Abstract
Objective
To measure local (peritoneal fluid) and systemic (plasma) cytokine profiles in patients with infection-inflammation of the vermiform appendix, a relatively mild, localized inflammatory process.
Summary Background Data
The systemic host response to invading microorganisms, often termed the systemic inflammatory response syndrome (SIRS), includes changes in heart rate, respiratory rate, body temperature, and circulating white blood cell numbers. Although these changes can be induced experimentally by administering proinflammatory cytokines, the mediators that appear in the bloodstream during early, localized infection in humans have not been defined.
Methods
The authors studied 56 patients with pathologically proven appendicitis. Blood was obtained before the induction of anesthesia, when 82% of the patients met the criteria for SIRS. Peritoneal fluid (PF) was obtained by intraoperative lavage. Cytokines were measured by immunoassay. To assess the net impact of the mediators within plasma, the authors studied the ability of patient plasma to augment or suppress bacterial lipopolysaccharide (LPS) stimulation of monocytes in vitro.
Results
Of the proinflammatory cytokines, tumor necrosis factor-alpha was present in PF but not in plasma, interleukin (IL)-1β and interferon-γ were found in low concentrations in both PF and plasma, and IL-12 (p70) was detectable in plasma but not PF. In contrast, IL-6 and IL-1 receptor antagonist (IL-1ra) were the most abundant cytokines in the PF and plasma, and the concentrations of IL-4 and IL-10 were also elevated in both compartments. Patients with more severe appendicitis had higher plasma levels of IL-6 and IL-10 and lower plasma levels of IL-12 and interferon-γ than did those with uncomplicated disease. Patient plasma inhibited LPS-induced stimulation of a monocyte cell line, and this inhibition was accentuated by complicated disease.
Conclusions
As judged from the pattern of soluble cytokines in plasma and the effect of the plasma on monocyte activation by LPS, mild, localized infection can induce a systemic response that is predominantly anti-inflammatory.
The body’s initial systemic reaction to noxious stimuli frequently includes tachycardia, tachypnea, fever or hypothermia, and leukocytosis or leukopenia. These responses have been termed the systemic inflammatory response syndrome (SIRS); when SIRS has a proven or suspected infectious etiology, it is called sepsis. 1 The SIRS/sepsis concept was based in part on studies in healthy humans injected with endotoxin, 2,3 in which the blood concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-1, and other proinflammatory mediators were noted to rise quickly, preceding the onset of tachycardia, leukocytosis, and fever. Blood concentrations of anti-inflammatory mediators increased later and in apparent response to the proinflammatory ones; these mediators seemed to reflect a delayed, compensatory, and even detrimental (immunosuppressive) reaction. 4
In the studies described here, we sought to characterize the mixture of pro- and anti-inflammatory cytokines that exists in different body compartments during the early course of localized inflammation in humans. We studied patients with acute appendicitis, a moderately severe yet localized infectious-inflammatory condition that is often accompanied by the clinical signs of SIRS. Several features make appendicitis a useful human model of acute, localized inflammation 5: the severity of appendiceal inflammation can be estimated from gross and microscopic pathologic findings, the periappendiceal fluid within the peritoneal cavity can be sampled easily, most patients are relatively young and healthy before developing appendicitis, and the duration of symptoms before presentation is typically less than 48 hours. We studied the cytokine profiles in the peritoneal fluid (PF; regional compartment) and plasma (systemic compartment), looking for differences that might reveal the nature of the inflammatory response in each compartment.
METHODS
Subject Enrollment, General Treatment Approach, and Clinical Data Collection
We recruited patients who were admitted to the emergency department at Parkland Memorial Hospital (Dallas, TX) with a clinical diagnosis of appendicitis and who were taken to the operating room for appendectomy. Detailed clinical data included the duration of symptoms, the use of anti-inflammatory drugs or antibiotics, and the presence of concurrent medical illnesses. Patients who returned for follow-up appointments were questioned regarding medication use during the postoperative recovery period. All subjects gave informed consent to be included in the study. The Institutional Review Board at the University of Texas Southwestern Medical Center approved this study and all the associated procedures. Patients in whom a laparoscopic appendectomy was performed were not enrolled.
The conduct of this study was based on our findings in a preliminary cohort of patients with acute appendicitis and a contemporary cohort of patients undergoing elective intra-abdominal surgery. 6 We measured plasma and PF cytokine concentrations in 19 patients who underwent appendectomy and in 18 patients who underwent laparotomy or laparoscopy for elective procedures. IL-1β, IL-4, IL-6, IL-8, IL-10, TNF-α, and interferon (IFN)-γ were not detectable in peritoneal aspirates obtained from the latter group; IL-1 receptor antagonist (IL-1ra) was detected at low concentrations in two of these patients (57 and 45 pg/mL). In the acute plasma of patients with pathologically proven appendicitis, in comparison to convalescent samples, we observed elevations in acute plasma IL-4, IL-6, and IL-10, but not TNF-α or IL-1β concentrations. In this preliminary sample, there were statistically insignificant differences in plasma and PF cytokine concentrations between patients with complicated and uncomplicated disease. We therefore sought to determine whether these differences were real by studying a new, larger cohort. This independent cohort of 56 patients is the subject of this report.
A pathologist (G.L.) blinded to other clinical data and to the experimental results confirmed the diagnosis of acute appendicitis and classified subjects as having complicated appendicitis if there was gross or microscopic evidence of gangrene, necrosis, or perforation of the appendix. We prospectively grouped patients with gross or microscopic evidence of perforation, gangrene, or necrosis together for analysis (complicated appendicitis) as we expected relatively few cases to meet these individual criteria and we had observed no clear differences in cytokine responses in the small number of patients (two with gross perforation and two with microscopic perforation or gangrene) who had these findings in the pilot sample.
Sample Collection
Venous blood samples were obtained (EDTA anticoagulant) immediately before general anesthesia was induced and at the time of clinic follow-up (7–10 days after surgery). Surgery residents under the guidance of a staff surgeon performed all appendectomies, obtaining the peritoneal aspirate using a standard approach that was developed during the preliminary study: after the peritoneum was incised through a right lower quadrant abdominal incision, 20 mL of 0.9% NaCl was instilled into the peritoneal cavity adjacent to the appendix and then aspirated. The PF and plasma samples were immediately centrifuged at 4°C at 3,000 rpm for 10 minutes and the supernatants were stored in aliquots at −70°C until analyzed.
Net Mediator Balance Assay
To evaluate the net pro- or anti-inflammatory impact of plasma obtained from patients with acute appendicitis, we measured the ability of patient plasma to modify the response of monocytes to bacterial lipopolysaccharide (LPS). The assay was similar to those used by Pugin et al. 7 and Brandtzaeg et al. 8 THP-1 monocytes were incubated with 0.05 μmol/L 1,25-dihydroxy vitamin D3 for 48 hours to induce expression of CD14, the binding receptor for LPS. Then 3.5 × 105 cells were suspended in 350 μL RPMI with 1% fetal calf serum and seeded in 24-well microtiter plates. Acute or recovery plasma (100 μL) was added, and 25 μL of LPS (Escherichia coli LCD25; List Biologic Laboratories, Campbell, CA) was added to stimulate the cells for 2 hours at 37°C in an atmosphere of 5% CO2. The concentration of IL-8 in the medium was then measured by enzyme-linked immunosorbent assay. In all assays, each experimental condition was tested in quadruplicate.
Cytokine Measurements
We used OptEIA Sets (Becton Dickinson-Pharmingen, San Diego, CA) for all cytokine measurements. The lower detection limits were 7.8 pg/mL (IFN-γ, IL-1β, IL-4, IL-6, IL-8, IL-10, and IL-12 [p70]), 5.0 pg/mL (TNF-α), and 20.6 pg/mL (IL-1Ra).
PF Protein Concentrations
The PF protein concentration was determined by the Bradford dye-binding procedure (Bio-Rad Protein Assay, Bio-Rad Laboratories, Hercules, CA). 9
Data Presentation and Statistical Analyses
Continuous data are presented as medians with the 25th to 75th percentiles, and categorical data as percentages. Many of the data were either not normally distributed or were recorded as nondetectable when below the detection threshold. Accordingly, the percentage of subjects with detectable concentrations of the various cytokines is also presented. Cytokine concentrations were compared by using the Wilcoxon rank sum test and repeated measures analysis of variance. Actual P values for the various comparisons are reported when possible.
RESULTS
Characteristics of Subjects
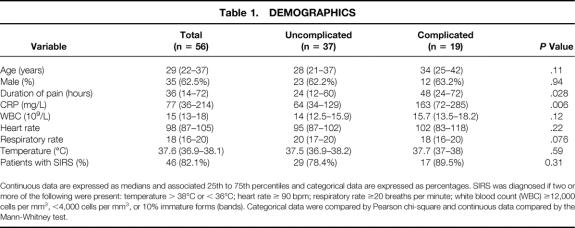
From August 8, 2000, to December 12, 2000, 85 patients underwent appendectomy for presumed acute appendicitis. We were unable to obtain informed consent in 25 patients. Four (7%) of the 60 remaining patients were excluded because they did not to have acute appendicitis on review of the pathologic specimen. Three of the four had an alternative pathologic diagnosis (two with cecal diverticulum and one with pelvic endometriosis), and the fourth patient was found to be HIV-positive and his samples were not analyzed. Therefore, this report is based on 56 patients with acute appendicitis. Of these 56 patients, 23 returned to clinic and agreed to follow-up blood sampling. Demographic features of the study cohort are shown in Table 1.
Table 1. DEMOGRAPHICS
Continuous data are expressed as medians and associated 25th to 75th percentiles and categorical data are expressed as percentages. SIRS was diagnosed if two or more of the following were present: temperature > 38°C or < 36°C; heart rate ≥ 90 bpm; respiratory rate ≥20 breaths per minute; white blood count (WBC) ≥12,000 cells per mm3, <4,000 cells per mm3, or 10% immature forms (bands). Categorical data were compared by Pearson chi-square and continuous data compared by the Mann-Whitney test.
Intensity of Systemic Response Reflects Severity of Local Inflammation
Most (82%) of the 56 patients met at least two criteria for SIRS (see Table 1); the respiratory criterion was met least frequently (34%) and the white blood cell count criterion most frequently (88%). Plasma C-reactive protein (CRP) concentrations were elevated (>10 mg/L) in all but two patients.
Nineteen of the 56 patients had complicated appendicitis. The CRP concentration was significantly higher in these patients (complicated, median = 163 mg/L; uncomplicated, median = 64 mg/L;P = .006), who were also more likely to have elevated white blood cell counts and to meet SIRS criteria. As expected, the duration of symptoms was related to the severity of appendicitis, although there was considerable overlap in this regard. For example, the median duration of symptoms as reported by the patient was 36 hours. One fourth of the patients had symptoms for 14 or fewer hours, and one (7%) of these had complicated disease. At the other extreme, 9 of 15 patients had uncomplicated disease despite reporting more than 48 hours of symptoms.
Peritoneal (Regional) Response Includes Both Pro- and Anti-Inflammatory Cytokines
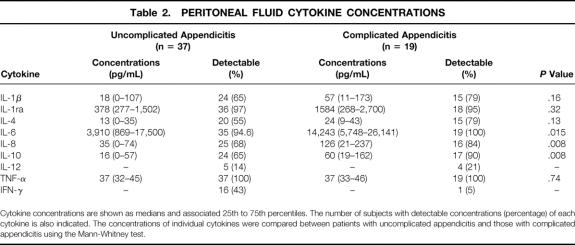
The concentration of each cytokine in PF aspirates and the percentages of patients with detectible levels are shown in Table 2. IL-6, the most markedly and consistently elevated cytokine, was detected in all but one patient. IL-1ra was present in approximately 30-fold excess relative to the concentrations of IL-1, and there was a significant correlation between the concentrations of these molecules (rho = 0.37, P = .006 by Spearman rank correlation). IL-12 and IFN-γ were detectable in less than half of the PF aspirates.
Table 2. PERITONEAL FLUID CYTOKINE CONCENTRATIONS
Cytokine concentrations are shown as medians and associated 25th to 75th percentiles. The number of subjects with detectable concentrations (percentage) of each cytokine is also indicated. The concentrations of individual cytokines were compared between patients with uncomplicated appendicitis and those with complicated appendicitis using the Mann-Whitney test.
The concentrations of IL-6, IL-8, and IL-10 were significantly higher in PF obtained from patients with complicated disease. These differences were also found when each cytokine concentration was normalized to the total protein concentration in the PF (data not shown). IFN-γ concentrations were lower in the PF aspirates of patients with complicated appendicitis, although relatively few patients in either group had detectible IFN-γ in PF.
The duration of symptoms was related to the concentration of IL-1β in PF. The IL-1β concentration was 17 pg/mL (range 0–38 pg/mL) in patients reporting 36 or fewer hours of symptoms and 79 pg/mL (range 33–173 pg/mL) in patients with more than 36 hours of symptoms (P = .01). There were no other associations between duration of symptoms and cytokine concentrations in the PF aspirates.
Plasma (Systemic) Cytokine Profile Appears to be Anti-Inflammatory
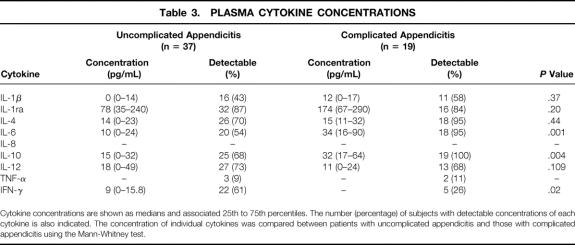
The concentrations of several cytokines that have predominantly anti-inflammatory activities (IL-1ra, IL-4, IL-6, and IL-10) were elevated in the preoperative plasma samples from most patients (Table 3). In contrast, although proinflammatory IFN-γ and IL-1β were detectable in half of the patients, their concentrations were low, and TNF was found in the plasma of only 10% of the patients. IL-12 was found in the plasma of most patients, but the levels were within the range found in normal subjects. The anti-inflammatory cytokine profile was more marked in patients with complicated appendicitis, who had higher plasma concentrations of IL-1Ra and IL-10 and lower levels of IFN-γ and IL-12 than did patients with uncomplicated disease.
Table 3. PLASMA CYTOKINE CONCENTRATIONS
Cytokine concentrations are shown as medians and associated 25th to 75th percentiles. The number (percentage) of subjects with detectable concentrations of each cytokine is also indicated. The concentration of individual cytokines was compared between patients with uncomplicated appendicitis and those with complicated appendicitis using the Mann-Whitney test.
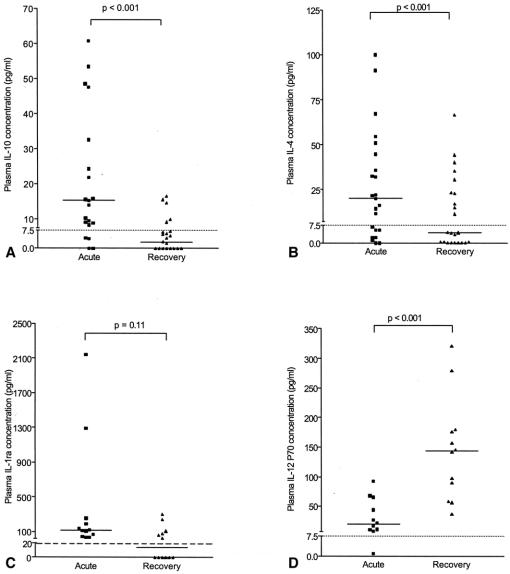
The concentrations of IL-10, and IL-4 were significantly lower in the recovery samples than in acute plasma (Fig. 1); a similar trend was found for IL-1Ra, whereas IL-12 levels tended to be higher. Plasma CRP was less than 10 mg/L in each of the nine patients from whom convalescent samples were assayed.
Figure 1. Acute and convalescent plasma cytokine concentrations. Sufficient acute and convalescent plasma was available to compare IL-10, and IL-4 in all 23 patients who returned for follow-up and to compare IL-1ra and IL-12 in 12 patients.
We also asked whether the absence of a SIRS response to appendicitis was associated with a different cytokine profile than that observed in the patients who met at least two SIRS criteria. Plasma IL-10 was lower (8.6 pg/mL; range 0–16 pg/mL) in the 10 patients without SIRS than in the 46 patients with SIRS (23 pg/mL; range 11–53 pg/mL, P = .004). There were no differences in any of the other plasma or PF cytokine concentrations according to whether the patient met SIRS criteria. Plasma cytokine concentrations were not related to the duration of symptoms.
Plasma From Patients With Appendicitis Suppresses In Vitro LPS-Induced IL-8 Production by Monocytes
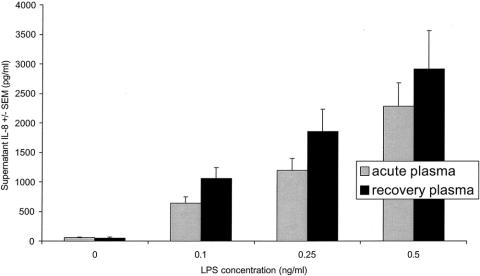
We first measured IL-8 concentrations in the medium of THP-1 cells that had been incubated with LPS in the presence of acute or recovery plasma (n = 14 patients). At each of the three LPS concentrations, THP-1 cells exposed to acute plasma released less IL-8 than did cells exposed to recovery phase plasma (Fig. 2).
Figure 2. Acute phase plasma inhibits LPS-induced IL-8 production by THP-1 monocytes in vitro. THP-1 cells were incubated with the indicated concentrations of LPS in the presence of acute or convalescent patient plasma. At each LPS concentration, monocytes exposed to acute plasma (grey bars) released less IL-8 than did cells exposed to plasma from the recovery period (solid black bars). (P = .01 by repeated measures ANOVA comparing the effects of acute and recovery plasma at 3 LPS exposure concentrations.)
We next asked if plasma from patients with complicated appendicitis has greater suppressive potency than does plasma from patients with less severe disease. We incubated THP-1 cells with 0.5 ng/mL LPS in the presence of preoperative plasma from 8 patients with complicated disease and 11 patients with uncomplicated disease. Cells incubated with plasma from patients with complicated appendicitis produced less IL-8 than did cells incubated with plasma from those with uncomplicated disease (Fig. 3).
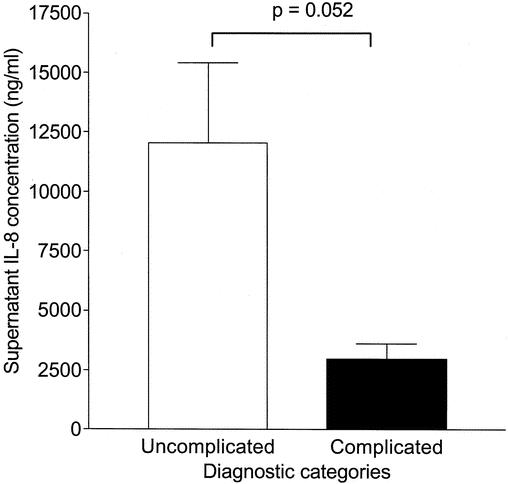
Figure 3. Plasma from patients with complicated appendicitis inhibits the monocyte response to LPS in vitro. Cells were stimulated with 0.5 ng/mL LPS after exposure to acute plasma from patients with uncomplicated appendicitis (n = 11) or complicated appendicitis (n = 8). Cells exposed to plasma from the less severely ill patients released more IL-8 (12,100 ± 3,350 [SEM] pg/mL) than did cells exposed to plasma from patients with complicated disease (2,480 ± 440 pg/mL).
DISCUSSION
According to the concept that has dominated clinical investigation for many years, the systemic response to local infection is initially proinflammatory. Elaboration of TNF-α, IL-1β, and other mediators at an infected site was thought to trigger “systemic inflammation” that resulted in tachycardia, tachypnea, fever, leukocytosis, and other clinical signs. Systemic inflammation would eventually resolve, in part, due to the action of compensatory anti-inflammatory forces. 10,11 Our observations in patients with acute appendicitis suggest that a systemic anti-inflammatory state may develop quite early in the course of a local infectious/inflammatory stimulus. In the plasma of patients with uncomplicated local infection/inflammation, we found high concentrations of several anti-inflammatory cytokines (IL-4, IL-6, IL-10, IL-1Ra), while the classical proinflammatory ones (TNF-α, IL-1β, IFN-γ, IL-12) were found in low concentrations, if at all. These findings were accentuated in patients with more severe local inflammation. We also found that the acute phase plasma suppressed the responses of cultured monocytes to LPS, a bacterial agonist, and that plasma from patients with complicated appendicitis was more suppressive than was plasma from patients with less severe disease. Although cytokine concentrations provide only a partial view of the body’s responses to injury and infection, our results point to an early, anti-inflammatory systemic response to localized infection.
An early anti-inflammatory systemic response to local infection may be beneficial, allowing the body to develop a proinflammatory local or regional environment, in which microbial invasion can be quelled, while preventing inflammation in other tissues. Although the association of elevated circulating anti-inflammatory cytokines with mortality in sepsis has raised the possibility that systemic anti-inflammation may be detrimental, 4,12,13 many observations suggest that the systemic response to a local stimulus results in changes that limit remote tissue injury. For instance, neutrophil recruitment to an inflammatory site requires adhesion to the vascular endothelium. Neutrophil–endothelial interactions are partly dependent on the neutrophil adhesion molecule L-selectin, which is shed from circulating neutrophils in response to mediators 14,15 that include cortisol and CRP, 16 one of the proteins found to be elevated in the plasma of appendicitis patients. In addition, many aspects of the evolutionarily conserved acute phase response are anti-inflammatory, supporting the notion that suppression of systemic inflammation is beneficial to host survival. For instance, CRP, α-1 antitrypsin, and other acute phase proteins have been shown to induce human monocytes to produce IL-1ra in 5- to 10-fold excess over IL-1β. 17 IL-4 and IL-10, additional anti-inflammatory mediators, were also clearly elevated in acute phase plasma, particularly in patients with complicated disease. IL-10 suppresses proinflammatory cytokine secretion 18,19 and decreases monocyte HLA-DR expression. 20 Although the role of IL-6 in inflammation is more controversial, there is evidence favoring important systemic anti-inflammatory effects in response to both systemic (intravenous) or localized (intraperitoneal) endotoxin administration. 21
Our findings raise the possibility that a systemic anti-inflammatory cytokine response may develop without an initially proinflammatory systemic state; to the contrary, an anti-inflammatory systemic milieu may be the usual and initial response to inflammatory stimuli. 11,22,23 Even in the most severe form of sepsis (septic shock), when circulating concentrations of TNF-α are often detectable, anti-inflammatory cytokine concentrations are elevated to a greater degree. 24 Moreover, an overall anti-inflammatory effect, similar to that shown in our monocyte reporter assay, has been shown in children with severe meningococcal sepsis 8 and in critically ill adults. 25 Support for the concept that local and systemic compartments can mount distinctly different responses comes from evidence that, in mice, mortality following intravenous administration of E. coli is ameliorated by systemic administration of anti-TNF-α antibody, whereas mortality associated with intraperitoneal E. coli is not. 26
While less is known about the response to infection or inflammation that occurs regionally, our findings are consistent with the results of other studies that measured cytokine responses in compartments such as the peritoneal cavity and lung. 7,27,28 Experimental peritonitis induces a marked peritoneal proinflammatory cytokine profile, accompanied by elevated plasma concentrations of chemokines and anti-inflammatory cytokines. 27 In human studies of severe peritonitis, TNF-α, IL-6, and IL-8 are among the mediators most markedly elevated in the peritoneal compartment. 29 This pattern is not unique to peritoneal infection or inflammation; other experimental and clinical models have also shown differences in local/regional and circulating cytokine responses. 7,28,30 For instance, Marie et al. demonstrated a more intense cytokine response in the pleural fluid, relative to plasma, in patients with pneumonia and acute lung injury; they suggested that these differences are evidence for compartmentalization of the inflammatory response. 28 Pugin et al. found markedly different local (alveolar lavage fluid) and systemic (plasma) inflammatory environments in patients with acute respiratory distress syndrome. 31 They reported that proinflammatory cytokine concentrations were lower (IL-8) or undetectable (TNF-α and IL-1β) in plasma, whereas the alveolar concentrations and activities of these mediators were elevated. Moreover, alveolar lavage fluid from patients with acute respiratory distress syndrome stimulated reporter A549 cells to express more intercellular adhesion molecule (ICAM)-1 than did alveolar fluid from patients with cardiogenic pulmonary edema. A549 cells also expressed more ICAM-1 when exposed to alveolar fluid than when exposed to plasma. While it is difficult to compare the effects of alveolar fluid and plasma, these studies suggest that the local environment is more proinflammatory than the systemic one. Unfortunately, we were unable to use our reporter assay to directly compare the effects of PF to those of plasma. PF from appendicitis patients often induced the reporter monocytes to produce IL-8 in the absence of added LPS (data not shown). Spontaneous bacterial peritonitis in patients with ascites and liver disease may represent an additional human model of local-regional infection that seems to induce an anti-inflammatory systemic response. 32 However, as TNF-α has been detected in low concentrations in the plasma of patients with liver disease and spontaneous bacterial peritonitis, the overall systemic inflammatory state in such patients is unknown. 33 This may, in part, be because the role that hepatocytes and resident macrophages play in the acute phase response may be altered by the changes imposed by chronic liver disease. As a result, the inferences that can be drawn about the systemic response from a model that is based on chronic liver disease to other conditions are likely limited.
A number of mechanisms may contribute to a systemic anti-inflammatory response to a local inflammatory stimulus such as acute appendicitis. 34 First, the acute phase response appears to include a number of anti-inflammatory influences and mediators. 35 As discussed above, CRP appears to prevent neutrophil adhesion to endothelial cells and also to limit free radical production. 16 That IL-6 is one of the important inducers of the acute phase response is of particular interest given the high levels of IL-6 that we observed in PF aspirates. Additional mechanisms that may limit systemic inflammation include stimulation of the sympathoadrenal system and parasympathetic nervous system. Experimental intravenous infusion of TNF-α increases circulating catecholamines, and efferent effects of the sympathoadrenal system may suppress inflammatory and immune responses. 36,37 For instance, experimental epinephrine infusion suppresses in vivo production of TNF-α but not IL-10 production. 38 Finally, there is interesting recent evidence supporting inflammation-modifying activities of the parasympathetic nervous system. In a series of elegant experiments, in vivo vagal nerve stimulation in rats reduced hepatic TNF synthesis and prevented the development of shock after LPS infusion. 39 Each of these mechanisms may contribute to the overall systemic response that we have characterized in patients with acute appendicitis.
A number of points must be considered when interpreting our findings. Cytokine measurements have known limitations, including an imperfect correlation between biologic activity and immunoassay results. 40 Moreover, single measurements may not accurately reflect temporal trends. It is possible, for example, that we failed to detect the peak plasma concentrations of TNF-α, IL-1β, and other proinflammatory cytokines that might have occurred before blood samples were drawn. This possibility will clearly be difficult to prove. However, some of our evidence suggests that early, transient elevations did not occur. First, in the patients with the shortest duration of symptoms, proinflammatory cytokines were not more prominent in the plasma than in patients who reported more prolonged symptoms. The only temporal pattern we noted was that PF IL-1β was higher in patients reporting a longer duration of symptoms. Nevertheless, we cannot exclude the possibility that TNF-α and other proinflammatory cytokines were elevated in plasma and possibly PF before our samples were obtained. If early and transient elevations in TNF-α, IL-1β, IFN-γ, and other proinflammatory mediators did occur, they might have contributed to changes that persisted for variable periods of time after their disappearance. However, and of importance here, the eventual effect of TNF-α increasing leukocyte apoptosis suggests that at least in isolation, TNF-α may eventually lead to a suppression of leukocyte activity—an anti-inflammatory effect. 41 It is also unlikely that circulating cytokine concentrations entirely reflect the prevailing inflammatory milieu. In vivo cytokine dose–response relationships may not be linear, and other mediators in addition to those measured here doubtless contribute to the inflammatory balance. Nonetheless, the demonstration, by others and ourselves, that plasma from patients with appendicitis and more severe illnesses suppresses monocyte function suggests that acute phase plasma constituents are at least in part responsible for a systemic anti-inflammatory state.
In summary, anti-inflammatory cytokines appear to dominate in the plasma of patients with acute appendicitis, within hours of the onset of symptoms. More severe local inflammation was associated with higher circulating concentrations of IL-4 and IL-10, mediators typically considered anti-inflammatory, but lower concentrations of two proinflammatory cytokines, IFN-γ and IL-12. Moreover, plasma from acutely ill patients had suppressive activity toward reporter monocytes. These data suggest that a local inflammatory stimulus may be sufficient to induce a systemic response that is anti-inflammatory and that a systemic “proinflammatory” state may not be a necessary precursor of this response.
Footnotes
Robert S Munford is supported by NIH grants AI18188 and AI38596 and the Jan and Henri Bromberg Chair in Internal Medicine.
Grant E O’Keefe is supported by NIH grant 5P50GM021681-370013.
Correspondence: Grant O’Keefe, MD, University of Washington, Department of Surgery, Box 359796, Harborview Medical Center, 325 Ninth Avenue, Seattle, WA 98104-2499.
E-mail: gokeefe@u.washington.edu
Accepted for publication August 5, 2002.
References
- 1.Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996; 24: 163–172. [DOI] [PubMed] [Google Scholar]
- 2.Hesse DG, Tracey KJ, Fong Y, et al. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988; 166: 147–153. [PubMed] [Google Scholar]
- 3.Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000; 127: 117–126. [DOI] [PubMed] [Google Scholar]
- 4.Sfeir T, Saha DC, Astiz M, et al. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit Care Med. 2001; 29: 129–133. [DOI] [PubMed] [Google Scholar]
- 5.Bittinger F, Brochhausen C, Kohler H, et al. Differential expression of cell adhesion molecules in inflamed appendix: correlation with clinical stage. J Pathol. 1998; 186: 422–428. [DOI] [PubMed] [Google Scholar]
- 6.Rivera-Chivez F, Munford RS, O’Keefe GE. Characterization of local and systemic cytokine responses during acute inflammation in humans. Shock. 2002; 13: 65. [Google Scholar]
- 7.Pugin J, Ricou B, Steinberg KP, et al. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996; 153: 1850–1856. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg P, Osnes L, Ovstebo R, et al. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996; 184: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 10.Botha AJ, Moore FA, Moore EE, et al. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery. 1995; 118: 358–364. [DOI] [PubMed] [Google Scholar]
- 11.Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999; 27: 749–755. [DOI] [PubMed] [Google Scholar]
- 12.van Dissel JT, van Langevelde P, Westendorp RG, et al. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998; 351: 950–953. [DOI] [PubMed] [Google Scholar]
- 13.Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000; 181: 176–180. [DOI] [PubMed] [Google Scholar]
- 14.Strausbaugh HJ, Green PG, Lo E, et al. Painful stimulation suppresses joint inflammation by inducing shedding of L-selectin from neutrophils. Nat Med. 1999; 5: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 15.Ferri LE, Swartz D, Christou NV. Soluble L-selectin at levels present in septic patients diminishes leukocyte-endothelial cell interactions in mice in vivo: a mechanism for decreased leukocyte delivery to remote sites in sepsis. Crit Care Med. 2001; 29: 117–122. [DOI] [PubMed] [Google Scholar]
- 16.Zouki C, Beauchamp M, Baron C, et al. Prevention of in vitro neutrophil adhesion to endothelial cells through shedding of L-selectin by C-reactive protein and peptides derived from C-reactive protein. J Clin Invest. 1997; 100: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilg H, Vannier E, Vachino G, et al. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993; 178: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Waal MR, Abrams J, Bennett B, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991; 174: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991; 147: 3815–3822. [PubMed] [Google Scholar]
- 20.Klava A, Windsor AC, Farmery SM, et al. Interleukin-10. A role in the development of postoperative immunosuppression. Arch Surg. 1997; 132: 425–429. [DOI] [PubMed] [Google Scholar]
- 21.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998; 101: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi T, Koido Y, Aiboshi J, et al. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999; 27: 1262–1264. [DOI] [PubMed] [Google Scholar]
- 23.Simovic MO, Bonham MJ, Abu-Zidan FM, et al. Anti-inflammatory cytokine response and clinical outcome in acute pancreatitis. Crit Care Med. 1999; 27: 2662–2665. [DOI] [PubMed] [Google Scholar]
- 24.Damas P, Canivet JL, de Groote D, et al. Sepsis and serum cytokine concentrations. Crit Care Med. 1997; 25: 405–412. [DOI] [PubMed] [Google Scholar]
- 25.Randow F, Syrbe U, Meisel C, et al. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995; 181: 1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bagby GJ, Plessala KJ, Wilson LA, et al. Divergent efficacy of antibody to tumor necrosis factor-alpha in intravascular and peritonitis models of sepsis. J Infect Dis. 1991; 163: 83–88. [DOI] [PubMed] [Google Scholar]
- 27.Walley KR, Lukacs NW, Standiford TJ, et al. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996; 64: 4733–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marie C, Losser MR, Fitting C, et al. Cytokines and soluble cytokine receptors in pleural effusions from septic and nonseptic patients. Am J Respir Crit Care Med. 1997; 156: 1515–1522. [DOI] [PubMed] [Google Scholar]
- 29.Holzheimer RG, Schein M, Wittmann DH. Inflammatory response in peritoneal exudate and plasma of patients undergoing planned relaparotomy for severe secondary peritonitis. Arch Surg. 1995; 130: 1314–1319. [DOI] [PubMed] [Google Scholar]
- 30.Kupper TS, Deitch EA, Baker CC, et al. The human burn wound as a primary source of interleukin-1 activity. Surgery. 1986; 100: 409–415. [PubMed] [Google Scholar]
- 31.Pugin J, Verghese G, Widmer MC, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999; 27: 304–312. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Ramos C, Galan F, Diaz F, et al. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001; 46: 1668–1676. [DOI] [PubMed] [Google Scholar]
- 33.Such J, Hillebrand DJ, Guarner C, et al. Tumor necrosis factor-alpha, interleukin-6, and nitric oxide in sterile ascitic fluid and serum from patients with cirrhosis who subsequently develop ascitic fluid infection. Dig Dis Sci. 2001; 46: 2360–2366. [DOI] [PubMed] [Google Scholar]
- 34.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001; 163: 316–321. [DOI] [PubMed] [Google Scholar]
- 35.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999; 340: 448–454. [DOI] [PubMed] [Google Scholar]
- 36.Tracey KJ, Lowry SF, Fahey TJ III, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987; 164: 415–422. [PubMed] [Google Scholar]
- 37.Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000; 21: 281–289. [DOI] [PubMed] [Google Scholar]
- 38.van der PT, Coyle SM, Barbosa K, et al. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest. 1996; 97: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000; 405: 458–462. [DOI] [PubMed] [Google Scholar]
- 40.Corti A, Poiesi C, Merli S, et al. Tumor necrosis factor (TNF) alpha quantification by ELISA and bioassay: effects of TNF alpha-soluble TNF receptor (p55) complex dissociation during assay incubations. J Immunol Methods. 1994; 177: 191–198. [DOI] [PubMed] [Google Scholar]
- 41.Avdi NJ, Nick JA, Whitlock BB, et al. Tumor necrosis factor-alpha activation of the c-Jun N-terminal kinase pathway in human neutrophils. Integrin involvement in a pathway leading from cytoplasmic tyrosine kinases apoptosis. J Biol Chem. 2001; 276: 2189–2199. [DOI] [PubMed] [Google Scholar]