Abstract
Changes in the firing pattern of midbrain dopamine neurons are thought to encode information for certain types of reward-related learning. In particular, the burst pattern of firing is predicted to result in more efficient dopamine release at target loci, which could underlie changes in synaptic plasticity. In this study, the effects of dopamine on the firing patterns of dopaminergic neurons in vivo and their electrophysiological characteristics in vitro were examined by using a genetic dopamine-deficient (DD) mouse model. Extracellular recordings in vivo showed that, although the firing pattern of dopamine neurons in normal mice included bursting activity, DD mice recordings showed only a single-spike pattern of activity with no bursts. Bursting was restored in DD mice after systemic administration of the dopamine precursor, l-3,4-dihydroxyphenylalanine (l-dopa). Whole-cell recordings in vitro demonstrated that the basic electrophysiology and pharmacology of dopamine neurons were identical between DD and control mice, except that amphetamine did not elicit a hyperpolarizing current in slices from DD mice. These data suggest that endogenously released dopamine plays a critical role in the afferent control of dopamine neuron bursting activity and that this control is exerted via a network feedback mechanism.
The activity of dopamine neurons has been shown to correlate with behavioral adaptations during reward-related learning in primates and rodents (1–4). Dopamine neurons fire spontaneously in vivo in a spectrum of patterns ranging from pacemaker, to random, to bursting modes (5, 6). Clusters of two to eight spikes characterize the burst mode (7, 8). The random mode is the most common pattern encountered in vivo and is characterized by bursts of spikes followed by single-spike activity (5, 9). The pacemaker pattern, encountered in ≈20% of neurons recorded in vivo, is characterized by single spikes firing in a clock-like manner, interrupted by infrequent bursts (2, 5, 6). Determining the origins and mechanisms responsible for burst firing in vivo is of interest because this firing pattern is thought to be responsible for large increases in dopamine release in the striatum that may mediate synaptic plasticity and contribute to reward-related learning (4, 10–17).
The only pattern recorded spontaneously in vitro is the single-spike, pacemaker pattern without bursts (18–20). This contrasts markedly with recordings in vivo where bursts can still be encountered even if a neuron is classified as firing in a pacemaker mode (2). This disparity between in vivo and in vitro recordings suggests that afferents play a critical role in the control of dopamine neuron firing pattern.
Release of dopamine in the basal ganglia and other projection areas may influence the afferent regulation of dopamine neurons through reciprocal and other long distance, multisynaptic connections (e.g., see ref. 21). This study investigates the effects of removing dopamine on the activity of dopamine neurons by using mice that were rendered dopamine-deficient (DD) by the selective removal of the tyrosine hydroxylase (Th) gene in dopamine neurons. This genetic lesion was achieved by inactivating the endogenous Th gene and then restoring Th function to noradrenergic and adrenergic cells by targeting the Th gene to the dopamine β-hydroxylase (Dbh) locus (22). In this mutant, the dopamine neurons are intact and seem to make normal connections (22). DD mice manifest a severe parkinsonian-like state beginning about 2 weeks after birth, and they will not survive without intervention; however, they can be rescued by daily administration of l-3,4-dihydroxyphenylalanine (l-dopa) (22). Here we assess the firing patterns and properties of dopamine neurons of DD mice in both their dopamine-depleted state and after restoration of dopamine to gain insights into how this neurotransmitter influences dopamine neuron activity.
Materials and Methods
Animals.
Mice were used in accordance with guidelines for animal care and use established by the National Institutes of Health, and the Animal Care Committees at the University of Washington and Oregon Health Sciences University. Control and DD mice (Th−/−; DbhTh/+), previously called DA−/− mice, were bred as described and maintained on a mixed C57BL/6 × 129/SvEv genetic background (22). Control mice included animals that had at least one intact Th and one intact Dbh allele; previous studies established that one Th or Dbh allele is sufficient for production of nearly normal levels of dopamine and norepinephrine (23, 24). DD mice were maintained from ≈2 weeks of age until experimentation by daily injections of l-dopa (50 mg/kg body weight, i.p.). DD and control mice used for recordings in vitro were 4–5 weeks old, and those used for recordings in vivo were 3 months old. All recordings were performed at least 24 h after the last daily l-dopa injection, when brain dopamine levels are <1.0% of control mice (22, 25).
Extracellular Recordings.
Twelve mice (seven DD mice and five control mice) were anesthetized (10 ml/kg body weight of 2.5% ketamine, 1% xylazine, and 0.5% acepromazine in normal saline) and placed in a stereotaxic frame. All wound margins and points of contact between the animal and stereotaxic apparatus were infiltrated with lidocaine (5%) ointment. A small hole was drilled and the dura was punctured at the following coordinates from Bregma (26): anterior, −3.1 to −3.5 mm; lateral: −0.9 to −1.3 mm. Glass electrodes (5–10 MΩ) filled with 1M NaCl were lowered 3.5 to 4.2 mm from the dural surface, and recordings were made at room temperature.
Single units were amplified with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA) and displayed on a Tektronix storage oscilloscope. Dopamine neurons were identified by their extracellular waveforms (characterized by a prominent notch in the initial positive phase and having durations of 2–5 ms), slow spontaneous activity, and sensitivity to apomorphine (0.75 mg/kg, i.p.) (8, 27). At the end of experiments, some mice were given a lethal overdose of anesthetic, and brain slices were examined for histological verification of the recording sites (n = 5).
Slice Recordings.
Midbrain horizontal slices (200–300 μm) were prepared from 25 mice (13 DD mice and 12 control mice) as described (28). Horizontal slices were placed in a chamber (0.5 ml) superfused with physiological saline (35°C) at a rate of 1.5 ml/min. The solution was equilibrated with 95% O2/5% CO2 (pH 7.4) and contained 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 1.4 mM NaH2PO4, 25 mM NaHCO3, and 11 mM d-glucose. The internal solution used for whole-cell recordings contained 115 mM K-methyl sulfate, 20 mM KCl, 1 mM MgCl2, 10 mM Hepes, 0.1 mM EGTA, 2 mM ATP, 0.3 mM GTP, and 10 mM creatine phosphate.
Patch recordings were made by using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). Midbrain dopamine neurons were identified by their electrical properties, which included slow spontaneous activity and a hyperpolarization-induced inward current (H-current; refs. 29 and 30).
Evoked Responses.
Iontophoretic pipettes (20–50 MΩ) were filled with l-aspartate (1 M, pH 7.5) and placed within 10 μm of the soma or proximal dendrite. Iontophoretic pulses (−50 nA, 50 ms) were applied once per minute. Picrotoxin (100 μM) and strychnine (1 μM) were used to block γ-aminobutyric acid type A and glycine receptors, respectively. The metabotropic glutamate receptor (mGluR) inhibitory postsynaptic potential was isolated by using 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) (5 μM), MK-801 (50 μM), and CGP 56999a (100 nM) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, N-methyl-d-aspartate, and γ-aminobutyric acid type B (GABAB) receptors, respectively.
Drugs.
Drugs were applied to the slice by superfusion, except aspartate (see above). For the experiments in vivo, drugs were administered intraperitoneally. Adenosine trisphosphate, (+)-amphetamine sulfate, baclofen, dopamine-HCl, guanosine trisphosphate, l-aspartate, l-dopa, quinpirole, picrotoxin, and strychnine were from Sigma. S(−)-eticlopride and MK-801 were from Research Biochemicals (Natick, MA). NBQX, (S)-α-methyl-4-carboxyphenylglycine (MCPG), and (S)-3,5-dihydroxyphenylglycine were from Tocris Cookson (St. Louis). CGP 56999a was a gift from Novartis Pharmaceuticals (Basel).
Data Analysis.
Values are given as means ± SEM. For all experiments, P < 0.05 was considered as a significant difference. For in vitro recordings, the change produced by a drug was calculated as the mean holding/evoked current amplitude ≈30 s after equilibrium had been reached relative to the holding current before drug superfusion. Unpaired comparisons between two groups were made with a Mann–Whitney U test, whereas paired comparisons were made by using a Wilcoxon signed-rank test.
For in vivo recordings, baseline firing rate and patterns were analyzed over 5 min of activity before any drug administration. Bursts were defined by using the previously established criteria of ≤80 ms interspike interval to signal the onset of a burst, and an interval of >160 ms to signal the end of a burst (7). Autocorrelation and first-order histograms were constructed from samples of spontaneous spike trains. The autocorrelation measures interspike intervals to the nth order. One property of this function is that it asymptotes to a constant limiting value so that highly regular spike trains reach this value more slowly than spike trains with a higher degree of variability. Thus, the number of peaks in the autocorrelogram that occur at integral multiples of the mean interspike interval, before reaching a constant limiting value, represent an index of regularity of firing (31). Neurons that exhibit an initial peak with a decay to a steady-state level are classified as bursty, whereas neurons that exhibit a random firing pattern display an autocorrelation function characterized by an initial trough that rises to a steady-state value (6). The coefficient of variation (standard deviation divided by mean interspike interval) was calculated as an additional measure of regularity of firing.
Results
Extracellular Recordings in Vivo.
Dopamine neurons were recorded in vivo to determine whether the firing patterns were different between DD and control mice. Spike trains recorded from control mice had many different firing patterns with bursts always occurring regardless of the overall firing rate or pattern. Fig. 1A shows a representative trace from a control mouse with burst firing, short interspike interval and an autocorrelogram indicative of bursting activity. The average firing rates and coefficients of variation (CV) obtained from six control neurons were 3.57 ± 1.3 spikes per s and 65 ± 8%, respectively. Many of the spikes (34%) were fired in bursts as defined in Materials and Methods.
Figure 1.
Firing patterns in vivo are different between DD and control mice. Sample raw trace (Left), first-order histogram (Center), and autocorrelogram (Right) of extracellular recordings from identified dopamine neurons displayed for a control mouse (A) and a DD mouse (B and C). (A) This dopamine neuron fired in a burst-firing pattern as indicated by the closely spaced spikes (Left), numerous interspike intervals <80 ms (Center), and the initial peak in the autocorrelogram with a decay to a steady-state level (Right). (B) Before any drug administration, this dopamine neuron from a DD mouse fired in a somewhat irregular single-spike pattern without any bursts (Left), as indicated by the relatively long interspike intervals (Center), and multiple peaks in the autocorrelogram (Right). (C) After administration of l-dopa, this same neuron shifted to a random firing pattern with some bursts revealed as dispersed interspike intervals (Center) and indicated by the initial rise to a steady-state level with no peaks in the autocorrelogram (Right). Autocorrelogram and first-order histogram have bin widths of 20 ms.
Fig. 1B shows a typical trace from a recording obtained from a DD mouse; note the absence of bursts and the relatively long interspike intervals. No bursts were recorded from 11 neurons from DD mice over a combined period of 80 min. The average firing rate of these 11 neurons was 0.97 ± 0.1 spikes per s with a CV of 28 ± 3%. The autocorrelograms of dopamine neurons in DD mice indicated either random or pacemaker patterns (Fig. 1B).
The effect of l-dopa was examined in both control and DD mice. After ≈5–10 min of extracellular recordings, mice were injected with l-dopa (50 mg/kg, i.p.). Sample spike trains were recorded beginning at 5, 10, 20, and 30 min after l-dopa administration. l-Dopa had no effect on the firing pattern of control mice (data not shown). Dopamine neurons recorded from DD mice began to show bursts by 15 min in two neurons, 20 min in eight neurons, and 30 min in one neuron. Fig. 1C is the same neuron shown in Fig. 1B 20 min after administration of l-dopa; note the presence of bursts, decrease in interspike interval, and change in the autocorrelogram. Overall, 4% of the spikes occurred in bursts after l-dopa administration with a CV of 40 ± 10% (n = 11). The average firing rate increased only modestly after l-dopa administration in DD mice (1.17 ± 0.16 spikes per s; P > 0.05), whereas there was a pronounced increase in variability of interspike intervals. The autocorrelograms of dopamine neurons in DD mice after l-dopa administration indicated random firing patterns.
Recordings in Vitro.
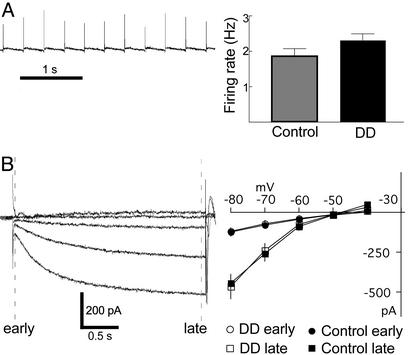
To assess whether the lack of bursting activity of dopamine neurons from DD mice reflects changes in their intrinsic properties, experiments were conducted in slices in which long-range afferents are removed. Dopamine neurons fire spontaneously in vitro in a clock-like, single-spike mode, sometimes referred to as a pacemaker pattern (18–20). A typical firing pattern of a dopamine neuron recorded in the cell-attached mode is shown in Fig. 2A. The average firing rates from DD and control mice were the same (DD: 2.3 ± 0.18 spikes per s, n = 10; control: 1.9 ± 0.21 spikes per s, n = 9; P > 0.05). The pacemaker activity is confirmed by a CV of 5 ± 1% (P < 0.05 compared to recordings from DD mice in vivo). Dopamine neurons also characteristically display a time-delayed inward current in response to a hyperpolarizing voltage command (H-current; 10 mV steps from −40 to −80) (18, 30, 32). The instantaneous and slow currents were measured and compared between DD and control mice. As shown in Fig. 2B, no difference in amplitude could be detected between the neurons in slices from DD or control mice. The time constants of H-currents measured at −80 mV were also the same between recordings from both genotypes (DD: τ = 595 ± 74 s, n = 7; control: τ = 658 ± 34 s, n = 7; P > 0.05). Thus, in the absence of afferent input, the basic electrophysiological properties were the same between dopamine neurons of DD and control mice.
Figure 2.
Firing pattern and membrane conductances in vitro are the same between DD and control mice. (A) Cell-attached voltage recording of dopamine neuron firing rate in a slice from a DD mouse. No differences were observed between DD (n=10) and control (n=9) mice. (B) Currents evoked from a dopamine neuron in response to steps from −40 to −80 mV (holding current = −50 mV) in a DD mouse (single traces). Currents were measured early and late (dashed lines in figure) during the voltage steps. The current–voltage relationships of DD (n=7) and control (n=7) mice are indistinguishable.
Response to Amphetamine.
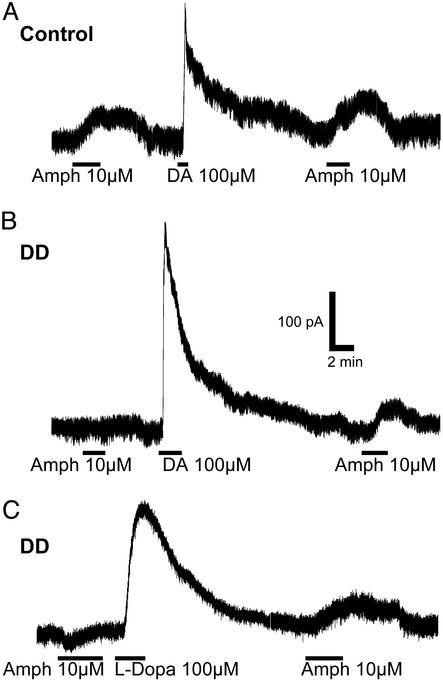
Amphetamine-induced release of dopamine in the midbrain is known to inhibit the activity of dopamine neurons through activation of D2 autoreceptors (33–35). Amphetamine induced an outward current of 47 ± 9 pA (n = 7; P < 0.05) in control mice (Fig. 3A) but had no effect on slices from DD mice (−2 ± 4 pA, n = 5). After superfusion of the slice with dopamine (100 μM, 1–5 min), amphetamine produced an outward current of 64 ± 24 pA, (n = 5) in neurons from DD mice, similar to control mice (25 ± 3 pA after washing the slice with dopamine, n = 4; P > 0.05) (Fig. 3B). The effect of amphetamine was reversed by bath application of the D2 receptor antagonist eticlopride (100 nM; data not shown).
Figure 3.
Dopamine neurons from DD mice do not respond to amphetamine. (A) Current recordings from a control mouse. An outward current was observed in response to amphetamine (10 μM) before and after application of dopamine (100 μM). (B) In a recording from a DD slice, amphetamine (10 μM) had no effect before the slice was superfused with dopamine but induced an outward current after dopamine wash. (C) Similarly, amphetamine had no effect before l-dopa but caused an outward current after the neuron converted l-dopa into dopamine inside the neuron. All traces are single traces.
Similarly, when slices from DD mice were treated with l-dopa (100 μM), a subsequent application of amphetamine caused an outward current. The average response to amphetamine of DD slices before l-dopa treatment was −9 ± 1 pA (n = 5), whereas in control mice the average response was an outward current of 40 ± 13 pA (n = 4; P < 0.05). After l-dopa (1–5 min), amphetamine caused an outward current of 39 ± 9 pA (n = 7) in slices from DD mice, similar to that in control mice (25 ± 6 pA, n = 4; P > 0.05) (Fig. 3C). Therefore, dopamine neurons from DD mice can produce, release, and respond to dopamine.
D2, GABAB, and mGluR Receptor Sensitivity.
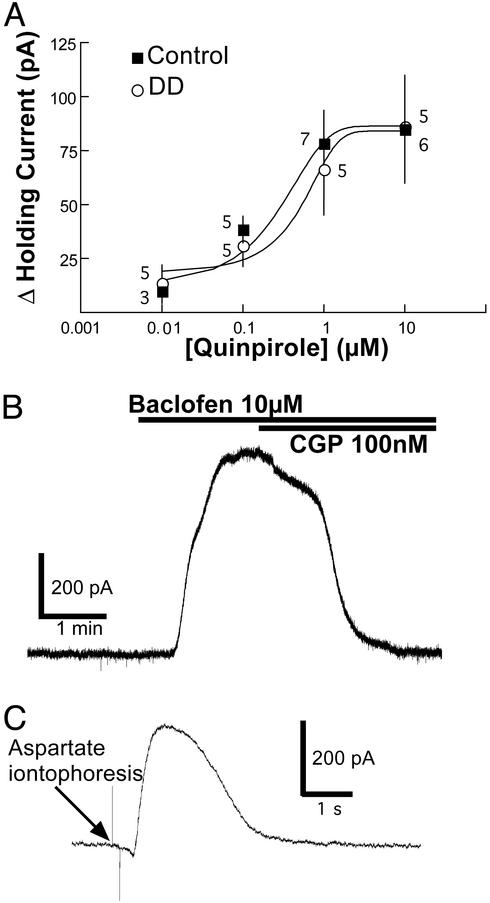
The sensitivity of dopamine neurons to D2 receptor activation was compared in DD and control mice. The dose-response curves to the D2 receptor agonist, quinpirole (0.01 to 10 μM), were identical in DD and control mice (Fig. 4A). The GABAB receptor agonist, baclofen (10 μM), caused an outward current in dopamine neurons from both DD and control mice. The effect of baclofen was reversed with the GABAB receptor antagonist CGP-56999a (100 nM). No differences were observed between the two genotypes (DD: 347 ± 57 pA; control: 297 ± 48 pA; n = 8, P > 0.05; Fig. 4B). Iontophoretic application of aspartate has been shown to activate mGluR on dopamine neurons to induce an outward current (36). There were no differences between the mGluR-mediated outward currents recorded from dopamine neurons of DD and control mice (DD: 102 ± 51 pA; control: 121 ± 24 pA; n = 4, P > 0.05) (Fig. 4C).
Figure 4.
Response of dopamine neurons to pharmacological agents are the same between DD and control mice. (A) Bath application of the D2 receptor agonist, quinpirole (0.01–10 μM), induced an outward current in dopamine neurons from both DD and control mice. There was no difference in the concentration-response curves. Numbers indicate the number of neurons tested per concentration. (B) Bath application of the GABAB receptor agonist, baclofen (10 μM), induced a large outward current in DD and control mice (single trace) that was blocked by the GABAB receptor antagonist, CGP56999a. (C) Iontophoretic application of aspartate (50 nA, 100 ms) induced equivalent mGluR-mediated outward currents in both DD and control mice (average of four traces).
Discussion
An important attribute of the DD mouse mutant is that dopamine neurons are intact and their ability to make dopamine can be controlled by the addition of l-dopa (22, 25). Starting at ≈2 weeks of age, DD mice require daily l-dopa injections for survival. In untreated DD mice, brain dopamine levels are <1.0% of normal levels; the mice are hypoactive and will die of starvation/dehydration (22). Administration of l-dopa (50 mg/kg) restores dopamine levels to ≈10% of wild-type levels, which is accompanied by behavioral activation and feeding that lasts ≈8 h; by 24 h dopamine levels fall to <1.0% again. This model provides a means to study the influence of dopamine on midbrain dopamine neuron characteristics in vitro and in vivo. There are three main findings: (i) the basic electrophysiological characteristics of dopamine neurons do not depend on dopamine tone; (ii) endogenously released dopamine directly influences the membrane physiology of dopamine neurons by activating a D2 receptor-mediated hyperpolarizing current; and (iii) endogenously released dopamine influences the burst firing of midbrain dopamine neurons via a network feedback mechanism.
The pacemaker-like firing pattern of midbrain dopamine neurons recorded in vitro from DD mice was indistinguishable from that of control mice. Voltage steps induced an H-current of the same magnitude and time-course in neurons from control and DD mice. Further, the activity of mGluR and GABAB metabotropic receptors were unaffected in DD mice. Thus, dopamine neurons do not depend on dopamine tone to maintain these intrinsic properties.
Exogenous application of dopamine induced outward currents of equal magnitude from DD and control mice that were blocked by a D2 receptor antagonist. The dose-response experiments indicated no difference in sensitivity to the D2 receptor agonist, quinpirole. This finding was unexpected because DD mice manifest both behavioral and biochemical hypersensitivity to D1 receptor agonists in vivo (37). Although DD mice show a more pronounced locomotor response to quinpirole than do controls (37), this behavioral assay is complicated because quinpirole inhibits dopamine release (and locomotion) in control mice via its actions on autoreceptors (38, 39), but this effect is inconsequential in mice lacking dopamine. Thus only the postsynaptic effects of dopamine are observed in DD mice. Our results reported here provide the first indication that D2 autoreceptors are not hypersensitive in DD mice but leave open the possibility that postsynaptic D2 receptors may be hypersensitive.
Amphetamine was used to verify the dopamine-depleted state of dopamine neurons and to show that dopamine neurons from DD mice were able to transport and store dopamine in vesicles after bath application of dopamine or l-dopa. As expected, amphetamine does not induce a D2 receptor-dependent outward current in slices from DD mice because there is no dopamine to release (22, 25). The lack of effect of amphetamine also indicates that other monoamines in these slice preparations do not affect the holding current. Although slices from DD mice failed to elicit an outward current with amphetamine, this property was restored within a few minutes by either bathing the slices with dopamine, which is taken up by dopamine transporter (37, 40), or with l-dopa, which is taken up by amino acid transporters (41, 42), and metabolized to dopamine inside the neuron. Thus, there was no permanent defect in the ability to take up or release dopamine.
Bursts recorded from dopamine neurons of control mice in vivo presumably result from phasic afferent inputs (for review, see ref. 43). The firing pattern in anesthetized DD mice is an irregular single spike pattern as indicated by the high CV (28 ± 3%) and lack of bursts. This activity is different from the pacemaker pattern encountered during in vitro recordings where the CV is lower (5 ± 1%), which indicates a higher degree of regularity. Thus, afferent regulation of firing still seems to be involved in the in vivo firing pattern of dopamine neurons in DD mice. Importantly, bursting activity begins to recover ≈20 min after treatment with l-dopa, which partially restores dopamine. Therefore, dopamine seems to be capable of coordinating afferents that elicit bursting.
The difference between the results in vivo and in vitro suggests that the bursting activity of dopamine neurons is regulated by network feedback mechanisms. Dopamine neurons can affect many target nuclei that have direct or indirect reciprocal connections with dopamine neurons. For example, dopamine neurons project to GABAergic neurons in the striatum (27, 44), which in turn project back to the dopamine neurons in the midbrain (45–49). Dopamine neurons also project to glutamatergic neurons in prefrontal cortex that can affect the firing pattern of dopamine neurons either directly (50–52) or through other nuclei such as the subthalamic nucleus (53). No attempt was made to determine the exact circuitry that normally supports bursting activity in this study. It might be possible to map where dopamine signaling is required for bursting activity by restoring dopamine signaling in selected brain regions. For example, infection of the caudate putamen with adeno-associated viruses carrying genes that permit local synthesis of l-dopa is sufficient to rescue DD mice (54). Would dopamine signaling in that restricted brain region be sufficient to restore dopamine bursting activity in the midbrain? It will be interesting to repeat these experiments in freely moving, awake animals, where we expect there would be many more opportunities for afferents to affect bursting activity.
Although there was a difference in the firing pattern between DD and normal mice found using in vivo extracellular recordings from midbrain dopamine neurons, no differences were discernible in pharmacology or electrophysiology in the brain slice preparation. Importantly, after treatment of DD mice with l-dopa the firing pattern began to resemble that of controls. These results suggest that dopamine is necessary for the normal afferent control of dopamine neuron firing. The severe behavioral abnormalities of DD mice may reflect both the absence of tonic dopamine signaling and phasic increases in dopamine release associated with bursting activity.
Acknowledgments
We thank Kristin Nagata and Nora Meneses for maintaining the DD mouse colony, and Dr. Greg Mark for help with animal surgery. Carlos Paladini is a National Alliance for Research on Schizophrenia and Depression Young Investigator Fellow. This work was supported in part by National Institutes of Health Grant DA04523.
Abbreviations
- CV
coefficient of variation
- l-dopa
l-3,4-dihydroxyphenylalanine
- DD
dopamine-deficient
- GABAB
γ-aminobutyric acid type B
- mGluR
metabotropic glutamate receptor
References
- 1.Hollerman J R, Schultz W. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- 2.Hyland B I, Reynolds J N, Hay J, Perk C G, Miller R. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 3.Schultz W. J Neurophys. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Schultz W, Dayan P, Montague P R. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C J, Young S J, Groves P M. Brain Res. 1977;136:243–260. doi: 10.1016/0006-8993(77)90801-0. [DOI] [PubMed] [Google Scholar]
- 6.Tepper J M, Martin L P, Anderson D R. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grace A A, Bunney B S. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanghera M K, Trulson M E, German D C. Neuroscience. 1984;12:793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- 9.Trulson M E, Trulson T J. Exp Neurol. 1987;96:68–81. doi: 10.1016/0014-4886(87)90169-5. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 11.Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Marschitz M, You Z B, Goiny M, Meana J J, Silveira R, Godukhin O V, Chen Y, Espinoza S, Pettersson E, Loidi C F, et al. J Neurochem. 1996;66:1726–1735. doi: 10.1046/j.1471-4159.1996.66041726.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonon F G. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds J N, Hyland B I, Wickens J R. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds J N, Wickens J R. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 16.Suaud-Chagny M F, Chergui K, Chouvet G, Gonon F. Neuroscience. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- 17.Williams G V, Millar J. Neuroscience. 1990;39:1–16. doi: 10.1016/0306-4522(90)90217-r. [DOI] [PubMed] [Google Scholar]
- 18.Kita T, Kita H, Kitai S T. Brain Res. 1986;372:21–30. doi: 10.1016/0006-8993(86)91454-x. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y, Kitai S T. Neurosci Res. 1993;18:209–221. doi: 10.1016/0168-0102(93)90056-v. [DOI] [PubMed] [Google Scholar]
- 20.Lacey M G. Prog Brain Res. 1993;99:251–276. doi: 10.1016/s0079-6123(08)61351-5. [DOI] [PubMed] [Google Scholar]
- 21.Tung C S, Grenhoff J, Svensson T H. J Neural Transm Gen Sect. 1991;84:53–64. doi: 10.1007/BF01249109. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Q Y, Palmiter R D. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 23.Rios M, Habecker B, Sasaoko T, Eisenhofer G, Tian H, Landis S, Chikaraishi D, Roffler-Tarlov S. J Neurosci. 1999;19:3519–3526. doi: 10.1523/JNEUROSCI.19-09-03519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas S A, Marck B T, Palmiter R D, Matsumoto A M. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
- 25.Szczypka M S, Rainey M A, Kim D A, Alaynick W A, Marck B T, Matsumoto A M, Palmiter R D. Proc Natl Acad Sci USA. 1999;96:12138–12143. doi: 10.1073/pnas.96.21.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin J B J, Paxinos G, editors. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 27.Deniau J M, Hammond C, Riszk A, Feger J. Exp Brain Res. 1978;32:409–422. doi: 10.1007/BF00238711. [DOI] [PubMed] [Google Scholar]
- 28.Williams J T, North R A, Shefner S A, Nishi S, Egan T M. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- 29.Mercuri N B, Bonci A, Calabresi P, Stefani A, Bernardi G. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S W, North R A. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perkel D H, Gerstein G L, Moore G P. Biophys J. 1967;7:391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grace A A, Onn S P. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fon E A, Pothos E N, Sun B C, Killeen N, Sulzer D, Edwards R H. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 34.Pierce R C, Kalivas P W. J Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones S R, Gainetdinov R R, Wightman R M, Caron M G. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fiorillo C D, Williams J T. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 37.Kim D S, Szczypka M S, Palmiter R D. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Usiello A, Baik J H, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza P V, Borrelli E. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Xu R, Sasoaka T, Tonegawa S, Kung M P, Sankoorikal E B. J Neurosci. 2000;20:8305–8314. doi: 10.1523/JNEUROSCI.20-22-08305.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman A F, Lupica C R, Gerhardt G A. J Pharmacol Exp Ther. 1998;287:487–496. [PubMed] [Google Scholar]
- 41.Sampaio-Maia B, Soares-da-Silva P. Life Sci. 2000;67:3209–3220. doi: 10.1016/s0024-3205(00)00903-6. [DOI] [PubMed] [Google Scholar]
- 42.Sugaya Y, Sasaki Y, Goshima Y, Kitahama K, Kusakabe T, Miyamae T, Kato T, Misu Y. Neuroscience. 2001;104:1–14. doi: 10.1016/s0306-4522(01)00008-2. [DOI] [PubMed] [Google Scholar]
- 43.Kitai S T, Shepard P D, Callaway J C, Scroggs R. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- 44.Guyenet P G, Aghajanian G K. Brain Res. 1978;150:69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- 45.Grofova I. Brain Res. 1975;91:286–291. doi: 10.1016/0006-8993(75)90550-8. [DOI] [PubMed] [Google Scholar]
- 46.Paladini C A, Celada P, Tepper J M. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- 47.Precht W, Yoshida M. Brain Res. 1971;32:229–233. doi: 10.1016/0006-8993(71)90171-5. [DOI] [PubMed] [Google Scholar]
- 48.Ribak C E, Vaughn J E, Roberts E. Brain Res. 1980;192:413–420. doi: 10.1016/0006-8993(80)90893-8. [DOI] [PubMed] [Google Scholar]
- 49.Somogyi P, Bolam J P, Totterdell S, Smith A D. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- 50.Somogyi P, Bolam J P, Smith A D. J Comp Neurol. 1981;195:567–584. doi: 10.1002/cne.901950403. [DOI] [PubMed] [Google Scholar]
- 51.Naito A, Kita H. Brain Res. 1994;637:317–322. doi: 10.1016/0006-8993(94)91252-1. [DOI] [PubMed] [Google Scholar]
- 52.Christie M J, Bridge S, James L B, Beart P M. Brain Res. 1985;333:169–172. doi: 10.1016/0006-8993(85)90140-4. [DOI] [PubMed] [Google Scholar]
- 53.Kita H, Kitai S T. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- 54.Szczypka M S, Kwok K, Brot M D, Marck B T, Matsumoto A M, Donahue B A, Palmiter R D. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]