Abstract
Objective
To assess whether postoperative detection of circulating tumor cells in peripheral blood influenced the prognosis of patients with colorectal cancer after radical surgery.
Summary Background Data
In a previous study, the authors demonstrated that baseline detection of blood circulating tumor cells does not have prognostic significance in patients with colorectal cancer. However, surgical procedures may increase tumor cell detachment and mobilization.
Methods
Sixty-six patients with histologically confirmed colorectal cancer operated on for cure were included in this study. Circulating tumor cells were detected by means of reverse transcriptase-polymerase chain reaction targeting to carcinoembryonic antigen messenger RNA in peripheral blood samples obtained 24 hours after surgery. Endpoints of the study were tumor recurrence, overall survival, and cancer-related survival. Univariate (Kaplan-Meier method) and multivariate (Cox regression model) analyses were performed.
Results
After a median follow-up of 36 months, 15 patients (23%) had tumor relapse and 14 had died (21%), 8 of them from a cancer-related cause. Cox regression analysis identified lymph node metastases and gender as independent predictors of tumor recurrence and cancer-related survival, whereas overall survival was dependent on the degree of differentiation of the primary tumor. More importantly, the presence of circulating tumor cells after surgery had no prognostic influence on tumor recurrence, overall survival, or cancer-related survival.
Conclusions
Postoperative detection of blood circulating tumor cells had no prognostic significance in patients with colorectal cancer operated on for cure.
Despite advances in surgical and adjuvant treatments, up to 30% to 40% of patients with resectable colorectal cancer (CRC) develop tumor relapse during follow-up. 1,2 Current staging methods based on pathologic characteristics, mainly in-depth tumor penetration and lymph node status, allow stratification of patients operated on for cure according to their risk of recurrence. 3 However, these well-established parameters are not accurate enough to predict the probability of tumor relapsing on a case-by-case basis.
Highly sensitive molecular procedures, such as the reverse transcription-polymerase chain reaction (RT-PCR) technique, permit us to detect tumor cells in different tissues and biologic fluids. A large body of evidence confirms the feasibility, reliability, and reproducibility of detecting circulating tumor cells in patients with different neoplasms, including CRC. 4–11 However, the role of neoplastic cell detection in peripheral blood in predicting tumor behavior remains controversial in patients with CRC. Although initial studies suggested a putative effect on disease recurrence and survival, 12–15 a recent analysis from our group including a large series of patients indicated that preoperative detection of circulating tumor cells does not seem to influence prognosis. 16
However, clinical studies showed that some surgical procedures, such as the “no-touch” technique, decrease the probability of CRC relapse, suggesting that manipulation of the tumor may be relevant to prognosis. In that sense, recent studies using RT-PCR techniques have demonstrated an increase in tumor cell detachment and mobilization during surgery. 17–21 Considering that circulating tumor cells after treatment denote the persistence of microscopic disease, it was tempting to hypothesize that postoperative detection of tumor cells in peripheral blood might constitute a biologic marker of tumor dissemination and, eventually, a predictor of prognosis. 22
In the current study, we evaluated the prognostic value of detecting tumor cells in the peripheral blood 24 hours after surgery in a cohort of patients with CRC operated on for cure using the same methodologic approach as in our previous work. 16
PATIENTS AND METHODS
Patients
Between January 1996 and November 1999, 66 patients with CRC operated on for cure were included in this study. Some of them were part of two previously reported series, 10,21 whose goals were limited to the evaluation of circulating tumor cells. Baseline characteristics of the patients are depicted in Table 1.
Table 1. BASELINE CHARACTERISTICS OF PATIENTS
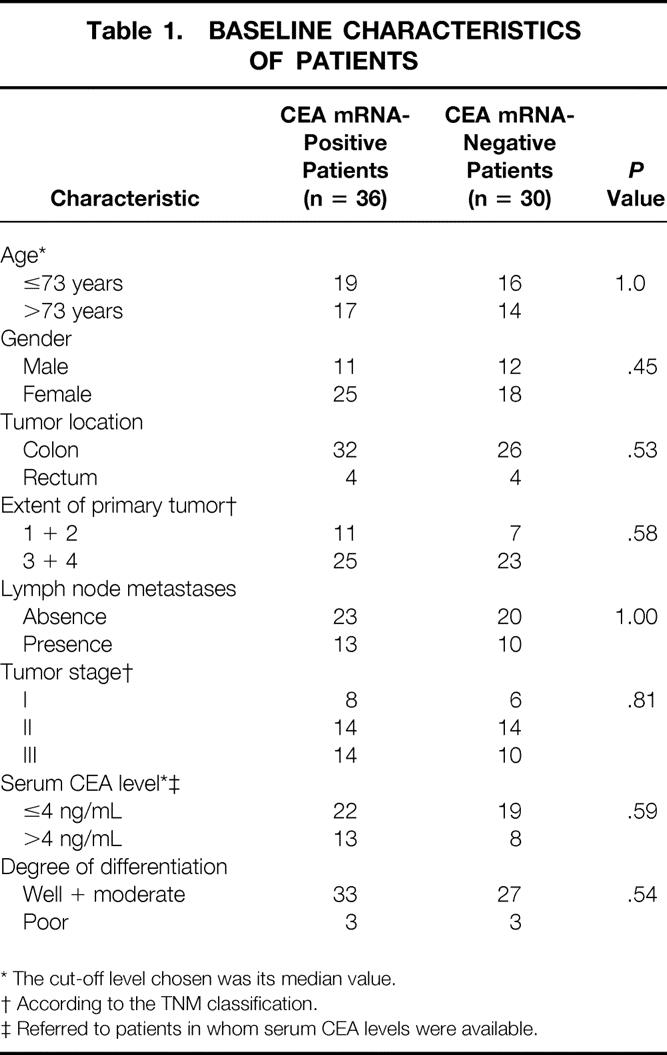
* The cut-off level chosen was its median value.
† According to the TNM classification.
‡ Referred to patients in whom serum CEA levels were available.
Peripheral blood samples were obtained before and 24 hours after surgery to determine the presence of circulating tumor cells by means of a RT-PCR technique targeting carcinoembryonic antigen (CEA) mRNA. In our previous investigation, the sensitivity limit of this technique was established at approximately one tumor cell per 107 white blood cells (5 cells per 10 mL blood). 10
All interventions were done by a single team with extensive experience in colorectal surgery, using “no-touch” techniques. After surgical resection, patients underwent standard therapeutic and follow-up measures according to recommended guidelines. 23 Postoperative adjuvant chemotherapy was given to all patients with stage II and III tumors, and radiation therapy (45 Gy) was indicated in patients with rectal cancer. Chemotherapy consisted of 5-fluorouracil by rapid intravenous injection (425 mg/m2) and leucovorin (20 mg/m2) daily for 5 days, every 28 days, for six cycles. No patient received preoperative chemoradiation treatment. Information regarding the presence of circulating tumor cells was not used to indicate adjuvant therapy in any patient. Postoperative surveillance consisted of medical history, physical examination, and laboratory studies including serum CEA levels every 3 months. Abdominal ultrasonography or computed tomography was performed every 6 months, and chest radiography and total colonoscopy were performed once a year. All recurrences were histologically confirmed. The type of recurrence was designated as locoregional (tumor growth restricted to the anastomosis or the region of primary operation) or distant (distant metastases or diffuse peritoneal seeding).
To assess the prognostic significance of circulating tumor cell detection, the long-term follow-up of the whole series was analyzed.
The protocol was approved by the institutional Ethics of Research Committee, and informed consent was obtained from each patient.
Detection of Tumor Cells in Peripheral Blood
The methodology for circulating tumor cell detection has been described in full elsewhere. 10 Briefly, peripheral venous blood samples (20 mL) were obtained with standard venipuncture techniques using heparinized tubes. Mononuclear cells from blood were isolated using a Ficoll gradient and stored at −80°C. Total RNA extraction was performed according to the method of Chirgwin. RT-PCR was done using CEA-specific oligonucleotide primers, which were selected to span an intron, to amplify products of different size from the CEA mRNA (388 bp) and any contaminating genomic DNA (1,020 bp). Samples were electrophoresed on 2% agarose gels and visualized by ethidium bromide staining. The integrity of RNA was confirmed by determining the presence of glyceraldehyde phosphate dehydrogenase mRNA in the same samples.
The investigator performing RT-PCR analysis was unaware of the clinical characteristics of patients. Positive and negative control samples were included in each experiment.
Statistical Analysis
The main endpoint of the study was cancer-related survival. For this purpose, a study population of 60 patients was calculated to be representative of an original population of 150 patients fitting the inclusion criteria with a confidence level of 0.8 (ε value = 0.05). Secondary endpoints were tumor recurrence and overall survival. All probabilities were measured from the date of surgical resection of the primary tumor. Data on patients who were alive or had no evidence of disease at the end of study were censored. The main endpoint of the study was assessment of tumor resectability.
The univariate analysis was done by constructing probability curves according to the Kaplan-Meier method and comparing them by the log-rank test. Variables included in this analysis were age, gender, tumor location, extent of primary tumor, lymph node metastases, degree of differentiation, and circulating tumor cell detection. For continuous variables, the cut-off level chosen was their median value. Variables actually reflecting a combination of independent parameters (i.e., tumor TNM stage) were not included as a single covariable but rather decomposed in the corresponding original counterparts. However, to confirm the absence of any potential confounding effect of tumor stage, survival analysis was repeated after stratifying patients according to the TNM classification. 3 Variables achieving a probability value of less than 0.1 in the univariate analysis were subsequently introduced in a multivariate stepwise proportional-hazard analysis (Cox model) to identify those variables independently associated with survival. 24
Categorical variables were compared by means of the chi-square test, applying the Yates correction when necessary. Length of follow-up was described as median and range.
Survival analysis was done in February 2002. All calculations were performed using the SPSS software package (SPSS Inc., Chicago, IL).
RESULTS
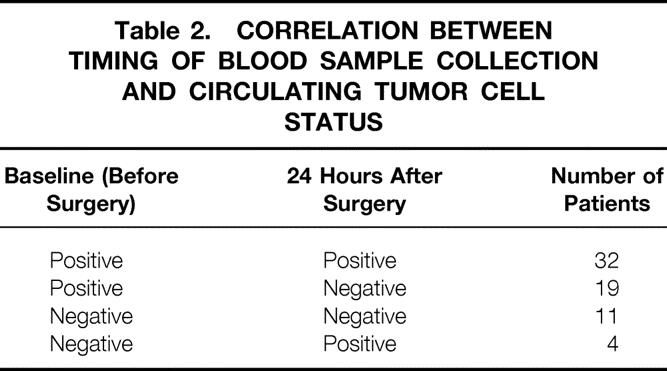
CEA mRNA expression was detected in peripheral blood samples from 36 of 66 (55%) patients with CRC 24 hours after radical surgery. This figure resulted from the positive conversion of 4 of 15 (27%) baseline CEA mRNA-negative patients, as well as negative conversion of 19 of 51 (37%) baseline CEA mRNA-positive patients (Table 2). Patients with positive and negative CEA mRNA expression after surgery were similar with regard to baseline characteristics (see Table 1).
Table 2. CORRELATION BETWEEN TIMING OF BLOOD SAMPLE COLLECTION AND CIRCULATING TUMOR CELL STATUS
Circulating Tumor Cells and Tumor Recurrence
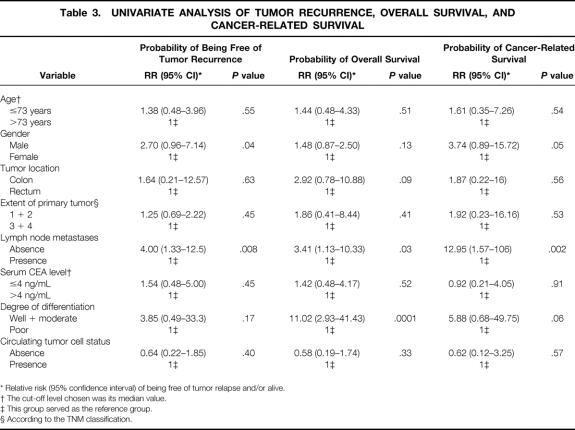
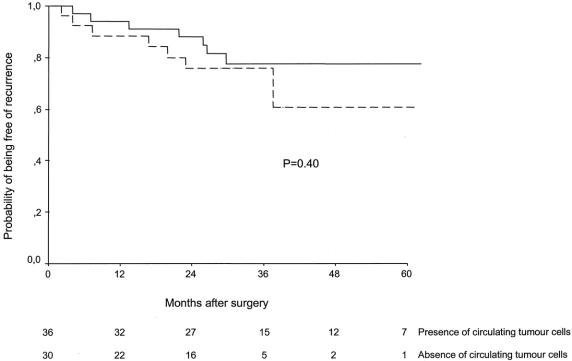
The median follow-up of the whole series was 36 months (range 14–73). No patient was lost to follow-up. Fifteen of 66 patients (23%) operated on for cure had tumor recurrence at the end of the study (distant metastases in 10, locoregional relapse in 5). The univariate analysis disclosed that the probability of tumor recurrence was significantly correlated with gender (log-rank = 3.87, P = .04) and the presence of lymph node metastases (log-rank = 7.04, P = .008). However, postoperative tumor cell detection in peripheral blood was not associated with a higher probability of tumor relapse (Table 3). Indeed, recurrence of the disease was observed in 8 of the 36 (22%) CEA mRNA-positive patients and in 7 of the 30 (23%) CEA mRNA-negative patients (log-rank = 0.70, P = .40) (Fig. 1). No patient experiencing positive conversion after surgery developed tumor relapse during follow-up. After adjusting for tumor TNM stage, the probability of tumor recurrence remained identical in both groups (log-rank = 1.54, P = .21) (Fig. 2). These results were also reproduced when either locoregional relapse (relative risk 0.23, 95% confidence interval 0.02–2.67; log-rank = 1.59, P = .20) or distant metastases (relative risk 0.58, 95% confidence interval 0.15–2.22; log-rank = 0.65, P = .41) were considered.
Table 3. UNIVARIATE ANALYSIS OF TUMOR RECURRENCE, OVERALL SURVIVAL, AND CANCER-RELATED SURVIVAL
* Relative risk (95% confidence interval) of being free of tumor relapse and/or alive.
† The cut-off level chosen was its median value.
‡ This group served as the reference group.
§ According to the TNM classification.
Figure 1. Probability of being free of tumor recurrence in patients with colorectal cancer operated on for cure according to the presence (continuous line) or absence (dotted line) of blood circulating tumor cells after surgery.
Figure 2. Probability of being free of tumor recurrence in patients with colorectal cancer operated on for cure after stratification by tumor TNM stage and according to the presence (continuous line) or absence (dotted line) of blood circulating tumor cells after surgery.
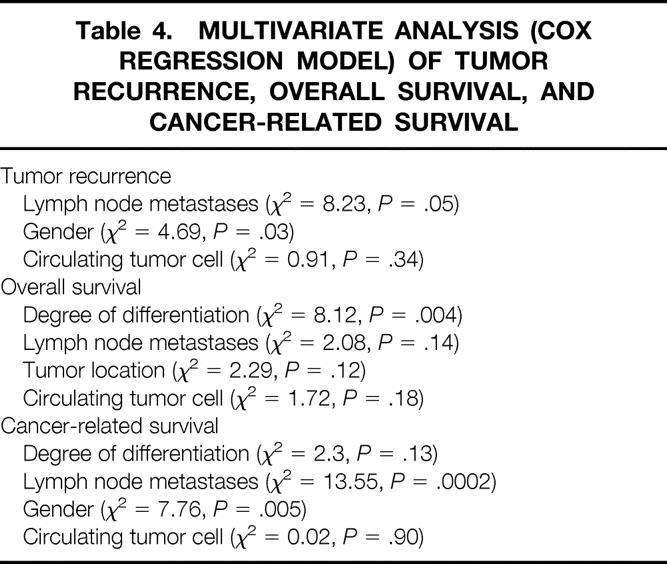
The Cox regression model, including those variables selected in the univariate analysis, as well as circulating tumor cell detection, identified the presence of lymph node metastases and gender as the variables independently associated with tumor recurrence (Table 4).
Table 4. MULTIVARIATE ANALYSIS (COX REGRESSION MODEL) OF TUMOR RECURRENCE, OVERALL SURVIVAL, AND CANCER-RELATED SURVIVAL
Circulating Tumor Cells and Overall Survival
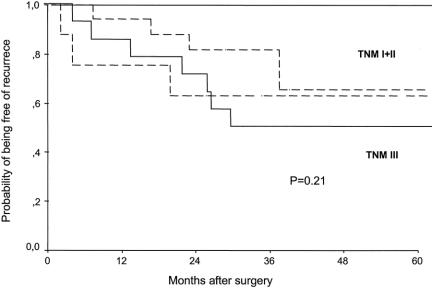
At the end of follow-up, 52 of 66 patients (79%) operated on for cure were alive. The univariate analysis disclosed that the probability of overall survival was significantly correlated with the degree of differentiation (log-rank = 19.88, P = .0001) and lymph node metastases (log-rank = 5.33, P = .03), whereas a trend was observed for tumor location (log-rank = 2.84, P = .09). No difference was observed when patients were classified according to the circulating tumor cell status after surgery (see Table 3). In fact, 29 (81%) CEA mRNA-positive patients and 23 (77%) CEA mRNA-negative patients were alive at the end of the study (log-rank = 0.39, P = .33) (Fig. 3). After adjusting for tumor TNM stage, the probability of overall survival remained identical in both groups of patients (log-rank = 2.27, P = .13).
Figure 3. Probability of overall survival in patients with colorectal cancer operated on for cure according to the presence (continuous line) or absence (dotted line) of blood circulating tumor cells after surgery.
Degree of differentiation was the only variable independently associated with overall survival in the Cox regression analysis, there being no confounding interactions with the presence of blood circulating tumor cells after surgery (see Table 4).
Circulating Tumor Cells and Cancer-Related Survival
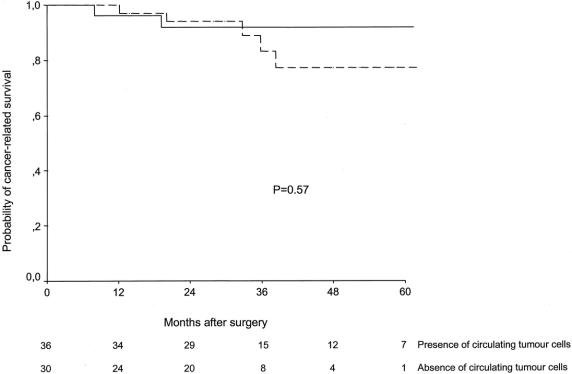
At the end of follow-up, 8 of 66 patients (12%) died of tumor progression. In the univariate analysis, cancer-related survival was dependent on lymph node metastases (log- rank = 9.25, P = .002) and gender (log-rank = 3.74, P = .05), whereas a trend was observed for degree of differentiation (log-rank = 3.35, P = .06) (see Table 3). Again, this parameter was unrelated to the presence of blood circulating tumor cells after surgery (log-rank = 0.31, P = .57) (Fig. 4). After adjusting for tumor TNM stage, the probability of cancer-related survival remained identical in both groups of patients (log-rank = 0.1, P = .92).
Figure 4. Probability of cancer-related survival in patients with colorectal cancer operated on for cure according to the presence (continuous line) or absence (dotted line) of blood circulating tumor cells after surgery.
Presence of lymph node metastases and gender were independent predictive factors for cancer-related survival (see Table 4).
DISCUSSION
The results of the current study indicate that postoperative detection of tumor cells in peripheral blood by means of RT-PCR targeting CEA mRNA has no prognostic significance in patients with CRC operated on for cure. The probability of tumor recurrence, overall survival, and cancer-related survival were not influenced by the presence of circulating tumor cells after surgery. This conclusion arises from the analysis of the long-term follow-up of a large series of patients with nonmetastatic CRC in which the prognostic value of detecting neoplastic cells in blood was assessed by using a multivariate methodology and after adjustment for tumor stage, thus making unlikely a potential bias due to confounding interactions among variables.
In recent years, great efforts have been made to identify patients at high risk of tumor relapse after surgery. Identification of this subset of patients is especially meaningful in CRC because of the existence of effective treatments. So far, prediction of tumor recurrence mainly relies on the histopathologic characteristics of the resected specimen, 3 indicating adjuvant chemotherapy or radiation therapy in patients with stage II and III tumors. However, not all patients with such lesions will develop tumor recurrence in the absence of these treatments, which are not exempt from conspicuous side effects. Accordingly, identification of more accurate predictors of tumor relapse on a case-by-case basis seems mandatory. Development of molecular techniques that can detect tumor cells in different fluids and tissues appeared to be a promising approach to accomplish this goal. In fact, the detection of minimal residual disease in lymph nodes and bone marrow has been demonstrated to predict the prognosis of patients with CRC. 25–28 Conversely, although the presence of blood circulating tumor cells has been associated with a poorer prognosis in other neoplasms, 29 no influence on survival was observed in the largest series of patients with CRC published so far, when detection was performed preoperatively. 16
To our knowledge, this is the first study evaluating the prognostic influence of blood circulating tumor cells in samples obtained after surgery. Several experimental 30 and clinical 17–21 studies have demonstrated that surgical procedures increase tumor cell detachment and mobilization in CRC. In addition, a recent study has observed a clearance of circulating tumor cells after excision of primary CRC, and the rate of positive samples did not change significantly during follow-up. 22 These observations provided the rationale to assess the role of postoperative detection of tumor cells in peripheral blood as a marker of tumor dissemination and, ultimately, as a predictor of prognosis. Unfortunately, the results of the current study argue against this interesting approach. Indeed, in a similar manner to that reported for preoperative detection, 16 the presence of blood circulating tumor cells detected by RT-PCR targeting CEA mRNA 24 hours after surgery did not correlate with tumor recurrence or survival in patients with CRC operated on for cure.
The apparently unequivocal results of this study, however, deserve some comment before we can draw definite conclusions against the utility of detecting blood circulating tumor cells in patients with CRC. First, different molecular targets (CEA, guanylyl cyclase C, cytokeratin 18, 19, and 20, among others) have been used for this purpose, reflecting the absence of selective and specific markers of colorectal tumor cells. The use of a multiple mRNA marker assay could overcome this important limitation, as has been shown for the detection of CRC cells in stool samples. 31,32 Second, identification of circulating tumor cells represents a stochastic event because cell shedding is an intermittent process and they circulate in non-homogeneously distributed clumps. 33 Blood sampling at different time points may help in improving this approach. However, in contrast to the previously mentioned study, 22 another longitudinal analysis 34 has observed that blood sampling within 40 days of surgery can lead to spurious and inconclusive results. In this latter study, only two of the eight positive patients 24 hours after surgery remained positive during follow-up, whereas two negative patients after surgery became positive during follow-up. Finally, circulating CEA mRNA-positive cells might not correspond to the cancer cell subpopulation involved in metastatic spread. In that sense, it is important to keep in mind that dissemination is a complex, multi-step process in which tumor cell detachment and mobilization are the first, necessary, but not sufficient stages of a highly inefficient process. Identification and further characterization of these mechanisms may provide new markers for the detection of circulating tumor cells in patients with CRC; consequently, the effectiveness of this approach for predicting prognosis will need to be revisited.
Acknowledgments
The authors thank Loreto Boix and Laura Gargallo for their excellent technical support.
Footnotes
Supported in part by grants from the Fondo de Investigaciones Sanitarias (FIS 01/0104-02), the Ministerio de Ciencia y Tecnología (SAF00-0038), and the Agència d’Avaluació de Tecnologia i Recerca Mèdiques of the Generalitat de Catalunya (026/16/2000). X.B. and V.P. are research fellows from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS).
Correspondence: Antoni Castells, MD, Department of Gastroenterology
Hospital Clínic, Villarroel 170, 08036 Barcelona, Catalonia, Spain.
E-mail: acastell@medicina.ub.es
Accepted for publication August 5, 2002.
References
- 1.Castells A, Kroser J, Rustgi AK. Gastrointestinal neoplasms. In: Beers MH, Berkow R, eds. The Merck Manual Of Geriatrics, 3d ed. West Point: Merck & Co.; 2000: 1134–1153.
- 2.Safi F, Beyer HG. The value of follow-up of curative surgery of colorectal carcinoma. Cancer Detec Prev. 1993; 17: 417–424. [PubMed] [Google Scholar]
- 3.Beahrs OH, Henson DE, Hutter RVP, et al. Manual for staging of cancer, 4th ed. Philadelphia: JB Lippincott, 1993.
- 4.Burchill SA, Bradbury MF, Pittman K, et al. Detection of epithelial cancer cells in peripheral blood by reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995; 71: 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PWM, Burchill SA, Selby PJ. The molecular detection of circulating tumour cells. Br J Cancer. 1995; 72: 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong LS, Bateman WJ, Morris AG, et al. Detection of circulating tumour cells with the magnetic activated cell sorter. Br J Surg. 1995; 82: 1333–1337. [DOI] [PubMed] [Google Scholar]
- 7.Gunn J, McCall JL, Yun K, et al. Detection of micrometastases in colorectal cancer patients with K19 and K20 reverse transcription polymerase chain reaction. Lab Invest. 1996; 75: 611–616. [PubMed] [Google Scholar]
- 8.Jonas S, Windeatt S, O-Boateng A, et al. Identification of carcinoembryonic antigen-producing cells circulating in the blood of patients with colorectal carcinoma by reverse transcriptase polymerase chain reaction. Gut. 1996; 39: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori M, Mimori K, Ueo H, et al. Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer. 1996; 68: 739–743. [DOI] [PubMed] [Google Scholar]
- 10.Castells A, Boix L, Bessa X, et al. Detection of colonic cells in peripheral blood of colorectal cancer patients by means of reverse transcriptase and polymerase chain reaction. Br J Cancer. 1998; 78: 1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustin SA, Gyselman VG, Williams NS, et al. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer. 1999; 79: 1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardingham JE, Kotasek D, Sage RE, et al. Detection of circulating tumor cells in colorectal cancer by immunobead-PCR is a sensitive prognostic marker for relapse of disease. Mol Med. 1995; 1: 789–794. [PMC free article] [PubMed] [Google Scholar]
- 13.Funaki NO, Tanaka J, Ohshio G, et al. Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer. 1998; 77: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyld DK, Selby P, Perren TJ, et al. Detection of colorectal cancer cells in peripheral blood by RT-PCR for cytokeratin 20. Cancer. 1998; 79: 288–293. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi T, Makino M, Suzuki K, et al. Prognostic significance of reverse transcriptase-polymerase chain reaction measurement of carcinoembryonic antigen mRNA levels in tumor drainage blood and peripheral blood of patients with colorectal carcinoma. Cancer. 2000; 89: 970–976. [PubMed] [Google Scholar]
- 16.Bessa X, Elizalde JI, Boix L, et al. Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology. 2001; 120: 1084–1092. [DOI] [PubMed] [Google Scholar]
- 17.Weitz J, Jienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998; 4: 343–348. [PubMed] [Google Scholar]
- 18.Hayashi N, Egami H, Kai M, et al. No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery. 1999; 125: 369–374. [PubMed] [Google Scholar]
- 19.Sales JP, Wind P, Douard R, et al. Blood dissemination of colonic epithelial cells during non-touch surgery for rectosigmoid cancer. Lancet. 1999; 354: 392. [DOI] [PubMed] [Google Scholar]
- 20.Weitz J, Koch M, Kienle P, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000; 232: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessa X, Castells A, Lacy AM, et al. Laparoscopic-assisted versus open colectomy for colorectal cancer: influence on neoplastic cell mobilization. J Gastrointest Surg. 2001; 5: 66–73. [DOI] [PubMed] [Google Scholar]
- 22.Patel H, Le Marer N, Wharton RQ, et al. Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg. 2002; 235: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman JM, Cheung RJ, Weinberg DS. Surveillance after colorectal cancer resection. Lancet. 2000; 355: 395–399. [DOI] [PubMed] [Google Scholar]
- 24.Castells A, Puig P, Mora J, et al. K-ras mutations in DNA extracted from plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J Clin Oncol. 1999; 17: 578–584. [DOI] [PubMed] [Google Scholar]
- 25.Lindemann F, Schlimok G, Dirschedl P, et al. Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet. 1992; 340: 685–689. [DOI] [PubMed] [Google Scholar]
- 26.Gerhard M, Juhl H, Kalthoff H, et al. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol. 1994; 12: 725–729. [DOI] [PubMed] [Google Scholar]
- 27.O’Sullivan GC, Collins JK, O’Brien F, et al. Micrometastases in bone marrow of patients undergoing curative surgery for gastrointestinal cancer. Gastroenterology. 1995; 109: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 28.Liefers GJ, Cleton-Jansen AM, Van de Velde CJH, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998; 339: 223–228. [DOI] [PubMed] [Google Scholar]
- 29.Mellado B, Colomer D, Castel T, et al. Detection of circulating neoplastic cells by reverse-transcriptase polymerase chain reaction in malignant melanoma: Association with clinical stage and prognosis. J Clin Oncol. 1996; 14: 2091–2097. [DOI] [PubMed] [Google Scholar]
- 30.Nishizaki T, Matsumata T, Kanematsu T, et al. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990; 49: 92–97. [DOI] [PubMed] [Google Scholar]
- 31.Ahlquist DA, Skoletsky JE, Boynton KA, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000; 119: 1219–1227. [DOI] [PubMed] [Google Scholar]
- 32.Dong SM, Traverso G, Johnson C, et al. Detecting colorectal cancer in stool with the use of multiple genetic targets. J Natl Cancer Inst. 2001; 93: 858–865. [DOI] [PubMed] [Google Scholar]
- 33.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991; 51: S5054–S5059. [PubMed] [Google Scholar]
- 34.Guadagni F, Kantor J, Aloe S, et al. Detection of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum markers. Cancer Res. 2001; 61: 2523–2532. [PubMed] [Google Scholar]