by AP-1-type transcription factors. The involvement of the AP-1/NF-κB signal transduction pathways is consistent with the involvement of stress response pathways, which is not surprising in itself. The prolonged nature of the changes may be more specific to severe burn trauma in light of the prolonged metabolic changes.
The effects of oxandrolone treatment on gene expression in burned children compared to untreated patients are perhaps more comprehensive in that a broad category of genes are suppressed by oxandrolone. For example, oxandrolone treatment suppressed the expression of GADD45, which was found to have been stimulated twofold in the untreated burned children. We found a similar inhibitory effect on the expression of the Jun B transcription factor (with a 13-fold decrease), suggesting that while beneficial to wound healing, oxandrolone may not be as beneficial to developmental processes. This is consistent with the marked increased expression of myogenic factor 6, which plays a dual role in early myogenesis and the regeneration of injured skeletal muscle, even though Fos/Jun proteins are involved in the regulation of muscle specific genes in vitro. 33
The participation of a large number of genes critical to inflammation and other stress response signal transduction pathways in response to oxandrolone treatment suggests a mechanism principally consisting of a quenching or amelioration of the stimulation of gene expression resulting from thermal trauma. We feel that alterations in the expression of these selective genes may help identify molecular changes for specific therapeutic and diagnostic purposes.
Acknowledgment
The authors thank Dr. Tom Wood (Genomics Core Facility, UTMB) for his valuable help in processing the samples for DNA microarray hybridizations.
Footnotes
Supported by grants from Shriners Hospitals for Children (#8660, #8490) and the National Institutes of Health (#2T32GM0825611, #1P50GM60338-01, #5R01GM5729503, and #1454GM06211901A1).
Correspondence: Dr. Robert E. Barrow, PhD, Shriners Hospitals for Children, 815 Market Street, Galveston, TX 77550.
E-mail: rbarrow@utmb.edu
Accepted for publication August 5, 2002.
References
- 1.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990; 125: 392–395. [DOI] [PubMed] [Google Scholar]
- 2.Klein GL, Herndon DN, Langman CB, et al. Long-term reduction in bone mass after severe burn injury in children. J Pediatr. 1995; 126: 252–256. [DOI] [PubMed] [Google Scholar]
- 3.Jahoor F, Desai M, Herndon DN, et al. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988; 37: 330–337. [DOI] [PubMed] [Google Scholar]
- 4.Brillon DJ, Zheng B, Campbell RG, et al. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol. 1995; 268: E501–513. [DOI] [PubMed] [Google Scholar]
- 5.Brown JA, Gore DC, Jahoor F. Catabolic hormones alone fail to reproduce the stress-induced efflux of amino acids. Arch Surg. 1994; 129: 819–824. [DOI] [PubMed] [Google Scholar]
- 6.Woolf PD. Hormonal responses to trauma. Crit Care Med. 1992; 20: 216–226. [DOI] [PubMed] [Google Scholar]
- 7.Streat SJ, Beddoe AH, Hill GL. Aggressive nutritional support does not prevent protein loss despite fat gain in septic intensive care patients. J Trauma. 1987; 27: 262–266. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SE, Barrow RE, Herndon DN. Growth hormone and IGF-I therapy in the hypercatabolic patient. Baillieres Clin Endocrinol Metab. 1996; 10: 447–463. [DOI] [PubMed] [Google Scholar]
- 9.Bessey PQ, Watters JM, Aoki TT, et al. Combined hormonal infusion simulates the metabolic response to injury. Ann Surg. 1984; 200: 264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw JH, Wolfe RR. An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients. Studies in the basal state and the response to total parenteral nutrition. Ann Surg. 1989; 209: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang CH, Tiao G, James H, et al. Burn injury stimulates multiple proteolytic pathways in skeletal muscle, including the ubiquitin-energy-dependent pathway. J Am Coll Surg. 1995; 180: 161–170. [PubMed] [Google Scholar]
- 12.Mansoor O, Beaufrere B, Boirie Y, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA. 1996; 93: 2714–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999; 229: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995; 222: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore DC, Honeycutt D, Jahoor F, et al. Effect of exogenous growth hormone on whole-body and isolated-limb protein kinetics in burned patients. Arch Surg. 1991; 126: 38–43. [DOI] [PubMed] [Google Scholar]
- 16.Knox J, Demling R, Wilmore D, et al. Increased survival after major thermal injury: the effect of growth hormone therapy in adults. J Trauma. 1995; 39: 526–530. [DOI] [PubMed] [Google Scholar]
- 17.Demling RH. Comparison of the anabolic effects and complications of human growth hormone and the testosterone analog, oxandrolone, after severe burn injury. Burns. 1999; 25: 215–221. [DOI] [PubMed] [Google Scholar]
- 18.Ferrando AA, Sheffield-Moore M, Wolf SE, et al. Testosterone administration in severe burns ameliorates muscle catabolism. Crit Care Med. 2001; 29: 1936–1942. [DOI] [PubMed] [Google Scholar]
- 19.Demling RH, DeSanti L. Oxandrolone, an anabolic steroid, significantly increases the rate of weight gain in the recovery phase after major burns. J Trauma. 1997; 43: 47–51. [DOI] [PubMed] [Google Scholar]
- 20.Strawford A, Barbieri T, Van Loan M, et al. Resistance exercise and supraphysiologic androgen therapy in eugonadal men with HIV-related weight loss: a randomized controlled trial. JAMA. 1999; 281: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 21.Hausmann DF, Nutz V, Rommelsheim K, et al. Anabolic steroids in polytrauma patients. Influence on renal nitrogen and amino acid losses: a double-blind study. JPEN J Parenter Enteral Nutr. 1990; 14: 111–114. [DOI] [PubMed] [Google Scholar]
- 22.Hart DW, Wolf SE, Ramzy PI, et al. Anabolic effects of oxandrolone after severe burn. Ann Surg. 2001; 233: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfeld RG, Attie KM, Frane J, et al. Growth hormone therapy of Turner’s syndrome: beneficial effect on adult height. J Pediatr. 1998; 132: 319–324. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DM, McCauley E, Brown DR, et al. Oxandrolone therapy in constitutionally delayed growth and puberty. Bio-Technology General Corporation Cooperative Study Group. Pediatrics. 1995; 96: 1095–1100. [PubMed] [Google Scholar]
- 25.Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000; 15: 12–17. [DOI] [PubMed] [Google Scholar]
- 26.Biolo G, Chinkes D, Zhang XJ, et al. Harry M. Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr. 1992; 16: 305–315. [DOI] [PubMed] [Google Scholar]
- 27.Biolo G, Maggi SP, Williams BD, et al. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995; 268: E514–520. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001; 98: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudoit S, Yang YH, Callow M, et al. Statistical methods for identifying differentially expressed genesin replicated cDNA microarray experiments. Department of Statistics, University of California at Berkeley, Technical report #578 2000.
- 31.Lee CK, Klopp RG, Weindruch R, et al. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999; 285: 1390–1393. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh SM, Hollander CM, Fornace AJ Jr. Role of GADD45 in apoptosis. Biochem Pharmacol. 2000; 59: 43–45. [DOI] [PubMed] [Google Scholar]
- 33.Kami K, Noguchi K, Senba E. Localization of myogenin, c-fos, c-jun, and muscle-specific gene mRNAs in regenerating rat skeletal muscle. Cell Tissue Res. 1995; 280: 11–19. [DOI] [PubMed] [Google Scholar]