Abstract
Objective
To investigate the initiation of a complex inflammatory response within the human intestinal muscularis intraoperatively so as to determine the clinical applicability of the inflammatory hypothesis of postoperative ileus.
Summary Background Data
Mild intestinal manipulation in rodents initiates the activation of transcription factors, upregulates proinflammatory cytokines, and increases the release of kinetically active mediators (nitric oxide and prostaglandins), all of which results in the recruitment of leukocytes and a suppression in motility (i.e., postoperative ileus).
Methods
Human small bowel specimens were harvested during abdominal procedures at various times after laparotomy. Histochemical and immunohistochemical techniques were applied to intestinal muscularis whole-mounts. Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed for interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Signal transducers and activators of transcription (STAT) protein phosphorylation was determined by electromobility shift assay. Organ bath experiments were performed on jejunal circular smooth muscle strips. GW274150C and DFU were used in vitro as iNOS and COX-2 inhibitors.
Results
Normal human muscularis externa contained numerous macrophages that expressed increased lymphocyte function associated antigen-1 (LFA-1) immunoreactivity as a function of intraoperative time. RT-PCR demonstrated a time-dependent induction of IL-6, IL-1β, TNF-α, iNOS, and COX-2 mRNAs within muscularis extracts after incision. Mediators were localized to macrophages with STAT protein activation in protein extracts demonstrating local IL-6 functional activity. DFU alone or in combination with GW274150C increased circular muscle contractility. Specimens harvested after reoperation developed leukocytic infiltrates and displayed diminished in vitro muscle contractility.
Conclusions
These human data demonstrate that surgical trauma is followed by resident muscularis macrophage activation and the upregulation, release, and functional activity of proinflammatory cytokines and kinetically active mediators.
The abdomen is a commonly accessed site of the body in surgery. Inpatient operations on the digestive system alone rank second after the cardiovascular system in the United States. 1 If combined with ambulatory surgeries, more than 10 million operations on the digestive system are performed in the United States per year, not including the multiple abdominal procedures performed in urology, gynecology, and other surgical specialties. 1,2 Open abdominal surgical procedures are so commonly complicated by postoperative ileus that ileus is generally thought to be an accepted iatrogenic consequence and a “physiologic” reaction of the bowel to the operative trauma. 3 The morbidity of postoperative ileus is widely acknowledged, 3–5 and its economic burden has been estimated at $1 billion per year in the United States. 6
A number of pathogenic mechanisms have been proposed for postoperative ileus. 3,7 Recent findings in a rodent model of postoperative ileus support the role of a local inflammatory reaction within the gut wall as a major causative factor. 5,8–10 These studies revealed that the normally resident dense network of macrophages within the muscularis is rapidly activated during abdominal surgery, and that this initiates an inflammatory cascade of events including the activation of transcription factors and the upregulation of cytokines, chemokines, and kinetically active substances. This local inflammatory milieu, in conjunction with an increase in the expression of adhesion molecules within the blood vessels that course through the muscularis externa, results in the recruitment and extravasation of leukocytes into the circular muscle layer. The leukocytic infiltration within the muscularis and the local release and secretion of various potent leukocytic products succeed in inhibiting muscularis function for sustained periods. 5,8–10
Small animal models can be helpful tools in the investigation of basic molecular mechanisms but always leave room for uncertainty and skepticism concerning the clinical applicability of the results. The goal of this work was to extend our previous basic findings from animal models to human surgical small bowel specimens obtained during surgery. Therefore, we sought to investigate the intraoperative: 1) activation state of resident muscularis macrophages, 2) molecular inflammatory cytokine upregulation and cellular localization, and 3) induction and production of kinetically active substances and their functional role in muscle function. These data demonstrate for the first time that surgical trauma of the human gut is followed by the activation of resident macrophages within the intestinal muscularis and the upregulation, local release, and functional activity of proinflammatory cytokines and kinetically active mediators, which participate in causing postoperative ileus.
METHODS
Human Small Bowel Specimens
Human small bowel surgical specimens were used according to an exempt protocol approved by the University of Pittsburgh Health Sciences Institutional Review Board (IRB#9509134: Human Smooth Muscle in Health and Disease). The exempt status of the IRB protocol precluded the specific identification of patient-related information beyond diagnosis. Specimens were obtained intraoperatively from patients who underwent elective bowel resections due to various underlying diseases. The specimens were taken from the resected ends of the macroscopically “normal” small bowel. The size of the specimens varied from 1 to 5 cm in length. Patients with underlying primary small bowel disease (i.e., inflammatory bowel disease) or sepsis or who had received preoperative anti-inflammatory medications (e.g., steroid or immunosuppressive drugs) were excluded from the study, and specimens were never used from diseased organs (Table 1).
Table 1. PROCEDURES AND DIAGNOSES
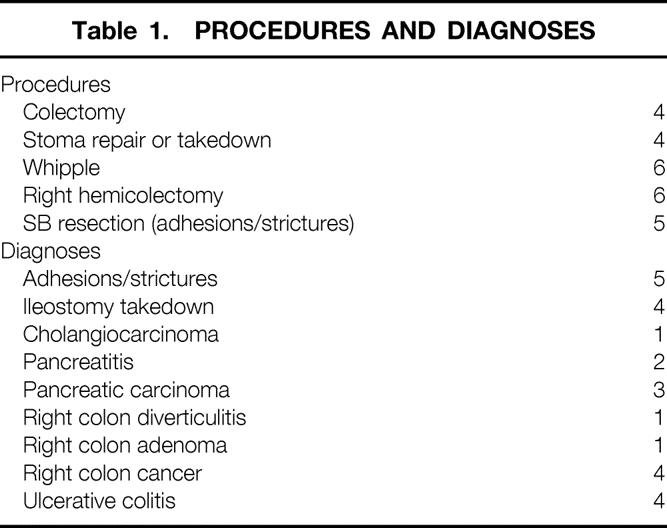
Due to the lack of definite human “control” specimens, we used small bowel specimens at two different time points into surgery. “Early” specimens were resected between 30 and 60 minutes after skin incision, and “late” specimens were resected greater than 3 hours after incision. Whenever possible, specimens were collected from the same patient at two different time points into surgery. For statistical analysis the following data could be documented using the exempt IRB protocol: underlying disease, operative procedure, time of skin incision and removal of the specimen, location and extent of resection, surgeon, and pathologic diagnosis (see Table 1).
Small Bowel Harvesting
The small bowel specimens were collected on site directly following the surgical resection. The bowel segments were transferred under sterile conditions into DMEM-F12 cell culture medium at 4°C and transported into the laboratory, where further dissection was carried out. The small intestine was transferred to a sterile beaker containing Hanks balanced salt solution (HBSS) (Sigma, St. Louis, MO) with 200 U/mL penicillin G and 200 μg/mL streptomycin. A segment of 1 to 2 cm in length was cut from the specimen, opened along the mesentery after removal of all adhesive tissue, and pinned down in a Sylgard-filled glass dish. The muscularis was separated from the mucosa and submucosa by microscopic dissection. The isolated gut wall layers were then either directly snap-frozen in liquid nitrogen and stored at −80°C for protein and RNA extraction or used for various experiments.
Histochemistry and Immunohistochemistry
Muscularis whole-mounts were prepared and fixed for histochemical and immunohistochemical stainings as previously described. 8–10 Neutrophils were visualized by a myeloperoxidase stain: ethanol-fixed whole-mounts were immersed in a mixture of 10 mg Hanker-Yates reagent (Sigma), 10 mL Krebs-Ringer’s buffer (KRB), and 100 μL 3% hydrogen peroxide (Sigma) for 20 minutes. Muscularis whole-mounts were also used for immunohistochemical analysis of the jejunal muscularis. Table 2 shows the primary and secondary antibodies used. Leukocytes were counted in five randomly chosen areas in each specimen at a magnification of 200×. Unspecific isotype-matched antibodies and primary antibody incubation without secondary antibody were used as negative controls.
Table 2. PRIMARY AND SECONDARY ANTIBODIES USED
Antibodies were used for whole-mount immunohistochemistry at the described concentrations, which may vary extensively in frozen or paraffin-embedded cross-sections. Specificity is given in accordance to the individual manufacturer’s information.
RNA Extraction and Semiquantitative Reverse Transcriptase–Polymerase Chain Reaction
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on total RNA extracts and quantified from frozen separated gut layers as previously described. 11 The sequences of the PCR primers are shown in Table 3. The specificity of the PCR primers was confirmed using restriction site analysis as described previously. 12 Extracted RNA from lipopolysaccharide (LPS)-stimulated human primary muscularis tissue cultures served as positive controls; water was added instead of DNA template as a negative control. To ensure equal reaction conditions, all samples received pooled reagents and comparisons were made only with simultaneously performed experiments.
Table 3. SEQUENCES OF PCR PRIMERS
Real-Time PCR
Cytokine expression of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) was quantified by a SYBR Green two-step real-time RT-PCR technique as previously described. 13 Sequences of the real-time-PCR primers are shown in Table 3. The specificity and equality of both primer efficiencies were confirmed in validation experiments. GAPDH was used as an endogenous control. Each sample was estimated in triplicate on an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). The real-time PCR data were plotted as the ΔRn fluorescence signal versus the cycle number. An arbitrary threshold was set to the midlinear portion of the log ΔRn cycle plot. The threshold cycle (CT) was defined as the cycle number at which the ΔRn crossed this threshold. Relative quantification was performed using the comparative CT method as described previously 14 (see also User Bulletin #2, PE Applied Biosystems, Foster City, CA).
Protein Extraction and Electromobility Shift Assay
Electromobility shift assay (EMSA) was performed using whole-tissue extracts of mucosa or muscularis cryostat sections, as described previously. 15 The activation of Stat3 was assessed using the hSIE (high-affinity serum-inducible element) duplex oligonucleotide that preferentially binds Stat3 and Stat1. 16 EMSA was performed on a 4% polyacrylamide gel and quantified as described. 17 Where indicated, EMSA binding reactions were incubated with antibodies specific for Stat3α (Santa Cruz Biotechnology, Santa Cruz, CA) or Stat3β (Charles River Pharmaservices, Southbridge, MA).
In Vitro Muscle Contractility
Mechanical activity was measured as previously described. 8 Briefly, a segment of the human small bowel was dissected into jejunal circular muscle strips (1 × 10 mm), which were positioned in a superfused organ chamber for isometric force transducer recordings (WPI, Sarasota, FL). Each strip was allowed to equilibrate for 1 hour and then was incrementally stretched to L0 (length at which maximal contraction occurs). Spontaneous circular muscle contractility and bethanechol dose-response curves were recorded. Integrated muscle contractions for 10-minute periods (Biopac A/D analysis system, Santa Barbara, CA) were calculated as g/mm2/s by converting weight and length of the strip to square millimeters of tissue. 18
Statistics
Data were compiled as mean ± standard error of the mean. Statistical analysis was performed using the unpaired Student t test and ANOVA. Statistical significance was set at P < .05.
Drugs and Solutions
A standard KRB was used (mmol/L): Na+, 137.4; K+, 5.9; Ca2+, 2.5; Mg2+, 1.2; Cl−, 134; HCO3−, 15.5; H2PO4−, 1.2; and glucose, 11.5. This physiologic solution was gassed with 97% O2/3% CO2 to establish a pH of 7.4. Muscle chamber temperatures were constantly monitored and maintained at 37 ± 0.5°C by the perfusion of the prewarmed KRB solution. KRB constituents and bethanechol were obtained from Sigma Chemical. Antibodies were diluted in 0.05 mol/L phosphate-buffered saline containing 0.2% bovine serum albumin (Sigma), 100 U/mL penicillin G, and 100 μg/mL streptomycin (Boehringer Mannheim, Indianapolis, IN).
RESULTS
Resident Leukocytes Within the Small Intestinal Muscularis
Our first objective was to determine the presence and phenotype of resident macrophages and other leukocytes within the human jejunal muscularis externa in normal, rapidly resected specimens (<1 hour after skin incision). As in rodents, Figure 1A demonstrates the dense presence of resident macrophages within the human jejunal muscularis within the first hour after skin incision. Macrophages were the most numerous type of leukocyte in the early resected “control” muscularis specimens. These cells were randomly distributed within each muscle layer. The macrophages were localized between the distinct lamina of the intermuscular bundles of each muscle layer (38.9 ± 2.78 cells/200× field as counted within whole-mounts of the longitudinal muscle layer). Morphologically, muscularis macrophages presented as oval or round cells, sometimes with pseudopodia or dendrites (see Fig. 1B). As depicted in Figs. 1A and C, a population of resident macrophages also encased the mesenteric blood vessels that coursed through the muscularis externa. In addition to the resident macrophages, there were a few neutrophils (0.2 ± 0.13 cells/200× field) but a relatively larger number of mast cells (20.4 ± 2.38 cells/200× field) in early specimens. Like the muscularis macrophages, neutrophils and mast cells appeared to be randomly distributed within the muscularis.
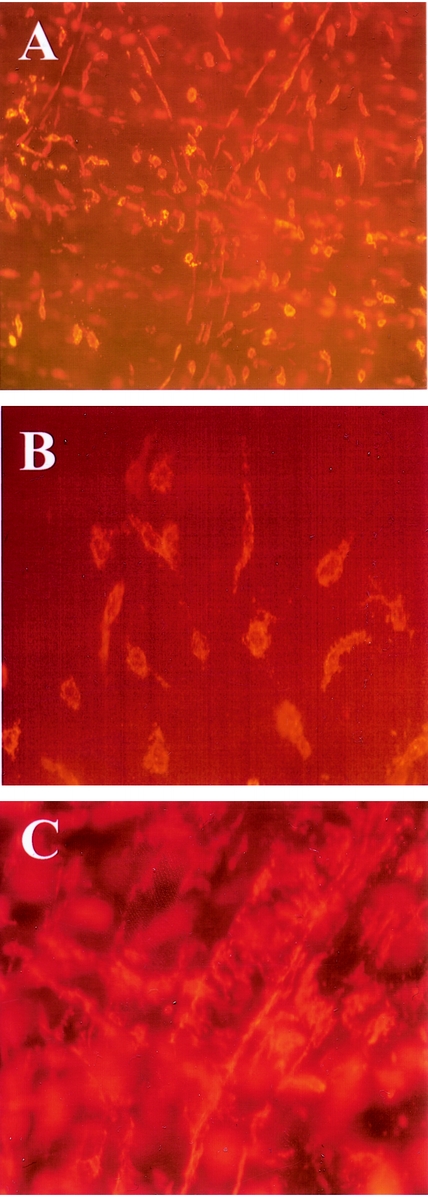
Figure 1. Ber-MAC3 immunohistochemistry for resident macrophages in human jejunal muscularis whole-mounts. (A) Dense network of resident macrophages between the muscle bundles in an early specimen (100×). The different shapes of oval, round, and sometimes flattened, elongated macrophages are easily visible. (B) Various macrophage morphologies (400×). (C) Lining of mesenteric vessels by the macrophages (200×).
Whole-mounts of the jejunal muscularis prepared from early surgical specimens showed a weak immunofluorescent signal for lymphocyte function associated antigen-1 (LFA-1), indicating that these cells were in an inactive state. 19 In late surgical specimens (>3 h), the resident muscularis macrophages were intensely positive for the activation marker LFA-1. These findings corroborated our observations in a rodent model of postoperative ileus.
Intestinal Cytokine Induction
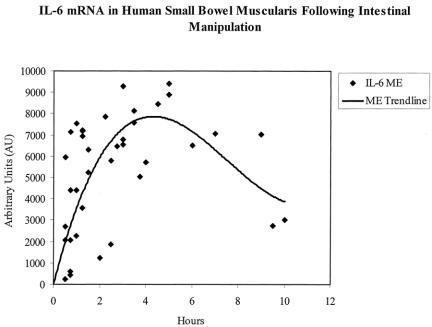
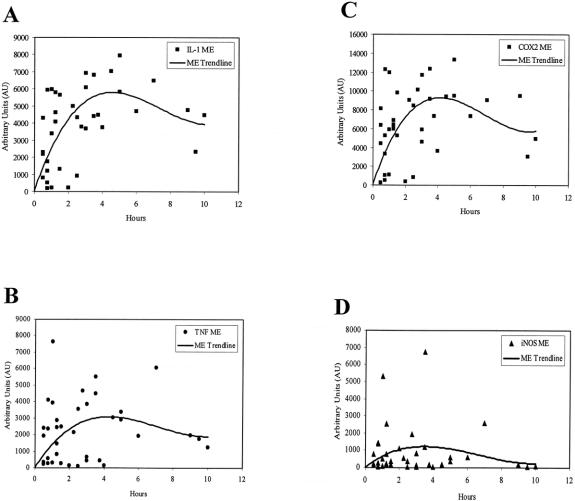
Next, we obtained human intestinal specimens at early and late time points after abdominal incision from the same patient during prolonged elective surgical procedures to investigate the expression of proinflammatory cytokines within the muscularis externa (n = 4). As shown in the RT-PCR gel of Figure 2, IL-6 mRNA was significantly induced within the muscularis during the intraoperative period of approximately 3 hours. Phosphorimaging of the radiolabeled gels demonstrated a significant 19.2-fold induction of IL-6 message. Mucosal IL-6 mRNA levels, however, were not altered during this same intraoperative period. Similarly, a significant intraoperative upregulation in mRNA was measured for COX-2 (3.4-fold) and iNOS (2.2-fold) by semiquantitative PCR. Mucosal extracts, by contrast, did not show a significant increase in mRNA expression. Within the confines of the variables of underlying disease, specific surgical procedure, and preoperative medications, we derived a time course for cytokine mRNA expression after surgical skin incision by collecting specimens at various time points into surgery from individual patients. The results for IL-6 mRNA expression in these specimens using semiquantitative RT-PCR are plotted in Figure 3. The temporal pattern of IL-6 induction intraoperatively in the human was similar to our finding using a rodent model of manipulation-induced ileus. The time course of other known proinflammatory cytokines and mediators 20,21 within the muscularis was also evaluated, and these expression profiles are shown in Figure 4. All except iNOS followed the general pattern of activation during the first few hours of the operation, followed by a plateau lasting to approximately 6 hours, and then a gradual decline.
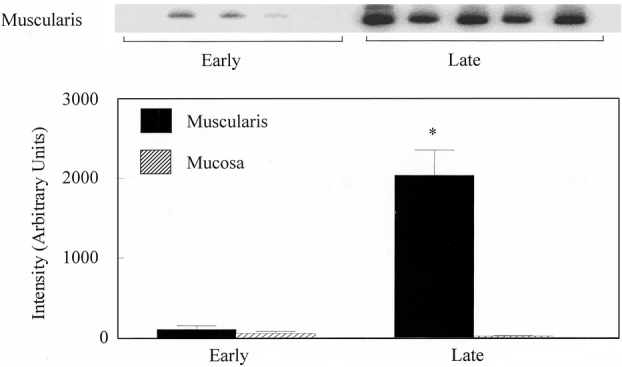
Figure 2. IL-6 mRNA induction in the human jejunal muscularis and mucosa at two different time points into surgery. The early specimens were removed within 1 hour after skin incision; late specimens were harvested after 3 or more hours. (Upper panel) Gel bands from semiquantitative RT-PCR. (Lower panel) Bar graph shows the quantitative measurement of the gel using phosphorimaging technology. Intraoperative IL-6 mRNA induction is evident in muscularis extracts but not in the mucosa. Data are expressed as mean ± SEM, n = 4.
Figure 3. Time course of IL-6 mRNA expression in isolated human jejunal muscularis extracts harvested at different time points into abdominal surgery. Message levels were measured by RT-PCR and quantified using laser densitometry. The trend line demonstrates an early induction of IL-6 mRNA, with a maximum intraoperative upregulation at 4 hours following skin incision. Even 10 hours after skin incision, cytokine mRNA levels were still significantly elevated. Every data point represents the analysis of each individual specimen harvested at a different time point into the surgical procedure.
Figure 4. Time courses for mRNA induction of the inflammatory mediators IL-1β (A) TNF-α (B), COX-2 (C), and iNOS (D) in isolated human jejunal muscularis extracts harvested at different time points into abdominal surgery. Message levels were measured by RT-PCR and quantified using laser densitometry. The expression profile shows an early induction with a maximum upregulation at approximately 4 hours after skin incision for all mediator mRNAs.
The semiquantitative PCR results were then confirmed using quantitative real-time PCR for the mediators that showed the most upregulation. These results were controlled for various interpatient variables by using early and late specimens taken during an operation in the same patient in two individual cases. The comparative real-time measurement of IL-6 in both patients between early and late specimens demonstrated a significant upregulation of IL-6 mRNA (patient 1, 221.8-fold increase from 30 minutes to 3.5 hours, patient 2, 298.2-fold increase from 45 minutes to 3 hours). IL-1β (8.8- and 35.4-fold) and COX-2 (4.5- and 7.3-fold) were also significantly upregulated in the specimens of the two patients.
Cytokine Cellular Localization
We next sought the cellular origin of the cytokines induced in the human jejunal muscularis during the operation. Immunohistochemical staining of human muscularis whole-mounts prepared from late surgical specimens demonstrated the distinct localization of IL-6 protein to a specific population of apparently resident cells within the muscularis (Fig. 5). These IL-6+ cells were multiform in shape but tended to be oval or round, sometimes elongated or stellate. They were distributed throughout the smooth muscle syncytium and were also observed to track along blood vessels that penetrated the muscularis externa. Our previous rat data, along with the configuration and distribution of these cells, prompted us to determine if these cells could be identified as resident muscularis macrophages. Indeed, immunohistochemistry using a specific human macrophage antibody (Ber-MAC3) labeled a population of cells with the similar multiform shape and distribution throughout the smooth muscle syncytium as observed for the IL-6+ cells. These resident leukocytes were also observed to encase the small vessels that coursed through the human intestinal muscle layer. Immunohistochemical-double labeling of the human muscularis whole-mounts with Ber-MAC3-RPE and IL-6-FITC identified the resident muscularis macrophages as the primary site of IL-6 protein in the late surgical specimens. Furthermore, the lower level of mRNA reported above for muscularis extracts from early human specimens was reflected in a lower intensity of IL-6 immunofluorescence in the early whole-mounts.
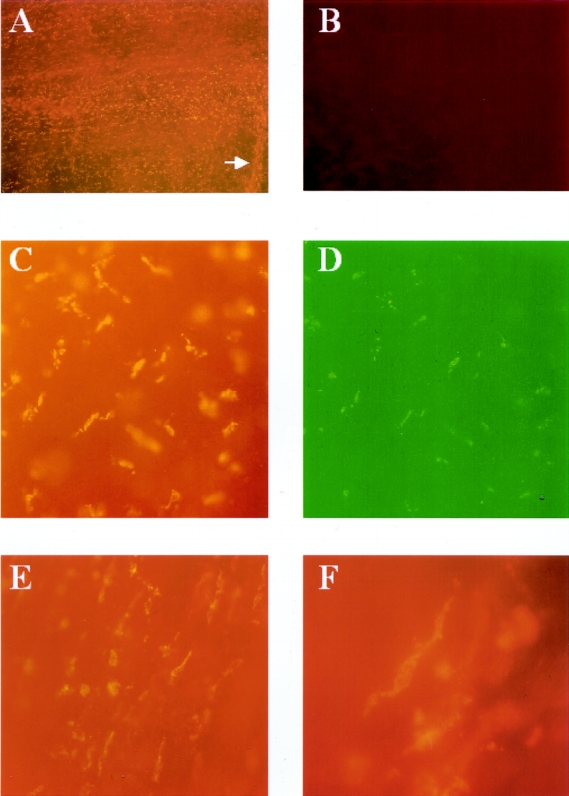
Figure 5. Fluorescence micrographs of human jejunal muscularis whole-mounts stained with human specific monoclonal antibodies against IL-6 and macrophages (Ber-MAC3). (A) A late surgical specimen at low magnification with a large number of IL-6+ cells within the intestinal muscularis whole-mount. The arrow points to an incoming mesenteric vessel that is lined by the IL-6+ cells. (B) An early specimen after IL-6 immunohistochemistry; no cellular signal is visible. Double-labeling of the same specimen with (C) Ber-MAC3-RPE (macrophages) and (D) IL-6-FITC. Both antibodies stain the same population of cells, which are muscularis macrophages. (E, F) Morphology of the IL-6+ cells, which resemble macrophages and are comparable in shape and distribution to the cells seen in Figure 1. (A, B: 40×; C–E, 200×; F, 400×)
Similar findings were seen in immunohistochemical stains for IL-1β, COX-2, and iNOS. All these mediators showed a strong signal in specimens after extended surgical procedures. Unlike the others, however, TNF-α immunohistochemistry demonstrated a strong signal in early specimens that was significantly weaker in later samples. To test the hypothesis that TNF-α might be stored preformed in the muscularis macrophages, we incubated unfixed muscularis whole-mounts of early specimens for 3 hours in cell culture medium (DMEM/F-12) or a buffered physiologic salt solution (HBSS) with or without the addition of the bacterial peptide fMLP, since bacterial products are known to activate macrophages and stimulate the release of TNF-α. 22,23 These whole-mounts were fixed after the incubation period and stained for TNF-α by immunohistochemistry. Whole-mounts that were incubated in media or HBSS still expressed a strong signal for TNF-α, while specimens incubated with fMLP almost completely lost the signal for TNF-α. These results suggest that TNF-α is stored in muscularis macrophages and is released after activation of these cells.
Signal Transduction
To demonstrate the local functional activity of the muscularis macrophage-derived IL-6, which is known to signal through the Stat3α isoform, STAT protein phosphorylation was determined using hSIE. 15 Figure 6A shows that intraoperative activation of the transcription factor Stat3 occurred within protein extracts of the human muscularis from two typical patients. Like the real-time PCR data, the protein extracts for this experiment were controlled for various interpatient variables by using early and late specimens taken during an operation in the same patient in two individual cases. The average intraoperative levels of Stat3 protein phosphorylation in the late human muscularis specimens demonstrated a 3.0-fold increase over early specimens (n = 5). The mobility characteristics of the hSIE/STAT band suggested that the phosphorylated STAT proteins consist primarily of the SIF-A complex. Quantification of the SIF-A complex (Stat3 homodimer) is shown in the histogram in Figure 6B.
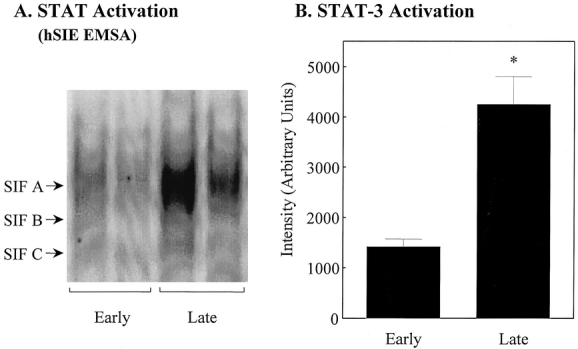
Figure 6. Stat3 activation in human jejunal muscularis protein extracts from early and late surgical specimens. (A) EMSA gel bands using the human SIE probe in two patients where the specimens were harvested at two different time points of the same procedure. Activation of the STAT complex was significantly increased in the late specimens compared to early samples. (B) Quantified activated STAT complex using laser densitometry from early and late specimens. Data are expressed as mean ± SEM, n = 5. *P < .05.
Functional Blockade of Kinetically Active Inflammatory Mediators
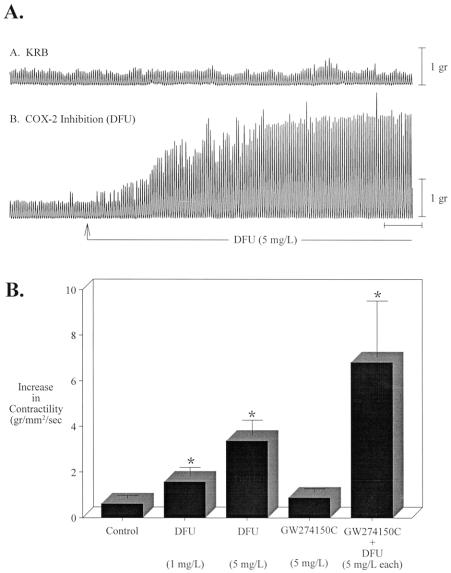
Nitric oxide and prostaglandins are known to be kinetically active substances secreted by macrophages that interfere with the muscle function of the gut. 18,24 To test the importance of NO and prostaglandins in human specimens, we investigated the functional significance of iNOS and COX-2 inhibition by the specific pharmacologic blockers GW274150C (iNOS) and DFU (COX-2). Both drugs were added to the perfusion solution (KRB) at a concentration of 5 mg/L. As shown in Figure 7, DFU caused a significant increase in spontaneous jejunal circular muscle contractility (3.4-fold), whereas GW274150C alone had only minimal effects. However there was a synergistic effect between the two inhibitors: spontaneous contractility increased 6.8-fold in the presence of both agents.
Figure 7. Pharmacologic inhibition of COX-2 and iNOS on spontaneous contractions recorded from human small intestinal circular smooth muscle. (A) Spontaneous mechanical activity recorded from two muscles prepared from the same patient. In the upper trace the muscle was maintained in KRB, while in the lower trace the muscle was exposed to DFU (5 mg/L). (B) Contractile areas recorded over a period of 15 minutes after 45 minutes of exposure to DFU, GW274150C, or both. Data are expressed as g/mm2/s, mean ± SEM, N = 4–11. *P < .05.
In Vitro Muscle Function Following Surgical Trauma
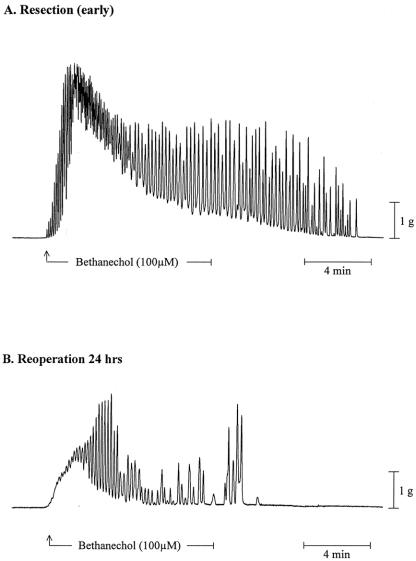
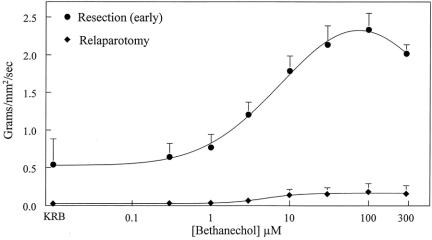
To investigate muscle function at later time points after the surgical insult, 9,25 we harvested specimens from three patients who were reoperated on 24 or 48 hours after an initial abdominal procedure. Early specimens were used as functional control samples (n = 4). Control circular muscle strips exhibited regular monophasic contractions with a frequency of 11.4 ± 0.49 events/min. Muscle strips from the reoperated small bowel demonstrated a marked reduction in spontaneous contractions (4.4 ± 0.22 events/min). Small intestinal circular muscle strips were also significantly less responsive to muscarinic stimulation with bethanechol (control: 2.18 ± 0.184, reoperated: 0.18 ± 0.110 g/mm2 per second at 100 μmol/L bethanechol). The alteration in circular muscle contractility after surgical trauma is illustrated in representative mechanical traces from a control and a reoperated specimen in Figure 8. Complete dose-response curves to bethanechol for control and reoperated specimens are shown in Figure 9. The calculated ED50 values from sigmoid fit curves of the averaged bethanechol dose-response curves generated from control and reoperated specimens were not different from each other (5.12 and 6.06 μmol/L). However, as seen in the individual mechanical traces in Figure 9 and in the dose-response plots in Figure 10, the maximal force generated at every bethanechol concentration was significantly less in the reoperated muscles compared to the early harvested control muscles. At a bethanechol concentration of 100 μmol/L, surgical trauma and subsequent reoperation led to a significant 91.7% reduction in contractile activity compared to controls.
Figure 8. Organ-bath recorded mechanical traces of bethanechol-stimulated (100 μmol/L) circular muscle strips from human small bowel specimens. (A) Recording from an early specimen; a typical marked increase in phasic and tonic contractile activities was charted in response to bethanechol (100 μmol/L). (B) Recording from a muscle harvested during a second surgery 24 hours after the initial procedure; a marked decrease in phasic and tonic contractile responses was charted in specimens obtained after reoperation. Both traces were taken from typical incremental dose-response curve experiments.
Figure 9. Bethanechol-stimulated dose-response curves generated from human jejunal circular muscle contractile activity recorded after early resection or after a second operative intervention at 24 or 48 hours following the primary resection. Data are expressed as mean ± SEM, n = 4 for each curve.
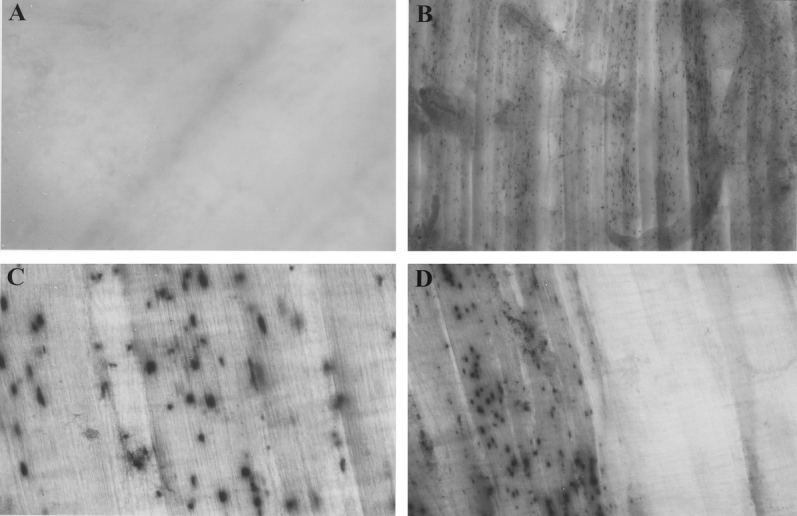
Figure 10. Myeloperoxidase staining with Hanker-Yates reagent in human muscularis whole-mounts prepared from resected jejunal specimens. (A) Myeloperoxidase staining in an early specimen with no visible positive cells. (B) Myeloperoxidase staining in a typical specimen that was harvested during a second surgery of a patient who had undergone a bowel resection 24 hours previously shows multiple positively stained neutrophils (A, B: original magnification 40×). (C) Infiltrated positively stained neutrophils (200×) in a patient reoperated on at 48 hours after the initial operation. (D) The right half of the circular muscle layer was removed to leave the longitudinal layer exposed before the staining procedure. This further dissection demonstrates that the majority of the infiltrated cells are recruited into the circular layer, a finding that we have also described in the rodent model (100×).
Leukocytic Infiltrates Following Surgical Trauma
Next, we sought to investigate the correlation between surgical trauma and cellular infiltration within the intestinal muscularis 8,25 by examining specimens from patients who underwent a relaparotomy 24 or 48 hours after the primary bowel operation. These specimens demonstrated a significant leukocytic infiltration of the muscularis with neutrophils and monocytes (see Fig. 10). Neutrophils and monocytes were nearly absent in early specimens. However, 24 or 48 hours after the initial surgery, the intestinal muscularis was infiltrated by a significant number of neutrophils and monocytes. These leukocytes were predominantly recruited to the circular muscle layer (the circular muscle layer was stripped off on the right half of the whole-mount, and the exposed longitudinal muscle layer is almost free of infiltrated neutrophils).
DISCUSSION
Postoperative ileus is an iatrogenic problem with significant morbidity and mortality. 3,5 The monetary impact of postoperative ileus in the United States alone is estimated at $1 billion per year. 6 The pathogenesis of postoperative ileus has been attributed to multiple factors, including the involvement of sympathetic reflexes, activation of inhibitory adrenergic and nitrergic nerves, inhibitory humoral agents, effects of anesthetic agents, and inflammation. 7,26,27 Rodent models of surgical trauma have provided some insight into the pathologic mechanisms that commonly occur after human abdominal operations. Currently, two mechanisms have received the most attention. Tache et al have proposed an elegant neural hypothesis involving the facilitation of inhibitory autonomic and enteric neural circuitry in the first few hours after operation. 28–30 In this scenario, cecal manipulation or psychological stress causes the central release of corticotropin-releasing factor in the paraventricular nucleus of the hypothalamus and the dorsal vagal complex. This central neural activity is hypothesized to then trigger an efferent inhibitory motor pathway. The importance of this early mechanism has been corroborated by the works of Raybould, De Winter, and Boeckxstaens. 27,31,32
A second mechanism, which hypothesizes the importance of inflammatory mediators in the pathogenesis of postoperative ileus, has also been proposed, although the genesis of the idea is difficult to attribute to a single group. 7 Our laboratory has explored the inflammatory hypothesis using a simple rodent model of intestinal manipulation. We envisage that even the gentlest surgical handling of the small intestine sequentially activates a typical acute inflammatory cascade within the muscle layers of the intestinal wall. Characteristic of this local inflammation is the activation of the resident macrophages that lie as sentinels within the enteric muscularis; when activated, phosphorylation of transcription factors results, with the subsequent induction of genes and the release of cytokines, chemokines, and kinetically active substances (nitric oxide and prostaglandins). This local inflammatory milieu then participates in the upregulation of adhesion molecules, which recruits degranulating leukocytes into the muscularis. Our animal data blocking leukocyte recruitment has demonstrated that the elaboration of leukocytic products (nitric oxide, prostaglandins, oxygen radicals, proteases) causes a sustained phase of both enteric neural and smooth muscle dysfunction. 9
These two hypothesized mechanisms are not mutually exclusive, and a combined overlay of these mechanisms 29 may better explain clinical postoperative ileus, which has a duration of several days. 7,33,34 In fact, the functional importance of each individual mechanism may be governed by the time after surgery. The clinical applicability of any of the proposed hypotheses has not been previously tested. Evidence presented here, however, lends support to the hypothesis that inflammation within the enteric musculature occurs at a predictable pace in humans as well as rodents, and that the inflammatory cascade is accompanied by impaired enteric muscle function.
As delineated above, this hypothesis focuses initially on the importance of the constitutively present network of muscularis macrophages. We have consistently referred to the muscularis macrophages as a network because although, in a limited anatomic interpretation, these resident phagocytes appear not to structurally interact, they certainly would have the potential to communicate through molecular mechanisms, thus creating a functional network of cells. The resident human muscularis macrophages appear to be less structurally organized compared to the rat, mouse, 35,36 and rabbit (A.J.B., unpublished observations). They appeared to be randomly distributed between the circular and longitudinal muscle bundles of the human muscularis externa. In contrast, in the rat, the muscularis externa macrophages are regularly arranged at the level of the myenteric plexus and between the circular muscle bundles. 36 Mikkelsen has made similar observations. 37 However, in addition to these muscularis externa macrophages within the human, a second population of resident macrophages, which structurally encase the small vessels that course through the muscle layers of the gut wall, can be readily observed. As in rodent models, 8,11 these human resident macrophages rapidly became activated intraoperatively, as demonstrated by the increased expression of LFA-1 and the increased expression of cytokines.
In this study, we deliberately analyzed the muscularis externa for a distinct set of cytokines (IL-6, IL-1β and TNF-α) and mediators (iNOS and COX-2). Both IL-6 and IL-1β were intraoperatively upregulated in a time-dependent manner within resident macrophages of the human muscularis. The functional activity of IL-6 protein could be surmised based on the significant increase in the phosphorylation of STAT proteins within the muscularis. Both IL-6 and IL-1β are known to alter enteric neurotransmission. Collins demonstrated that enteric release of both acetylcholine and norepinephrine is decreased in the presence of these two cytokines. 38 The potential motility-modulating capacity of TNF-α has also been demonstrated in a model of sepsis, in which the beneficial effect of TNF binding protein on LPS-induced suppression of rat longitudinal muscle contractility in vitro was demonstrated. 39 Thus, the observed intraoperative induction of TNF-α within the human muscularis could conceivably play a similar role in contributing to postoperative ileus.
Prostaglandins are some of the most potent modulators of intestinal motility. 40–42 We have recently shown that COX-2-generated prostaglandins play a significant inflammatory and direct smooth muscle inhibitory role in our rodent model of postoperative ileus. 24 Similarly, COX-2 has been demonstrated to participate in sepsis-induced ileus. 43 The human data presented here illustrate not only that COX-2 mRNA is increased intraoperatively, but also that the pharmacologic blockade of this pathway with DFU leads to a significant increase in human jejunal circular muscle contractility.
Nitric oxide is one of the most important regulators of intestinal motility. Typically, NO is released in a controlled manner from the enteric nervous system to produce programmed patterns of motility. 44 However, large amounts of NO are also generated through iNOS in many cells during inflammation and spewed forth in a relatively indiscriminate manner. 45 The key role for iNOS in both postoperative ileus 10,46 and sepsis-induced ileus 47–50 has already been delineated in rodent models. In the human tissues obtained in this study, iNOS blockade with the selective inhibitor GW274150C elicited only a small increase in jejunal circular muscle contractions. However, iNOS blockade in combination with COX-2 inhibition potentiated a significant increase in contractility compared to that measured in the presence of either iNOS or COX-2 inhibition alone.
Our final aim was to determine if the surgically induced inflammatory milieu causes the recruitment of leukocytes into the intestinal muscularis and if this has a functional consequence to the in vitro recorded contractions of the human jejunal circular smooth muscle. In muscularis whole-mounts of the human jejunum obtained 24 or 48 hours after an initial surgery, a marked infiltration of the muscularis with myeloperoxidase-positive cells was observed. However, it is impossible to determine the exact stimuli for this leukocytic recruitment, but we propose that at least some of the infiltrate is the result of the prior surgery.
Leukocytes are known to release many substances, many of which can potently alter smooth muscle function (e.g., NO, prostaglandins, reactive radical intermediates, proteases). 20 In rats, intestinal manipulation causes a sustained suppression in jejunal circular muscle contractility. 8 The blockade of the manipulation-induced leukocyte recruitment nearly completely prevents the suppression in jejunal muscle contractility, as observed 24 hours after intestinal manipulation. 9 Again, in parallel with rodent studies, the polymorphonuclear neutrophil-infiltrated human intestinal specimens generated significantly less spontaneous and bethanechol-stimulated contractility.
A general interpretation of the above data would support the gut as a major cytokine-producing organ in numerous disease states. This concept has been popularized by Deitch et al, who see the gut as the “motor” of multiple organ dysfunction following shock and trauma. 51–53 The prevailing belief would suggest that the mucosa is the primary generator of these systemically active substances, because the mucosa hosts a large number of leukocytes and epithelial cells, all of which are capable of producing inflammatory mediators. 20,54,55 Our results show that the local production of inflammatory mediators during intestinal surgery is mainly localized to the muscularis externa. The stimulus for the intraoperative induction of the various inflammatory mediators is undoubtedly the physical manipulation of the gut, but a component of the upregulation could be exposure of the muscularis to small quantities of bacterial products, which could come from the patient or the operative field. In contrast to the muscularis, the mucosal cytokine and mediator expression was only minimally altered within the human bowel wall following surgical trauma compared to the muscularis, thus proposing the intestinal muscularis as an important immunologically active structure within the body.
In conclusion, our human study documents for the first time that simple abdominal surgery leads to an activation of the dense network of resident muscularis macrophages and a subsequent upregulation of cytokines and kinetically active substances, which inhibit human jejunal smooth muscle contractility. Furthermore, the results suggest that the inflammatory milieu created by surgical trauma participates in the recruitment of leukocytes into the muscularis and a further suppression in muscle function. These findings in human intraoperative specimens strongly support the inflammatory-based hypothesis for postoperative ileus, in which the resident muscularis macrophages play a seminal role. The establishment of this mechanism opens up the potential for new therapeutic strategies in the prevention of this surgical complication, which is currently mostly looked on as a “normal” physiologic response of the intestine to the surgeon’s hand.
Footnotes
Supported in part by grants from the Deutsche Forschungsgemeinschaft Ka 1270/1-1 (J.C.K.), Schw 745/1-1 (N.T.S.), Tu 116/2-1 (A.T.) and from the National Institutes of Health R01-GM58241 and P50-GM-53789.
Correspondence: Anthony J. Bauer, PhD, University of Pittsburgh Medical School, Department of Medicine, S-849 Scaife Hall, 3550 Terrace Street, Pittsburgh, PA 15261.
E-mail: tbauer@pitt.edu
Accepted for publication September 9, 2002.
References
- 1.Hall MJ, Popovic JR. Summary: national hospital discharge survey. Advance Data. 2000;1998;316:1–17. [PubMed]
- 2.Hall MJ, Lawrence L. Ambulatory surgery in the United States, 1996. Advance Data. 1998; 300: 1–16. [PubMed] [Google Scholar]
- 3.Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000; 87: 1480–1493. [DOI] [PubMed] [Google Scholar]
- 4.Thompson JS, Quigley EM. Prokinetic agents in the surgical patient. Am J Surg. 1999; 177: 508–514. [DOI] [PubMed] [Google Scholar]
- 5.Kehlet H. Postoperative ileus. Gut. 2000; 47 (Suppl 4): iv85–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad M, Matthews JB. Deflating postoperative ileus. Gastroenterology. 1999; 117: 489–492. [DOI] [PubMed] [Google Scholar]
- 7.Livingston EH, Passaro EP. Postoperative ileus. Dig Dis Sci. 1990; 35: 121–132. [DOI] [PubMed] [Google Scholar]
- 8.Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in paralytic ileus. Ann Surg. 1998; 228: 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999; 117: 378–387. [DOI] [PubMed] [Google Scholar]
- 10.Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000; 118: 316–327. [DOI] [PubMed] [Google Scholar]
- 11.Eskandari MK, Kalff JC, Billiar TR, et al. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol. 1997; 273: G727–G734. [DOI] [PubMed] [Google Scholar]
- 12.Wan Y, Freeswick PD, Khemlani LS, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun. 1995; 63: 2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuerler A, Schwarz NT, Tuerler E, et al. Rat intestinal monocyte chemoattractant protein-1 initiates leukocyte recruitment and smooth muscle dysfunction in endotoxemic ileus. Am J Physiol. 2002; 282: G145–155. [DOI] [PubMed] [Google Scholar]
- 14.Schmittgen TD, Zakrajsek BA, Mills AG, et al. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000; 285: 194–204. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer C, Kalff JC, Audolfsson G, et al. Molecular and functional contractile sequelae of rat intestinal ischemia/reperfusion injury. Transplantation. 1999; 68: 1244–1254. [DOI] [PubMed] [Google Scholar]
- 16.Wagner BJ, Hayes TE, Hoban CJ, et al. The SIF binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990; 9: 4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborty A, White SM, Schaefer TS, et al. Granulocyte colony-stimulating factor activation of Stat3-α and Stat3β in immature normal and leukemic human myeloid cells. Blood. 1996; 88: 2442–2449. [PubMed] [Google Scholar]
- 18.Türler A, Moore BA, Pezzone MA, et al. Colonic postoperative inflammatory ileus in the rat. Ann Surg; 2002. [DOI] [PMC free article] [PubMed]
- 19.Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today. 1987; 8: 151–158. [DOI] [PubMed] [Google Scholar]
- 20.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987; 79: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan I, Collins SM. Expression of cytokines in the longitudinal muscle myenteric plexus of the inflamed intestine of rat. Gastroenterology. 1994; 107: 691–700. [DOI] [PubMed] [Google Scholar]
- 22.Sredni-Kenigsbuch D, Kambayashi T, Strassmann G. Neutrophils augment the release of TNF-alpha from LPS-stimulated macrophages via hydrogen peroxide. Immunol Lett. 2000; 71: 97–102. [DOI] [PubMed] [Google Scholar]
- 23.Vulcano M, Alves Rosa MF, Minnucci FS, et al. N-formyl-methionyl-leucyl-phenylalanine (fMLP) inhibits tumour necrosis factor-alpha (TNF-alpha) production on lipopolysaccharide (LPS)-stimulated human neutrophils. Clin Exp Immunol. 1998; 113: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz NT, Kalff JC, Engel BM, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001; 121: 1354–1371. [DOI] [PubMed] [Google Scholar]
- 25.Kalff JC, Eskandari MK, Hierholzer C, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999; 126: 498–509. [PubMed] [Google Scholar]
- 26.Bueno L, Ferre JP, Ruckebusch Y. Effects of anesthesia and surgical procedures on intestinal myoelectric activity in rats. Dig Dis Sci. 1978; 23: 690–695. [DOI] [PubMed] [Google Scholar]
- 27.De Winter BY, Boeckxstaens GE, De Man JG, et al. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br J Pharmacol. 1997; 120: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barquist E, Zinner M, Rivier J, et al. Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 1992; 262: G616–G620. [DOI] [PubMed] [Google Scholar]
- 29.Tache Y, Monnikes H, Bonaz B, et al. Role of CRF in stress-related alterations of gastric and colonic motor function. Ann NY Acad Sci. 1993; 697: 233–243. [DOI] [PubMed] [Google Scholar]
- 30.Nozu T, Martinez V, Rivier J, et al. Peripheral urocortin delays gastric emptying: role of CRF receptor 2. Am J Physiol. 1999; 276: G867–G874. [DOI] [PubMed] [Google Scholar]
- 31.Zittel TT, Lloyd KC, Rothenhofer I, et al. Calcitonin gene-related peptide and spinal afferents partly mediate postoperative colonic ileus in the rat. Surgery. 1998; 123: 518–527. [DOI] [PubMed] [Google Scholar]
- 32.Boeckxstaens GE, Pelckmans PA, Herman AG, et al. Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology. 1993; 104: 690–697. [DOI] [PubMed] [Google Scholar]
- 33.Seenu V, Goel AK. Early oral feeding after elective colorectal surgery: is it safe? Trop Gastroenterology. 1995; 16: 72–73. [PubMed] [Google Scholar]
- 34.Reissman P, Agachan F, Wexner, SD. Outcome of laparoscopic colorectal surgery in older patients. Am Surgeon. 1996; 62: 1060–1063. [PubMed] [Google Scholar]
- 35.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995; 10: 719–736. [PubMed] [Google Scholar]
- 36.Kalff JC, Schwarz NT, Walgenbach KJ, et al. Leukocytes of the intestinal muscularis externa: their phenotype and isolation. J Leukoc Biol. 1998; 63: 683–691. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res. 1992; 270: 273–279. [DOI] [PubMed] [Google Scholar]
- 38.Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996; 111: 1683–1699. [DOI] [PubMed] [Google Scholar]
- 39.Lodato RF, Khan AR, Zembowicz MJ, et al. Roles of IL-1 and TNF in the decreased ileal muscle contractility induced by lipopolysaccharide. Am J Physiol. 1999; 276: G1356–G1362. [DOI] [PubMed] [Google Scholar]
- 40.Sanders KM. Endogenous prostaglandin E and contractile activity of isolated ileal smooth muscle. Am J Physiol. 1978; 234: E209–E212. [DOI] [PubMed] [Google Scholar]
- 41.Thor P, Konturek JW, Konturek SJ, et al. Role of prostaglandins in control of intestinal motility. Am J Physiol. 1985; 248: G353–G359. [DOI] [PubMed] [Google Scholar]
- 42.Eberhart CE, Dubois RN. Eicosanoids and the gastrointestinal tract. Gastroenterology. 1995; 109: 285–301. [DOI] [PubMed] [Google Scholar]
- 43.Hori M, Kita M, Torihashi S, et al. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol. 2001; 280: G930–G938. [DOI] [PubMed] [Google Scholar]
- 44.Stark MB, Bauer AJ, Szurszewski JH. Effect of nitric oxide on circular muscle of the canine small intestine. J Physiol. 1991; 444: 743–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billiar TR. Nitric oxide: novel biology with clinical relevance. Ann Surg. 1995; 221: 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moojen TM, Van Gulik TM, Hoek FJ, et al. Possible role of nitric oxide in postoperative ileus: a comparative study. Neurogastroenterology Motil. 1999; 11: 403–408. [DOI] [PubMed] [Google Scholar]
- 47.Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol. 1999; 277: G478–G486. [DOI] [PubMed] [Google Scholar]
- 48.Hassoun HT, Weisbrodt NW, Mercer DW, et al. Inducible nitric oxide synthase mediates gut ischemia/reperfusion-induced ileus only after severe insults. J Surg Res. 2001; 97: 150–154. [DOI] [PubMed] [Google Scholar]
- 49.Cullen JJ, Mercer D, Hinkhouse M, et al. Effects of endotoxin on regulation of intestinal smooth muscle nitric oxide synthase and intestinal transit. Surgery. 1999; 125: 339–344. [PubMed] [Google Scholar]
- 50.Weisbrodt NW, Pressley TA, Li YF, et al. Decreased ileal muscle contractility and increased NOS II expression induced by lipopolysaccharide. Am J Physiol. 1996; 271: G454–G460. [DOI] [PubMed] [Google Scholar]
- 51.Deitch EA, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994; 1: 141–145. [DOI] [PubMed] [Google Scholar]
- 52.Mainous MR, Ertel W, Chaudry IH, et al. The gut: a cytokine-generating organ in systemic inflammation. Shock. 1995; 4: 193–199. [PubMed] [Google Scholar]
- 53.Michalsky MP, Deitch EA, Ding J, et al. Interleukin-6 and tumor necrosis factor production in an enterocyte cell model (Caco-2) during exposure to Escherichia coli. Shock. 1997; 7: 139–146. [DOI] [PubMed] [Google Scholar]
- 54.Auger MJ, Ross JA. The biology of the macrophage. In: Lewis CE, McGee JO, eds. The Macrophage. New York: Oxford University Press, 1992: 3–74.
- 55.van Furth R. Origin and turnover of monocytes and macrophages. In: Iversen OH, ed. Current Topics in Pathology. Heidelberg: Springer, 1989: 125–150. [PubMed]