Abstract
Brief forebrain ischemia in rodents induces selective and delayed neuronal death, particularly of hippocampal CA1 pyramidal neurons. Neuronal death is preceded by down-regulation specific to CA1 of GluR2, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit that limits Ca2+ influx. This alteration is hypothesized to cause neurodegeneration by permitting a lethal influx of Ca2+ and/or Zn2+ through newly formed GluR2-lacking AMPA receptors. Two days of mild hypothermia induced 1 h after ischemia potently and lastingly protects against ischemic injury. We examined molecular mechanisms underlying hypothermia-induced neuroprotection. We report that hypothermia rescues most hippocampal CA1 neurons from ischemia-induced cell death and attenuates ischemia-induced down-regulation of mRNA encoding the AMPA receptor subunit GluR2. Ischemia induced a marked down-regulation of GluR2 mRNA and a small down-regulation of GluR1 mRNA in CA1 at 2 days, as assessed by quantitative in situ hybridization. The ischemia-induced changes in gene expression were cell-specific in that GluR2 was not significantly altered in CA3 or dentate gyrus. After ischemia treated by hypothermia GluR2 mRNA expression was modestly reduced at 2 days and exhibited complete recovery to control levels at 7 days. Hypothermia prevented ischemia induced changes in GluR1 mRNA expression. These findings suggest that intervention at the level of transcriptional regulation of the GluR2 gene may be a mechanism by which prolonged postischemic cooling rescues CA1 neurons otherwise “destined to die.”
Keywords: cerebral ischemia‖neuroprotection‖GluR1‖global ischemia‖ hippocampus
Brief forebrain ischemia results in degeneration of hippocampal CA1 pyramidal neurons typically after 2–4 days (1, 2) depending on the duration of ischemia and species (3). Although there is considerable controversy over the mode of cell death (apoptosis or necrosis), it is generally agreed that excess Ca2+ influx is involved (4). During ischemia, CA1 pyramidal neurons depolarize, exhibit a transient rise in intracellular Ca2+ and possibly Zn2+ and become inexcitable, but within minutes of reperfusion, ionic gradients and excitability are restored. Ultimately, intracellular Ca2+ (and Zn2+) again rises in vulnerable CA1 neurons and neuronal death ensues.
Considerable evidence implicates Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (AMPARs) in ischemia-induced neuronal death (5, 6). Forebrain ischemia suppresses GluR2 mRNA and protein expression in CA1 neurons (7–10) and enhances AMPAR-mediated Ca2+ influx into CA1 neurons before death (11). Ischemia induces prolonged, Ca2+-dependent AMPA excitatory postsynaptic currents at CA1 synapses, which are sensitive to Joro spider toxin and 1-naphthyl acetyl spermine, channel blockers selective for Ca2+-permeable AMPARs (12–14). The presence of edited GluR2 subunits in heteromeric AMPARs greatly limits Ca2+ permeability (15, 16), voltage-dependent block by polyamines (17, 18), and single channel conductance (19). Thus, these findings indicate postischemic expression of GluR2-lacking, Ca2+-permeable receptors at CA1 synapses and predict enhanced vulnerability of CA1 neurons to ambient glutamate (5, 6). Consistent with this model, knockdown of the GluR2 gene by in vivo administration of antisense oligonucleotides, even in the absence of an ischemic insult, causes selective death of pyramidal neurons (20). Antisense-induced cell death is fully prevented by 1-naphthyl acetyl spermine, suggesting mediation by Ca2+-permeable AMPA receptors.
Prolonged postischemic hypothermia affords robust and sustained neuronal survival and greatly reduces ischemia-induced cognitive deficits in rodents (21, 22). For example, 5 min of forebrain ischemia in gerbils killed ≈98% of CA1 neurons, but prolonged, delayed hypothermia (induced 1 h after ischemia and maintained for 48 h) permanently (i.e., 60-day survival) salvaged most neurons (22–24). Hypothermia can protect when initiated as late as 12 h after ischemia, although neuroprotection is significantly better when cooling is initiated sooner. These findings support the clinical usefulness of postischemic hypothermia as a mode of intervention after global ischemia associated with cardiac arrest in humans (25, 26).
Here we show that hypothermia sufficient to protect CA1 neurons against delayed neurodegeneration attenuates the down-regulation of GluR2 mRNA observed 2 days after forebrain ischemia and is followed by complete recovery of GluR2 mRNA at 7 days. These results suggest that attenuation of GluR2 down-regulation is one mechanism by which prolonged postischemic cooling rescues CA1 neurons, and support the hypothesis that reduction of GluR2 expression and formation of Ca2+-permeable AMPA receptors play a causal role in the selective death of CA1 pyramidal cells after global ischemia.
Methods
Subjects.
Female and male Mongolian gerbils (58.8 ± 7.0 g SD at time of ischemia or sham operation) were obtained locally (University of Calgary) or from High Oak Ranch (Baden, Ontario). Surgical procedures were in accordance with the Canadian Council on Animal Care guidelines and the principles and procedures of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by a local animal care committee at the University of Calgary and the Biosciences Animal Policy and Welfare Committee at the University of Alberta.
Brain Temperature Probe Implantation.
All gerbils were implanted (under 65 mg/kg sodium pentobarbital anesthesia) with a 5.0-mm guide cannula ≈1 mm anterior and 1 mm to the left of bregma as described (27). Two days later a telemetry probe (model XM-FH, MiniMitter, Bend, OR), which measured the temperature of dorsal striatum, was inserted under brief halothane anesthesia. The brain temperature on the following day (day before ischemia or sham operation) served as baseline.
Ischemia/Sham Insult.
On the second day after probe insertion, gerbils were anesthetized with halothane (1.5–2% maintenance in 60% air/40% O2), and a midline neck incision was made followed by isolation of the common carotid arteries. Animals were subjected to either 5 min of normothermic bilateral carotid artery occlusion (with nontraumatic micro clips; ischemia) or sham occlusion (no ischemia; sham). Brain temperature was maintained very near normothermia (≈36.0°C) via an infrared lamp. Arteries were inspected to ensure adequate reflow, and then anesthesia was discontinued.
Postischemic Temperature Measurement and Control.
Gerbils either regulated their own postischemic temperature, or were subjected to prolonged mild hypothermia by an automated exposure technique by using fans, fine water spray, and infrared lamps that typically keeps temperature to within 0.3°C of the desired value in awake freely moving rodents (27). Hypothermia was induced slowly starting 1 h after reperfusion, by 1°C/30 min, to 32°C. Brain temperature was maintained at 32°C for 24 h, warmed to 34°C (by 1°C/30 min), maintained at 34°C for 24 h, warmed to 35°C (except for 48-h survival groups) and then held between 35°C and 36°C for 12 h. This established regimen affords near-total preservation of CA1 neurons when induced up to 6 h after ischemia in rat and gerbil (22–24). The ischemia 2-day survival group regulated their own postischemic temperature except for the first hour when temperature was kept above 37°C. In an additional four gerbils, ischemia was produced with core temperature controlled at normothermia, but without brain temperature recording, and animals were allowed to survive for 7 days to simply illustrate the near-complete loss of GluR1 and GluR2 mRNA in CA1 neurons that have undergone massive cell death.
In Situ Hybridization.
To examine the effect of hypothermia on GluR1 and GluR2 mRNA expression, animals were subjected to sham operation (2-day survival), sham operation followed by hypothermia (2- and 7-day survival times), global ischemia (2- and 7-day survival times), or global ischemia followed by hypothermia (2- and 7-day survival times). At that end of the scheduled survival time animals were anesthetized with 3–4% halothane and decapitated. Brains were promptly removed, frozen in isopentane and maintained at −80°C until overnight shipment (packed in dry ice) from Alberta, Canada, to the Bronx, NY, where co-investigators were blinded to the identity of the treatment groups. Glutamate receptor mRNA expression was assessed by in situ hybridization on sections at the level of the dorsal hippocampus as described (20). Briefly, cryosections (18 μm) were hybridized (overnight at 50°C) with 35S-labeled GluR1 or GluR2 riboprobes (106 cpm per section, 1 ng/ml) and apposed to Kodak XAR 5 film for 48 h.
For quantification of mRNA expression, autoradiograms were scanned at 2,500 dpi, and mean optical densities (background subtracted) in regions of maximal labeling of individual hippocampal subfields were averaged for two to four sections per animal for four to six animals. Optical density values were expressed as grand means (±SDs) of individual means. A few samples were lost because of problems with histological procedures unrelated to the surgical treatment. Global ischemia does not alter GluR1 or GluR2 mRNA expression in the dentate gyrus (DG) (7, 11); therefore to correct for variation in thickness of brain sections and efficiency of hybridization, optical density values were expressed as ratios of values for CA1 or CA3 to that for DG (CA1/DG and CA3/DG).
Histological Analysis.
Additional gerbils were allowed to survive 7 days for determination of hippocampal CA1 cell damage from Cresyl violet-stained 10-μm frozen coronal sections. These groups included sham-operated gerbils with (n = 3) and without hypothermia (n = 6), untreated normothermic ischemia (n = 4), and ischemia plus delayed hypothermia as described earlier (n = 6). Several others gerbils were excluded because of technical problems (e.g., telemetry probe failure). Animals were randomly assigned to groups. The number of viable looking CA1 neurons was counted and summed in medial, middle and lateral CA1 sectors at −1.7 mm to Bregma as done previously (21).
Statistics.
Data are presented as mean ± SD. The CA1 cell counts and GluR1 and GluR2 ratios were analyzed with ANOVA with Scheffé post hoc comparisons with SPSS except for comparisons among sham groups for which planned contrasts were used without α correction. An α correction was not used for comparisons among shams to minimize the chance of a type II error. P values <0.05 are considered statistically significant.
Results
Brain Temperature.
Baseline brain temperature was ≈36.2°C with only trivial group differences (averages ranged from 36.0 to 36.4°C). Temperature during and after ischemia/sham operation was regulated as described in Methods (Fig. 1A).
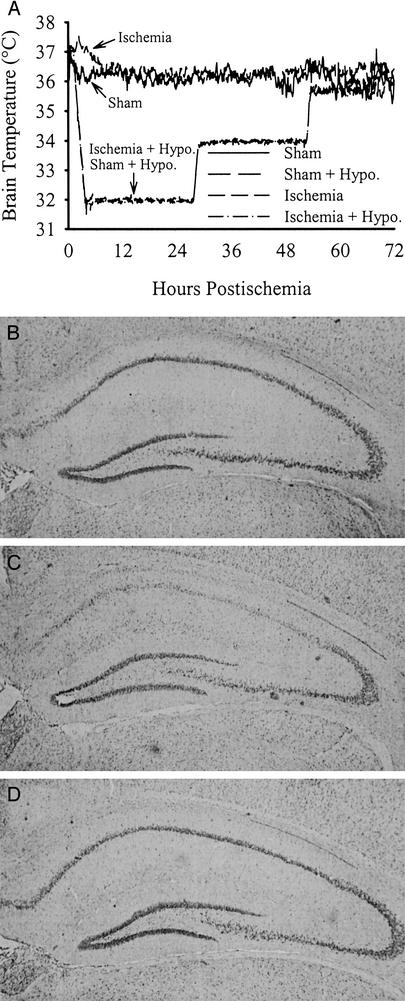
Figure 1.
(A) Brain temperature (averaged every 5 min, sampled every 30 s) in gerbils subjected to 5 min of forebrain ischemia or sham operation. (B) Representative photomicrograph of a normal gerbil's CA1 sector 7 days after sham operation. (C) The CA1 sector from a gerbil subjected to ischemia without treatment (7-day survival) illustrating near-total destruction of CA1 neurons with reactive gliosis evident. (D) Near-total CA1 neuronal survival at 7 days after ischemia treated with hypothermia delayed by 1 h. As discussed, postischemic hypothermia provides lasting protection to most CA1 neurons.
Hypothermia Prevents Ischemia-Induced Delayed Neurodegeneration in CA1.
Normothermic and hypothermia-treated sham groups had 362.2 ± 28.6 SD and 364.7 ± 23.1 CA1 cells total for the medial, middle, and lateral sectors at −1.7 mm to Bregma (Fig. 1B), respectively, and were combined for subsequent statistical analyses. The normothermic (Fig. 1C) and delayed hypothermia-treated ischemia groups (Fig. 1D) had 52.3 ± 89.2 (i.e., 14.4% of sham; P < 0.001 vs. sham) and 341.5 ± 73.3 (94.1% of sham; P = 0.791 vs. sham; P < 0.001 vs. untreated ischemic group) CA1 neurons remaining, respectively.
GluR2 and GluR1 mRNA Levels in Hippocampus After Ischemia.
Relative abundance of GluR2 and GluR1 mRNA was assessed by in situ hybridization and is reported as a ratio of the optical density in film autoradiograms for CA1/DG and CA3/DG (Figs. 2 and 3). In normothermic sham-operated animals, which were killed after 2 days, both GluR2 and GluR1 mRNAs were abundant in CA1, CA3, and DG (ratio of CA1/DG for GluR2: 1.13 ± 0.06 and for GluR1: 1.04 ± 0.06; ratio of CA3/DG for GluR2: 0.99 ± 0.05 and for GluR1: 1.07 ± 0.04). Hypothermia after sham operation did not affect CA1/DG ratios for GluR2 or GluR1 at 2 days (GluR2, 1.08 ± 0.12, P = 0.235; GluR1, 1.07 ± 0.05, P = 0.464) or 7 days (GluR2, 1.19 ± 0.06, P = 0.228; GluR1, 1.11 ± 0.04, P = 0.114). Therefore, data for GluR1 or GluR2 mRNA expression for the groups of sham-operated (control) animals were pooled.
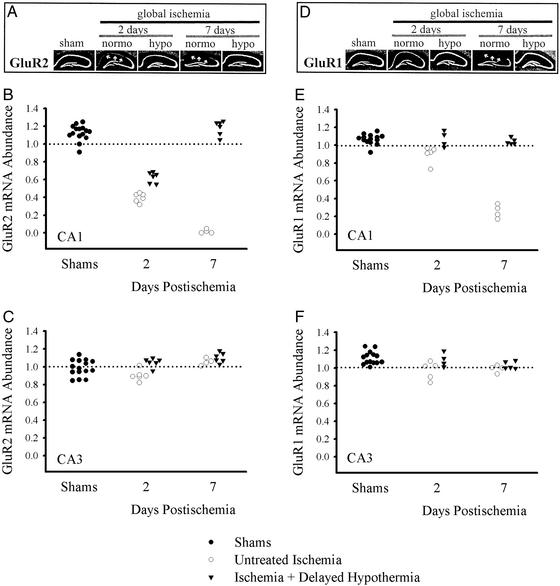
Figure 2.
Hypothermia significantly reduced ischemia-induced down-regulation of AMPA receptor mRNA in CA1 pyramidal neurons. (A) Film autoradiograms of GluR2 mRNAs detected by in situ hybridization in sham, ischemia, and ischemia plus hypothermia gerbils 2 and 7 days after ischemia. (B and C) Quantitative analysis of GluR2 mRNA expression in CA1 and CA3 is shown. Mean densities were reduced dramatically in CA1 after ischemia but not in CA3. Hypothermia treatment reduced the down-regulation of GluR2 at 2 and 7 days after ischemia. (D–F) GluR1 mRNA remained stable, although slightly reduced after ischemia, and recovered to control levels with hypothermia treatment. (D) Film autoradiograms of GluR1 mRNA expression in gerbils 2 and 7 days after sham operation, ischemia, and ischemia plus hypothermia. Quantitative analysis of GluR1 mRNA levels in CA1 and CA3 are shown in E and F, respectively. The decline in GluR1 at 2 days after untreated ischemia is much less than that for GluR2. Data are reported as a ratio of CA1/DG and CA3/DG as indicated in Methods.
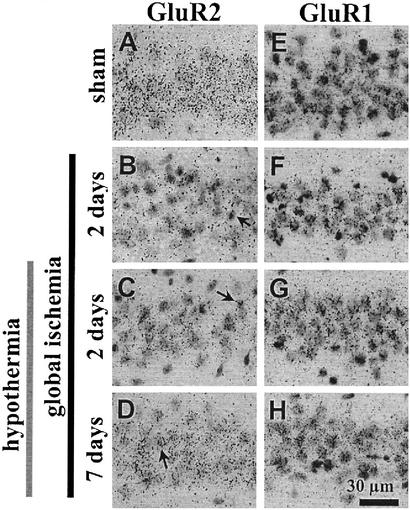
Figure 3.
Emulsion-dipped coronal sections of the hippocampal CA1 zone demonstrate GluR2 (A–D) and GluR1 (E–H) expression in pyramidal cells. (B–D) For GluR2, the number of silver grains overlying individual pyramidal cells in CA1 (arrows) was reduced markedly after ischemia (B), but with hypothermia treatment the reduction in labeling was reduced at 2 days (C) and recovery was marked at 7 days (D). (F–H) The modest down-regulation of GluR1 mRNA expression in CA1 2 days after untreated ischemia (F) was prevented by hypothermia (G and H).
Untreated forebrain ischemia induced a pronounced decrease in GluR2 mRNA expression in the CA1 sector at 2 days, which is before neuronal degeneration, (CA1/DG: 0.40 ± 0.05, P < 0.001 vs. pooled sham −1.13 ± 0.09, Figs. 2 A and B and 3B) and near total loss at 7 days, when almost all neurons have degenerated (CA1/DG: 0.02 ± 0.02, P < 0.001 vs. ischemia 2 days and shams). Compared to untreated ischemia, the decrease in GluR2 at 2 days in ischemic gerbils treated with delayed cooling was significantly attenuated (Figs. 2 A and B and 3C; CA1/DG: 0.63 ± 0.06; P < 0.001 vs. untreated ischemic animals at 2 days; P < 0.001 vs. combined shams), and the abundance of GluR2 had returned to normal by 7 days (Figs. 2 A and B and 3D; CA1/DG: 1.19 ± 0.08; P = 0.488 vs. combined sham groups).
Forebrain ischemia, followed by normothermia, induced a slight, but significant, decrease in GluR1 mRNA expression in the CA1 (CA1/DG) at 2 days (normothermic ischemia, 0.90 ± 0.09; shams, 1.07 ± 0.06, P = 0.001; Figs. 2 D and E and 3F). At 7 days, GluR1 mRNA expression in CA1 had declined to near background levels, consistent with essentially complete loss of CA1 neurons (0.26 ± 0.09, P < 0.001 vs. untreated ischemia and shams; Fig. 2 D and E). In contrast, in animals subjected to ischemia followed by hypothermia, GluR1 mRNA expression remained stable at sham levels in CA1, as assessed at 2 and 7 days (Figs. 2 D and E and 3 G and H; 2 days, 1.07 ± 0.09; 7 days, 1.05 ± 0.03; P ≥ 0.949 vs. combined shams).
Pyramidal neurons in CA3 survive brief forebrain ischemia that kills CA1 pyramidal cells. Indeed, there were no signs of cell death in CA3 or DG as expected with this model. There were no significant changes in CA3/DG ratios for GluR1 (means ranged from 0.97 to 1.15; P = 0.07; Fig. 2 D and F). There were only small group differences for GluR2 ratios of CA3/DG (Fig. 2 A and C) with the ischemia plus hypothermia 7-day group (1.11 ± 0.06) having a slightly but significantly (P = 0.026) larger ratio than shams (0.99 ± 0.09). The untreated ischemia 2-day group (0.90 ± 0.06) was not significantly (P = 0.177) different from the shams.
Discussion
Delayed and prolonged hypothermia is a highly effective neuroprotective strategy against global ischemia-induced behavioral deficits and neuronal death (21–24). Brief forebrain ischemia in gerbils induces delayed and selective death of CA1 pyramidal neurons; by 96 h, neuronal death is near total (Fig. 1; see refs. 6 and 24). Prolonged postischemic cooling, as presently used, rescued most CA1 neurons at a 7-day survival. Indeed, most CA1 neurons salvaged with early and prolonged hypothermia appear morphologically normal by ultrastructural examination (24) and survive for protracted periods (i.e., at a 6-mo survival; ref. 21). Although many processes undoubtedly contribute to CA1 neuronal death, accumulating evidence implicates Ca2+-permeable AMPARs as a key mediator (reviewed in ref. 6). The present study examined the molecular mechanisms underlying hypothermia-induced neuroprotection. The conclusions of our study are: (i) Ischemia induced a pronounced down-regulation of GluR2 mRNA expression and a small, but statistically significant, down-regulation of GluR1 mRNA expression in the hippocampal CA1 sector at 2 days, consistent with expression of GluR2-lacking, Ca2+-permeable AMPARs in CA1 neurons. (ii) Ischemia-induced changes in gene expression were cell-specific in that neither GluR1 nor GluR2 mRNA expression was significantly altered in CA3. (iii) In animals subjected to forebrain ischemia followed by prolonged cooling, GluR2 mRNA expression was modestly reduced at 2 days and exhibited complete recovery at 7 days. The results presented here suggest that prolonged postischemic cooling rescues neurons otherwise “destined to die,” at least in part by attenuating down-regulation of the GluR2 gene in these neurons, thereby limiting formation of Ca2+-permeable AMPARs.
Prolonged postischemic hypothermia affords near-total and persistent preservation of CA1 neurons (21–24) whereas brief hypothermia may not (28). As noted, prolonged hypothermia attenuated the decline in GluR2 mRNA expression and the small down-regulation of GluR1 at 2 days postischemia and promoted the full recovery of AMPAR mRNA expression by 7 days. In contrast, less efficacious neuroprotective strategies such as NBQX (3, 29) or brief hypothermia (28, 30), which do not afford permanent neuroprotection, do not attenuate or attenuate transiently the ischemia-induced alterations in gene expression. Indeed, Friedman et al. (30) report no effect of brief (3 h) postischemic hypothermia on ischemia-induced alterations in glutamate receptor gene expression.
Transcriptional Regulation of GluR2.
Recent studies (31, 32) reveal the presence of an RE1 (restrictive element-1) regulatory element within the proximal promoter region of the GluR2 gene and indicate that GluR2 gene expression is under control of REST, a gene silencing transcriptional factor, which binds the RE1 site and suppresses GluR2 gene expression in mammalian cells. Recent findings from our laboratory indicate that global ischemia activates a REST-initiated molecular signaling cascade that suppresses GluR2 transcription in CA1 neurons (33). Our findings are consistent with a model whereby the early rise in intracellular free Ca2+ triggered by the ischemic insult activates a Ca2+-dependent CREB/CaRE regulatory element in the proximal promoter of the REST gene (34) to induce REST mRNA expression. A likely point of intervention of hypothermia is inhibition of the REST-initiated molecular signaling cascade implicated in down-regulation of the GluR2 gene.
Specificity of Ischemia-Induced Alterations in Glutamate Receptor Expression.
We have reported selective suppression of AMPAR GluR2 subunit mRNA and protein expression (present study; refs. 7, 10, and 11) and enhanced AMPAR-mediated Ca2+-influx (11) in CA1 neurons before the onset of neuronal death. However, two groups report nonselective alterations in glutamate receptor gene expression after global ischemia. Friedman et al. (30) report nonselective down-regulation of mRNAs for GluR1, -2, and -3 and of the N-methyl-d-aspartate receptor subunit NR1 throughout the hippocampal CA1 and CA3 and (to a lesser extent) DG at 24 h after global ischemia in rats. Diemer and coworkers (35, 36) report similar nonselective down-regulation of GluR1, -2, and -3 mRNAs and proteins in CA1 and CA3 at 24 h after global ischemia in rats. Possible explanations for the discrepancies between these findings and those reported by us (present study; refs. 7, 10, and 11) are (i) early onset of neuronal death because of severity of the ischemic insult, and/or (ii) loss of mRNA and protein in tissue samples because of prolonged storage. Both groups used the relatively severe model of global ischemia involving a combination of systemic hypotension and bilateral common carotid artery occlusion (12 min). In addition, Diemer and coworkers (35) used another model involving a combination of hypotension and neck cuff inflation (15 min). Both of these models afford insults much greater than that required to selectively kill CA1 neurons with a delay of 2–4 days. Thus, the nonselective loss of GluR1–3 in the CA1 and CA3 subfields reported in these studies may reflect early onset of cell death at 24 h. However, these papers do not illustrate the time course of histologically detectable neuronal death.
Conclusions
Forebrain ischemia in gerbils induced a marked reduction in GluR2 mRNA expression, with only a very modest reduction in GluR1 mRNA expression, as shown previously (7–9, 11) and consistent with a role for Ca2+-permeable AMPARs in ischemia-induced neuronal death. Prolonged postischemic hypothermia significantly attenuated the decline in GluR2 mRNA and promoted its full recovery. Changes to GluR1 were completely prevented. Thus, these data suggest that delayed and prolonged hypothermia reduces ischemic CA1 damage through reducing the formation of modified GluR2-lacking AMPARs, which otherwise leads to influx of toxic levels of Ca2+ and possibly Zn2+. Further studies delineating the mechanisms by which prolonged postischemic hypothermia affects GluR2 subunit alterations and downstream events are warranted.
Acknowledgments
This work was supported by National Institutes of Health Grants NS31282 (to R.S.Z.) and NS-05712 (to M.V.L.B.), the Heart and Stroke Foundation of Canada (to A.M.B.), and the Alberta Heritage Foundation for Medical Research (to F.C.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA-type glutamate receptor
- DG
dentate gyrus
References
- 1.Kirino T. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 2.Pulsinelli W A, Brierley J B, Plum F. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 3.Colbourne F, Li H, Buchan A M. Stroke. 1999;30:662–668. doi: 10.1161/01.str.30.3.662. [DOI] [PubMed] [Google Scholar]
- 4.Choi D W. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 5.Pellegrini-Giampietro D E, Gorter J A, Bennett M V L, Zukin R S. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Grooms S Y, Bennett M V L, Zukin R S. Brain Res. 2000;886:190–207. doi: 10.1016/s0006-8993(00)02951-6. [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini-Giampietro D E, Zukin R S, Bennett M V L, Cho S, Pulsinelli W A. Proc Natl Acad Sci USA. 1992;89:10499–10503. doi: 10.1073/pnas.89.21.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard H, Heron A, Moreau J, Ben-Ari Y, Khrestchatisky M. Neuroscience. 1993;57:545–554. doi: 10.1016/0306-4522(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 9.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Brain Res. 1994;659:67–74. doi: 10.1016/0006-8993(94)90864-8. [DOI] [PubMed] [Google Scholar]
- 10.Opitz T, Grooms S Y, Bennett M V L, Zukin R S. Proc Natl Acad Sci USA. 2000;97:13360–13365. doi: 10.1073/pnas.97.24.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorter J A, Petrozzino J J, Aronica E M, Rosenbaum D M, Opitz T, Bennett M V L, Connor J A, Zukin R S. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsubokawa H, Oguro K, Robinson H P, Masuzawa T, Kirino T, Kawai N. Neuroscience. 1992;49:807–817. doi: 10.1016/0306-4522(92)90358-9. [DOI] [PubMed] [Google Scholar]
- 13.Tsubokawa H, Oguro K, Masuzawa T, Kawai N. J Neurophysiol. 1994;71:1190–1196. doi: 10.1152/jn.1994.71.3.1190. [DOI] [PubMed] [Google Scholar]
- 14.Tsubokawa H, Oguro K, Masuzawa T, Nakaima T, Kawai N. J Neurophysiol. 1995;74:218–225. doi: 10.1152/jn.1995.74.1.218. [DOI] [PubMed] [Google Scholar]
- 15.Hollmann M, Hartley M, Heinemann S. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 16.Verdoorn T A, Burnashev N, Monyer H, Seeburg P H, Sakmann B. Science. 1991;252:1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]
- 17.Bowie D, Mayer M L. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 18.Donevan S D, Rogawski M A. Proc Natl Acad Sci USA. 1995;92:9298–9302. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson G T, Kamboj S K, Cull-Candy S G. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oguro K, Oguro N, Kojima T, Grooms S Y, Calderone A, Zheng X, Bennett M V L, Zukin R S. J Neurosci. 1999;19:9218–9227. doi: 10.1523/JNEUROSCI.19-21-09218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colbourne F, Corbett D. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbourne F, Li H, Buchan A M. J Cereb Blood Flow Metab. 1999;19:742–749. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Colbourne F, Auer R N, Sutherland G. Brain Res. 1998;803:69–78. doi: 10.1016/s0006-8993(98)00612-x. [DOI] [PubMed] [Google Scholar]
- 24.Colbourne F, Sutherland G R, Auer R N. J Neurosci. 1999;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernard S, Gray T, Buist M, Jones B, Silvester W, Gutteridge G, Smith K. N Engl J Med. 2002;346:557–613. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 26.The Hypothermia After Cardiac Arrest Study Group. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 27.Colbourne F, Sutherland G R, Auer R N. J Neurosci Methods. 1996;67:185–190. [PubMed] [Google Scholar]
- 28.Dietrich W D, Busto R, Alonso O, Globus M Y-T, Ginsberg M D. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 29.Nurse S, Corbett D. J Cereb Blood Flow Metab. 1996;16:474–480. doi: 10.1097/00004647-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Friedman L K, Ginsberg M D, Belayev L, Busto R, Alonso O F, Lin B, Globus M Y-T. Mol Brain Res. 2001;86:34–47. doi: 10.1016/s0169-328x(00)00252-7. [DOI] [PubMed] [Google Scholar]
- 31.Palm K, Belluardo N, Metsis M, Timmusk T. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers S J, Peters J, Huang Y, Comer M B, Barthel F, Dingledine R. J Neurosci. 1998;18:6723–6739. doi: 10.1523/JNEUROSCI.18-17-06723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calderone, A., Jover, T., Noh, K.-M., Tanaka, H., Yokota, H., Grooms, S. Y., Regis, R., Bennett, M. V. L. & Zukin, R. S. (2003) J. Neurosci., in press. [DOI] [PMC free article] [PubMed]
- 34.Koenigsberger C, Chicca J J, II, Amoureux M C, Edelman G M, Jones F S. Proc Natl Acad Sci USA. 2000;97:2291–2296. doi: 10.1073/pnas.050578797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank L, Diemer N H, Kaiser F, Sheardown M, Rasmussen J S, Kristensen P. Acta Neurol Scand. 1995;92:337–343. doi: 10.1111/j.1600-0404.1995.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 36.Kjoller C, Diemer N H. Neurochem Int. 2000;37:7–15. doi: 10.1016/s0197-0186(00)00008-5. [DOI] [PubMed] [Google Scholar]