Abstract
In addition to neurological deficits, Huntington's disease (HD) patients and transgenic mice expressing mutant human huntingtin exhibit reduced levels of brain-derived neurotrophic factor, hyperglycemia, and tissue wasting. We show that the progression of neuropathological (formation of huntingtin inclusions and apoptotic protease activation), behavioral (motor dysfunction), and metabolic (glucose intolerance and tissue wasting) abnormalities in huntingtin mutant mice, an animal model of HD, are retarded when the mice are maintained on a dietary restriction (DR) feeding regimen resulting in an extension of their life span. DR increases levels of brain-derived neurotrophic factor and the protein chaperone heat-shock protein-70 in the striatum and cortex, which are depleted in HD mice fed a normal diet. The suppression of the pathogenic processes by DR in HD mice suggests that mutant huntingtin promotes neuronal degeneration by impairing cellular stress resistance, and that the body wasting in HD is driven by the neurodegenerative process. Our findings suggest a dietary intervention that may suppress the disease process and increase the life span of humans that carry the mutant huntingtin gene.
Keywords: apoptosis‖diabetes‖Huntington's disease‖striatum
Huntington's disease (HD) is an inherited neurodegenerative disorder characterized by degeneration of neurons in the striatum and cerebral cortex resulting in abnormal involuntary movements (chorea), and psychiatric and cognitive abnormalities (1). The genetic defect involves expansion of CAG trinucleotide repeats in exon 1 of the HD gene resulting in polyglutamine expansions in the huntingtin protein (2–4). Neither the normal function of huntingtin nor the mechanism whereby polyglutamine expansions result in selective loss of striatal neurons is known, although impaired energy metabolism (5, 6), excitotoxicity (7), and oxidative stress (8) are implicated. It has been proposed that the mutant huntingtin causes neuronal dysfunction and death by altering the transcription of certain genes, including those encoding neurotransmitters and neurotrophic factors (9). Transgenic mice expressing polyglutamine expanded full-length or N-terminal fragments of huntingtin exhibit neurodegenerative changes in the striatum, progressive motor dysfunction, and premature death (10, 11). Oxidative stress and apoptosis are suggested in the pathogenic process because antioxidants (12) and caspase inhibitors (13) can slow disease progression in huntingtin mutant mice.
Deficits in striatal and cortical glucose metabolism precede the appearance of symptoms in HD patients (14–16). Many HD patients and huntingtin mutant mice also exhibit hyperglycemia, apparently as the result of decreased insulin production and/or sensitivity (17, 18). A deficit in cellular energy metabolism may contribute to disease onset and progression because administration of creatine, an agent that reduces ATP depletion, delays the onset of symptoms and increases the survival times of huntingtin mutant mice (19). Another alteration in HD is decreased production of brain-derived neurotrophic factor (BDNF) (20, 21), a neurotrophin that increases the resistance of neurons to metabolic, excitotoxic, and oxidative insults (22, 23) and can protect striatal neurons in animal models relevant to HD (24–26).
The alterations in energy metabolism and the deficit in BDNF levels in HD led us to test the hypothesis that dietary restriction (DR) during adult life might be effective in delaying the onset and progression of HD. DR increases the life span of normal mice, rats, and monkeys (27), improves glucose regulation by increasing insulin sensitivity (28), reduces levels of oxidative stress in various tissues including the brain (29), and enhances cellular stress resistance (30, 31). Recent studies have shown that DR can increase the resistance of neurons to excitotoxic, metabolic, and oxidative insults (32–34), processes relevant to the neurodegenerative cascades of HD. Moreover, levels of BDNF are increased in the striatum, cerebral cortex and hippocampus of rats and mice maintained on a DR diet (23, 35). Here, we show that, when huntingtin mutant mice are maintained on an intermittent fasting DR regimen, their glucose regulation and brain BDNF levels are normalized, the onset of motor dysfunction is delayed, and they live longer.
Materials and Methods
Mice and Behavioral Testing.
Breeding pairs of HD-N171-82Q mice were kindly provided by D. R. Borchelt (Johns Hopkins University, Baltimore). These mice express a human N-terminal truncated huntingtin with 82 polyglutamine repeats driven by a mouse prion protein promoter (11). Transgenic mice were mated continuously to hybrid (C3H/HEJxC57BL/6J F1; Taconic Farms) mice, and lines were maintained on the hybrid background. Transgenic mice were identified by PCR analysis of tail DNA. Eight-wk-old HD-N171-82Q (+/−, HD) mice and nontransgenic mice were fed ad libitum (AL) or were maintained on a DR regimen in which they were fasted every other day. Disease progression and survival status were monitored daily; the first day on which limb tremors were detected was designated the day of disease onset. Mice were scored by a trained observer blind to the genotype and age of the mice. Body weight and food intake were recorded weekly. Motor function was evaluated, and blood glucose levels were measured every 4 wk. Motor performance was assessed with a rotary rod apparatus by using a protocol similar to that described (36, 37). The rotadrum was filled with water to a level just below the bottom of the rod; in preliminary studies, we found that the presence of the water increases the motivation of the mouse to stay on the rod. The mice were placed on the rotating rod, and the time until they fell off was recorded. This procedure was repeated (with a rest period that increased by 5 s with each fall) until the total time on the rod was 5 min. Both the total time spent on the rotating rod and the total number of falls for each mouse were recorded. These procedures were approved by the institutional Animal Care and Use Committee of the National Institute on Aging.
Measurements of Blood Glucose, Insulin, and Leptin Levels, and of Brain BDNF Levels.
Mice were fasted overnight, and blood samples were collected by venipuncture. Blood glucose concentrations were measured by using a glucometer (LifeScan, Mountain View, CA). The glucose tolerance test was performed on mice after an overnight fast: mice were given 2 mg of d-glucose per gram body weight orally, and the blood glucose concentration was measured in samples taken 0, 30, 60, and 120 min after glucose administration. Insulin levels were determined in duplicate in 10-μl serum samples by using a mouse insulin ELISA kit (ALPCO Diagnostics, Windham, NH) according to the manufacturer's protocol. Serum leptin levels were determined in duplicate by using 100 μl of serum diluted in saline (1:4). The assay was performed by using a leptin EIA kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer's protocol. Assays for insulin and leptin were performed in 96-well plates, and absorbance was measured at 450 nm with reference at 650 nm by using a microplate scanning spectrophotometer (PowerWave-X, Bio-Tek, Burlington, VT). Levels of BDNF in brain tissue were quantified by using an ELISA method (Promega) described (23). The concentrations of BDNF in each sample were determined in triplicate, and the average of the three values was used as the value for that mouse. Values are expressed as pg of BDNF per mg of protein.
Immunoblot and Immunostaining Methods.
These methods were similar to those described (33). Briefly, 50 μg of solubilized proteins were separated by SDS/PAGE and transferred to a nitrocellulose membrane. The membrane was incubated overnight at 4°C in the presence of 5% nonfat milk, and then incubated for 2 h with a primary antibody (mouse monoclonal anti-HSP-70, 1:5,000, Sigma; mouse monoclonal anti-caspase-1, 1:1,000, PharMingen; and mouse monoclonal anti-β actin, 1:5,000, Sigma). The membrane was then exposed for 1 h to horseradish peroxidase-conjugated secondary antibody (1:3,000; Jackson ImmunoResearch), and immunoreactive proteins were visualized by using a chemiluminescence-based detection kit according to the manufacturer's protocol (ECL kit; Amersham Pharmacia). Densitometric analyses of relative protein band intensities were performed by using nih image 1.62 software. For immunostaining of mutant human N-terminal fragment of huntingtin, mice were perfused through the ascending aorta with saline, followed by 4% paraformaldehyde in PBS (pH 7.4). Fixed brains were cryoprotected in a 30% sucrose solution, coronal brain sections (30 μm) were cut on a freezing microtome, and free-floating sections were preblocked in 5% normal horse serum in PBS containing 0.2% Triton X-100. The sections were incubated with mouse monoclonal antibody mEM48 (1:500) at room temperature for 24 h, then incubated with biotinylated anti-mouse IgG for 1 h at room temperature. The immunoreactive product was detected by using a biotinylated secondary antibody, peroxidase-labeled avidin, and diaminobenzadine.
Results
Dietary Restriction Delays Disease Onset and Increases Survival of Huntingtin Mutant Mice.
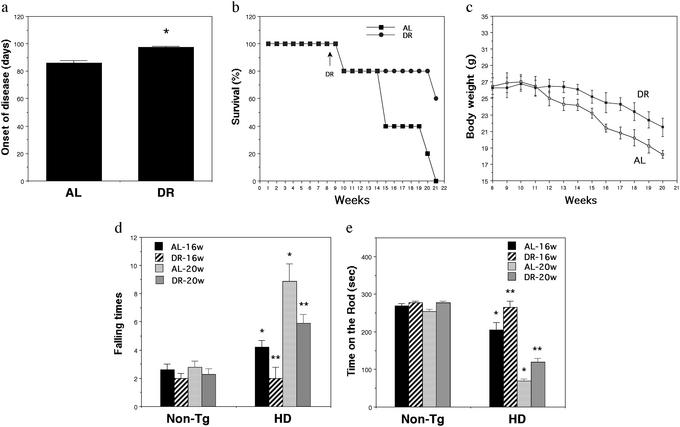
Beginning at 8 wk of age, HD mice were divided into two groups (15 mice per group); one group of mice was fed AL, and the other group was maintained on an intermittent fasting DR regimen in which they were deprived of food for a 24-h period every other day. Previous studies have shown that the same DR regimen extends lifespan in WT mice (38) and increases the resistance of their hippocampal neurons to excitotoxic injury (23). The onset of behavioral symptoms was significantly delayed by an average of 12 days in the HD mice on DR (Fig. 1a). DR significantly extended survival of HD mice. Whereas all of the mice fed AL had died by 21 wk of age, only 40% of the HD mice maintained on DR had died (Fig. 1b). The DR regimen increased the survival of HD mice by ≈2 wk, an amount similar to the delay in the time to onset of motor dysfunction. Previous studies have shown that HD mice exhibit a progressive weight loss (10, 11, 13). Surprisingly, HD mice maintained on the DR feeding regimen lost less weight than did HD mice fed AL (Fig. 1c).
Figure 1.
DR delays the onset of motor dysfunction, reduces weight loss, and increases the survival of HD N171-82 Q mice. (a) Eight-week-old male HD mice were maintained on an alternative day fasting regimen (DR) or an AL feeding regimen for 3 mo. Onset of disease was scored as the first date when limb tremors were detected. Values are the mean and SE (n = 12 mice per group); *, P < 0.001 (paired t test). (b) Survival plots for HD mice maintained on AL or DR diets. (c) Body weights for HD mice maintained on AL or DR diets. Values are the mean and SE (12 mice per group). (d and e) Motor behavioral performance was evaluated by using a rotarod apparatus in 16- and 20-wk-old nontransgenic (Non-Tg) and HD mice maintained on AL or DR diets. The number of times the mouse fell from the rod (d) and the total time spent on the rod (e) during a 5-min period were recorded. Values are the mean and SE (n = 6–15 mice per group). *, P < 0.01, compared with the non-Tg AL value; **, P < 0.01 compared with the HD AL value (ANOVA with Scheffé post hoc tests).
We next evaluated motor function in 16- and 20-wk-old HD and nontransgenic mice that had been on AL or DR feeding regimens beginning at 8 wk of age. There was a progressive impairment in performance of HD mice on the rotarod apparatus (increased numbers of falls and reduced time spent on the rotarod) compared with nontransgenic mice (Fig. 1 d and e). HD mice maintained on DR exhibited highly significant improvements in motor performance.
Dietary Restriction Reduces Brain Atrophy, Huntingtin Aggregate Formation, and Caspase Activation.
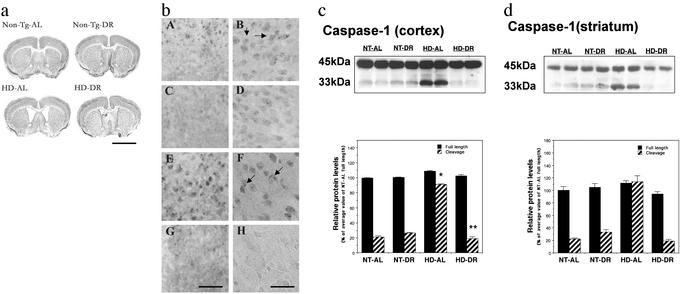
To determine whether the improved motor function and increased survival of HD mice maintained on DR resulted from a slowed progression of the neurodegenerative process in the brain, we performed histological and biochemical analyses of the brains of HD and nontransgenic mice that had been maintained on AL or DR diets. As disease progressed, brain atrophy occurred in the HD mice as indicated by an increase in the size of the lateral ventricles and a thinning of the cerebral cortex compared with nontransgenic mice (Fig. 2a). The magnitude of ventricular enlargement was significantly decreased in HD mice that had been maintained on DR. Intranuclear inclusions of aggregated huntingtin protein in striatal and cortical neurons are present in HD patients and in huntingtin mutant mice (10, 11). We found that the number of neurons exhibiting such inclusions was significantly decreased in HD mice that had been maintained on DR (Fig. 2b).
Figure 2.
DR reduces brain atrophy, decreases huntingtin aggregate formation, and inhibits caspase-1 activation in HD mice. (a) Photomicrographs of brain sections of 20-wk-old HD mice and nontransgenic mice (Non-Tg) that had been maintained on AL or DR regimens for 3 mo. Brain sections were stained with cresyl violet. The enlargement of lateral ventricles in HD mice is reduced in HD mice maintained on DR. Scale bar: 4 mm. The examples presented are representative of all six mice examined in each group. (b) Brain sections from 20-wk-old mice were immunostained with EM-48 antibody, which recognizes mutant human huntingtin N-terminal fragment. Intranuclear inclusions of mutant huntingtin (arrows) were abundant in the cortex (bA and bB) and striatum (bE and bF) of HD mice fed AL. The number of cells with intranuclear inclusions was decreased in the cortex (bC and bD) and striatum (bG and bH) of HD mice that had been maintained on DR compared with those fed AL. Scale bars: 125 μm in bG (bA, bC, and bE), 50 μm in bH (bB, bD, and bF). The results presented are representative of all six mice examined in each group. (c and d) Immunoblots of proteins in samples of cortical and striatal tissue (50 μg per lane) from the indicated genotype and diet groups. (Upper) Representative blots with caspase-1 antibody, which recognize full length of caspase-1 (45 kDa) and cleavage product (33 kDa) in cortex (c) and striatum (d). (Lower) Densitometric analysis results of blots (n = 4 mice per group). *, P < 0.01 compared with the value for nontransgenic (NT)-AL 33-kDa cleavage product; **, P < 0.01 compared with the value for the HD-AL 33-kDa cleavage product. ANOVA with Scheffé post hoc tests.
Striatal neurons may die by a form of programmed cell death called apoptosis, and studies of cultured cells and transgenic mice expressing mutant huntingtin have provided evidence that the mutant protein can trigger an apoptotic cascade involving activation of caspases (39). We performed immunoblot analyses of caspase-1 proteins in lysates of cerebral cortex and striatum of nontransgenic and HD mice that had been maintained on DR or AL diets. There was a marked increase in the amount of the 33-kDa caspase-1 cleavage product in samples for striatum and cortex from HD mice fed AL compared with HD mice on DR, and to nontransgenic mice on either AL or DR diets (Fig. 2 c and d). The latter result suggests that there is a much greater amount of activated caspase-1 in striatal and cortical cells of HD mice, and that DR can suppress this caspase activation.
Dietary Restriction Normalizes Blood Glucose Regulation in Huntingtin Mutant Mice.
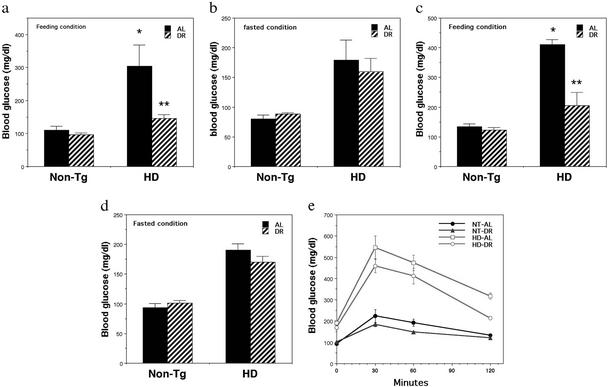
One widely documented physiological effect of DR is to increase insulin sensitivity as indicated by reduced blood glucose and insulin levels. Because many HD patients and huntingtin mutant mice are hyperglycemic, we determined whether this metabolic abnormality could be ameliorated by DR in the HD mice. HD mice exhibited large 2- to 3-fold increases in levels of blood glucose compared with nontransgenic mice, which were evident after feeding, as well as after an overnight fast (Fig. 3 a–d). HD mice that had been maintained on DR exhibited reduced blood glucose levels after feeding, but not after an overnight fast, compared with HD mice that had been maintained on an AL diet. Similar abnormalities in blood glucose levels, and normalization by DR, was observed in 16- and 20-wk-old mice (Fig. 3 a–d). We next performed glucose tolerance tests in nontransgenic and HD mice that had been maintained on AL or DR diets. HD mice that had been maintained on either diet exhibited a much greater peak elevation of blood glucose levels after an oral bolus of glucose compared with nontransgenic mice (Fig. 3e). However, the peak blood glucose level was lower, and the recovery toward baseline more rapid in the HD mice that had been maintained on DR, compared with the HD mice that had been fed AL.
Figure 3.
DR ameliorates hyperglycemia in HD N171-82Q mice. Eight-week-old male HD mice and nontransgenic control mice (Non-Tg) were maintained on DR or AL feeding regimens for 3 mo. Blood glucose levels were measured either under feeding conditions (a and c) or after an overnight fast (b and d) in 16-wk-old mice (a and b) and 20-wk-old mice (c and d). The values are the mean and SE of determinations made in six mice. *, P < 0.01 compared with the value of nontransgenic (NT)-AL group. **, P < 0.01 compared with the value of HD-AL group. ANOVA with Scheffé post hoc tests. (e) Mice were fasted overnight and then administered d-glucose orally (2 g/kg body weight); blood samples were taken before and at 30, 60, and 120 min after glucose administration. The values are the mean and SE of determinations made in six mice per group.
To further examine the effects of mutant huntingtin and diet on energy regulation, we measured levels of insulin and leptin in serum samples from nontransgenic and HD mice that had been maintained on AL or DR diets. As expected, insulin levels were significantly reduced in nontransgenic mice that had been maintained on DR (Table 1). Surprisingly, insulin levels in HD mice fed AL were lower than levels in nontransgenic mice fed AL, and DR had no significant effect on insulin levels in the HD mice. Serum leptin levels were slightly elevated in HD mice compared with nontransgenic mice fed AL. Leptin levels significantly decreased in nontransgenic mice on the DR diet, but were not significantly decreased in HD mice on the DR diet (Table 1).
Table 1.
Serum insulin and leptin levels in nontransgenic and HD mice maintained on AL or DR diets
| Group | Insulin, ng/ml | Leptin, ng/ml |
|---|---|---|
| NT-AL | 0.503 ± 0.015 | 0.605 ± 0.121 |
| NT-DR | 0.338 ± 0.012* | 0.296 ± 0.051* |
| HD-AL | 0.393 ± 0.010* | 0.854 ± 0.158 |
| HD-DR | 0.454 ± 0.030 | 0.607 ± 0.116** |
Nontransgenic mice (NT) and HD mice were maintained on an alternative- day feeding regimen (DR) or AL feeding regimen for 3 mo. Serum insulin and leptin levels were measured by ELISA.
, P < 0.01 compared to the values for the NT-AL group.
, P < 0.5 compared with the NT-DR group. ANOVA with Scheffe post hoc tests.
Deficits in Brain BDNF and Protein Chaperone Levels in Huntingtin Mutant Mice Are Restored by Dietary Restriction.
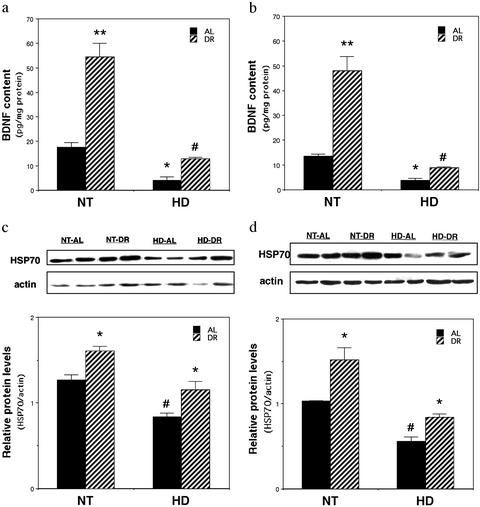
Levels of BDNF are decreased in the brains of HD patients and in huntingtin mutant mice (20, 21). We therefore determined whether DR would normalize BDNF levels in the brains of HD mutant mice. Levels of BDNF protein, measured by ELISA analysis, were increased 3- to 4-fold in the striatum and cerebral cortex of nontransgenic mice that had been maintained on DR for 3 months (Fig. 4 a and b). BDNF levels were significantly decreased, by 70–80% in the striatum and cortex of HD mice fed AL compared with nontransgenic mice fed AL. Levels of BDNF were significantly increased by 3- to 4-fold in HD mice maintained on DR compared with HD mice fed AL (Fig. 4 a and b).
Figure 4.
DR normalizes BDNF levels and up-regulates HSP-70 protein levels in brains of HD N171-82Q mice. Eight-week-old male HD mice and nontransgenic mice (NT) were maintained on DR or AL feeding regimens for 3 mo. (a and b) BDNF protein levels were measured in striatal (a) and cortical (b) tissues by ELISA. The values are the mean and SE, n = 6. *, P < 0.01, **, P < 0.001 compared with value for NT-AL; #, P < 0.01 compared with the value for HD-AL. ANOVA with Scheffé post hoc tests. (c and d) HSP-70 protein levels were measured in striatal (c) and cortical (d) tissues by immunoblot analysis. (Upper) Representative immunoblots. (Lower) The densitometric analysis of samples from four different mice per group. The values are the mean and SE. *, P < 0.01 compared with the value for the corresponding AL group; #, P < 0.01 compared with the value for the NT-AL group. ANOVA with Scheffé post hoc tests.
Beneficial effects of DR have been associated with increased production of protein chaperones such as heat-shock protein-70 (HSP-70) in several tissues including the brain (33, 34, 40). Immunoblot analyses of protein samples from the striatum and cerebral cortex of nontransgenic and HD mice that had been maintained for 3 mo on AL or DR diets revealed reduced levels of HSP-70 in striatum and cortex of AL-fed HD mice compared with AL-fed nontransgenic mice (Fig. 4 c and d). Levels of HSP-70 were significantly increased in the striatum and in the cortex of nontransgenic and HD mice maintained on DR compared with mice fed AL. Thus, DR increases levels of two neuroprotective proteins, BDNF and HSP-70, in HD mice.
Discussion
Intermittent fasting suppressed neuropathological alterations and motor dysfunction, and increased the survival of huntingtin mutant mice. The onset of motor dysfunction and the survival of the HD mice were increased by ≈2 wk. It will therefore be important to determine the maximum amount of time that disease can be delayed and survival increased in HD mice by using DR regimens. Previous studies have suggested two ways in which DR extends lifespan and protects against age-related disease: it reduces mitochondrial free radical production (27) and it increases cellular stress resistance (31). The decreased levels of BDNF and HSP-70 in the brains of HD mice suggest a reduced ability of neurons to cope with stress in HD. The increased levels of BDNF and HSP-70 that occurred in HD mice maintained on DR suggest a plausible mechanism whereby DR reduced brain atrophy and apoptosis in the mice. Indeed, previous studies have shown that BDNF (25, 26) and HSP-70 (41, 42) can protect neurons against excitotoxic and oxidative insults in models relevant to HD. Neuroprotective effects of BDNF may result from induction of the expression of antiapoptotic Bcl-2 family members (43) and antioxidant enzymes (44). The importance of BDNF in neuroprotection and enhancement of neurogenesis in mice maintained on DR was recently demonstrated in studies that used BDNF-neutralizing antibodies and BDNF-deficient mice (23, 45). A direct interaction of HSP-70 with mutant huntingtin has been demonstrated (46), which could be a mechanism whereby this protein chaperone protects neurons.
The abnormalities in the regulation of energy metabolism in some HD patients and huntingtin mutant mice may be the result of adverse effects of the mutant huntingtin protein in brain cells. We found that BDNF levels are decreased in the cerebral cortex and striatum of huntingtin mutant mice, and that DR simultaneously increases BDNF levels and improves glucose tolerance in the HD mice. Recent findings suggest that BDNF signaling in the brain plays an important role in regulating glucose metabolism. Intraventricular administration of BDNF reduces blood glucose levels in diabetic mice (47), and conditional deletion of BDNF from the brains of postnatal mice results in increased plasma levels of glucose, insulin and leptin and obesity (48). It is not known how BDNF signaling in the CNS regulates peripheral glucose metabolism, but actions on the sympathetic nervous system and/or hypothalamus are likely given the role of these systems in regulating energy metabolism (49) and their responsiveness to BDNF (50, 51). Interestingly, although blood glucose levels were greatly increased in HD mice, and DR normalized their blood glucose levels, DR did not decrease insulin and leptin levels in the HD mice as it did in nontransgenic mice. The restoration of glucose levels by DR without significant reductions in insulin and leptin levels reveals a novel feature of energy dyshomeostasis in HD mice.
HD patients typically undergo progressive weight loss over an extended time period, which is associated with a hypermetabolic state in which they cannot maintain weight even with increased calorie intake (52), and increased food intake does not improve symptoms or survival of HD patients (53). An intriguing feature of the huntingtin mutant mice identified in the present study is that they lose less weight when maintained on a DR diet than they do when fed AL. This seemingly paradoxical result might be explained by the ability of DR to suppress the pathogenic process that underlies tissue wasting in these mice. The hyperglycemic state of the huntingtin mutant mice was normalized by DR, suggesting an improved ability to use glucose. However, additional data in our study suggest that the abnormality in energy metabolism in HD mice is not the same as the insulin resistance syndrome that occurs in type 2 diabetes. Non insulin-dependent diabetics exhibit elevated levels of glucose, insulin, and leptin. In contrast, the HD mice had low insulin levels despite their being hyperglycemic; leptin levels were slightly increased in the HD mice. The reason for this unusual metabolic profile remains to be established. Analysis of another line of huntingtin mutant mice (R6/2) revealed reduced plasma insulin levels and marked reductions in levels of insulin and glucagons in pancreatic islet cells (13), suggesting that a deficit in insulin production may account for their hyperglycemic state. Our findings suggest that the ability of DR to reduce brain pathology and increase the survival times of HD mice is due mainly to its beneficial effects on neurons, such as increasing BDNF and HSP-70 levels. In support of the latter conclusion, it was reported that, in a line of huntingtin mutant mice in which only some individuals develop diabetes, there was no correlation between diabetes and motor impairment or survival (54).
Recent studies have shown that dietary supplementation with creatine (19) and behavioral enrichment (55) can reduce pathology and increase the survival of HD mice. However, the magnitude of the beneficial effects of the DR regimen used in the present study was considerably greater than that achieved with creatine or behavioral enrichment. The robustness of the effects of DR in this mouse model of HD is consistent with its remarkable ability to increase lifespan by 30–50% (27, 38). HD is an inherited disorder in which individuals who will almost surely develop the disease can be identified early in life before the development of symptoms (56). Our findings therefore suggest that a DR regimen might be effective in delaying disease onset and increasing the lifespan of those individuals that inherit the mutant huntingtin gene.
Acknowledgments
We thank Dr. D. R. Borchelt for providing breeding pairs of huntingtin mutant mice and for critical comments on the manuscript.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- DR
dietary restriction
- HD
Huntington's disease
- HSP-70
heat-shock protein-70
- AL
ad libitum
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Reiner A, Albin R L, Anderson K D, D'Amato C J, Penney J B, Young A B. Proc Natl Acad Sci USA. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew S E, Goldberg Y P, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman M A, et al. Nat Genet. 1993;7:513–519. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 3.Benitez J, Fernandez E, Garcia Ruiz P, Robledo M, Ramos C, Yebenes J. Hum Genet. 1994;94:563–564. doi: 10.1007/BF00211028. [DOI] [PubMed] [Google Scholar]
- 4.Brandt J, Bylsma F W, Gross R, Stine O C, Ranen N, Ross C A. Neurology. 1996;46:527–531. doi: 10.1212/wnl.46.2.527. [DOI] [PubMed] [Google Scholar]
- 5.Gu M, Gash M T, Mann V M, Javoy-Agid F, Cooper M, Schapira H V. Ann Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins B G, Koroshetz W J, Beal M F, Rosen BR. Neurology. 1993;43:2689–2695. doi: 10.1212/wnl.43.12.2689. [DOI] [PubMed] [Google Scholar]
- 7.Beal M F, Kowall N W, Ellison D W, Mazurek M F, Swartz K J, Martin J B. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- 8.Nakao N, Brundin P. Neuroscience. 1997;76:749–761. doi: 10.1016/s0306-4522(96)00223-0. [DOI] [PubMed] [Google Scholar]
- 9.Cha J H. Trends Neurosci. 2000;23:387–392. doi: 10.1016/s0166-2236(00)01609-x. [DOI] [PubMed] [Google Scholar]
- 10.Davies S W, Turmaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 11.Schilling G, Becher M W, Sharp A H, Jinnah H A, Duan K, Kotzuki J A, Slunt H H, Ratovitski T, Cooper J K, Jenkins N A, et al. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 12.Schilling G, Coonfield M L, Ross C A, Borchelt D R. Neurosci Lett. 2001;315:149–153. doi: 10.1016/s0304-3940(01)02326-6. [DOI] [PubMed] [Google Scholar]
- 13.Ona V O, Li M, Vonsattel J P, Andrews L J, Khan S Q, Chung W M, Frey A S, Menon A S, Li X J, Stieg P E, et al. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- 14.Antonini A, Leenders K L, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, Sanchez-Pernaute R, de Yebenez J G, Boesiger P, Weindl A, Maguire R P. Brain. 1996;119:2085–2095. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 15.Kuwert T. J Neurol. 1993;241:31–36. doi: 10.1007/BF00870669. [DOI] [PubMed] [Google Scholar]
- 16.Martin W R, Clark C, Ammann W, Stoessl A J, Shtybel W, Hayden M R. Neurology. 1992;42:223–229. doi: 10.1212/wnl.42.1.223. [DOI] [PubMed] [Google Scholar]
- 17.Farrer L. Clin Genet. 1985;27:62–67. doi: 10.1111/j.1399-0004.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 18.Hurlbert M S, Zhou W, Wasmeier C, Kaddis F G, Hutton J C, Freed C R. Diabetes. 1999;48:649–651. doi: 10.2337/diabetes.48.3.649. [DOI] [PubMed] [Google Scholar]
- 19.Andreassen O A, Dedeoglu A, Ferrante R J, Jenkins B G, Ferrante K L, Thomas M, Friedlich A, Browne S E, Schilling G, Borchelt D R, et al. Neurobiol Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- 20.Ferrer I, Goutan E, Marin C, Rey M J, Ribalta T. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 21.Zuccato C, Ciammola A, Rigamonti D, Leavitt B R, Goffredo D, Conti L, MacDonald M E, Friedlander R M, Silani V, Hayden M R, et al. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 22.Cheng B, Mattson M P. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- 23.Duan W, Guo Z, Mattson M P. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- 24.Nakao N, Kokaia Z, Odin P, Lindvall O. Exp Neurol. 1995;131:1–10. doi: 10.1016/0014-4886(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Navarro E, Canudas A M, Akerund P, Alberch J, Arenas E. J Neurochem. 2000;75:2190–2199. doi: 10.1046/j.1471-4159.2000.0752190.x. [DOI] [PubMed] [Google Scholar]
- 26.Bemelmans A P, Horellou P, Pradier L, Brunet I, Colin P, Mallet J. Hum Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- 27.Weindruch R, Sohal R S. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanagat J, Allison D B, Weindruch R. Toxicol Sci. 1999;52:S35–S40. doi: 10.1093/toxsci/52.2.35. [DOI] [PubMed] [Google Scholar]
- 29.Dubey A, Forster M J, Lal H, Sohal R S. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Ersoz A, Butterfield D A, Mattson M P. J Neurochem. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- 31.Yu B P, Chung H Y. Ann NY Acad Sci. 2001;928:39–47. doi: 10.1111/j.1749-6632.2001.tb05633.x. [DOI] [PubMed] [Google Scholar]
- 32.Bruce-Keller A J, Umberger G, McFall R, Mattson M P. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 33.Duan W, Mattson M P. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z F, Mattson M P. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 35.Lee J, Seroogy K B, Mattson M P. J Neurochem. 2000;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 36.Duan W, Ladenheim B, Cutler R G, Kruman I I, Cadet J L, Mattson M P. J Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 37.Hengemihle J M, Long J M, Betkey J, Jucker M, Ingram D K. Neurobiol Aging. 1999;20:9–18. doi: 10.1016/s0197-4580(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 38.Goodrick C L, Ingram D K, Reynolds M A, Freeman J R, Cider N. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- 39.Mattson M P. Nature. 2002;415:377–379. doi: 10.1038/415377a. [DOI] [PubMed] [Google Scholar]
- 40.Heydari A R, Conrad C C, Richardson A. J Nutr. 1995;125:410–418. doi: 10.1093/jn/125.3.410. [DOI] [PubMed] [Google Scholar]
- 41.Dedeoglu A, Ferrante R J, Andreassen O A, Dillmann W H, Beal M F. Exp Neurol. 2002;176:262–265. doi: 10.1006/exnr.2002.7933. [DOI] [PubMed] [Google Scholar]
- 42.Jana N R, Tanaka M, Wang G, Nukina N. Hum Mol Genet. 2000;9:2009–2018. doi: 10.1093/hmg/9.13.2009. [DOI] [PubMed] [Google Scholar]
- 43.Schabitz W R, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- 44.Mattson M P, Lovell M A, Furukawa K, Markesbery W R. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Duan W, Mattson M P. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhou H, Li S H, Li X J. J Biol Chem. 2001;276:48417–48424. doi: 10.1074/jbc.M104140200. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa T. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 48.Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan R M, Jaenisch R. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 49.Peters A, Schweiger U, Fruhwald-Schultes B, Born J, Fehm H L. Exp Clin Endocrinol Diabetes. 2002;110:199–211. doi: 10.1055/s-2002-33068. [DOI] [PubMed] [Google Scholar]
- 50.Schober A, Wolf N, Huber K, Hertel R, Krieglstein K, Minichiello L, Kahane N, Widenfalk J, Kalcheim C, Olson L. J Neurosci. 1998;18:7272–7284. doi: 10.1523/JNEUROSCI.18-18-07272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kernie S G, Liebl D J, Parada L F. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratley R E, Salbe A D, Ravussin E, Caviness J N. Ann Neurol. 2000;47:64–70. [PubMed] [Google Scholar]
- 53.Sanberg P R, Fibiger H C, Mark R F. Med J Aust. 1981;1:407–409. doi: 10.5694/j.1326-5377.1981.tb135681.x. [DOI] [PubMed] [Google Scholar]
- 54.Luesse H G, Schiefer J, Spruenken A, Puls C, Block F, Kosinski C M. Behav Brain Res. 2001;126:185–195. doi: 10.1016/s0166-4328(01)00261-3. [DOI] [PubMed] [Google Scholar]
- 55.Hockly E, Cordery P M, Woodman B, Mahal A, van Dellen A, Blakemore C, Lewis C M, Hannan A J, Bates G P. Ann Neurol. 2002;51:235–242. doi: 10.1002/ana.10094. [DOI] [PubMed] [Google Scholar]
- 56.Beilby J, Chin C Y, Porter I, Walpole I R, Goldblatt J. Med J Aust. 1994;161:356–360. doi: 10.5694/j.1326-5377.1994.tb127486.x. [DOI] [PubMed] [Google Scholar]