Abstract
The present study investigated whether activation of presynaptic N-methyl-d-aspartate (NMDA) receptors in the spinal cord produces a retrograde nociceptor sensitization (hypernociception) to mechanical nonnoxious stimulus. By using an electronic version of the von Frey hair test (pressure meter), s.c. intraplantar administration of prostaglandin E2 (PGE2) (50–400 ng per paw) evoked a dose-related ipsilateral paw hypernociception. In contrast, intrathecal (i.t.) administration of NMDA (5–80 ng) and PGE2 (15–150 ng) evoked dose-related bilateral paw hypernociception. The s.c. intraplantar administration of dipyrone (80–320 μg per paw) or morphine (3 and 9 μg per paw), usually used to antagonize peripheral PGE2 (100 ng per paw), induced hypernociception and also antagonized the ipsilateral (without affecting the contralateral) paw hypernociception induced by i.t. injections of NMDA (40 ng) or PGE2 (50 ng). These doses of drugs did not modify the basal mechanical sensitivity of control paws. This result shows that intraspinal NMDA or PGE2 produces sensitization of the primary sensory neuron in response to mechanical stimulation. In a second series of experiments it was shown that the i.t. treatment with NaV1.8 (SNS/PN3) sodium channel antisense oligodeoxynucleotides, but not mismatch oligodeoxynucleotides, decreased the mRNA expression of sodium tetrodotoxin-resistant channels on the dorsal root ganglia and abolished the mechanical hypernociception induced by i.t. administration of NMDA. Thus, our results support the suggestion that glutamate release in the spinal cord during inflammation causes retrograde hypernociception of nociceptors associated with sodium tetrodotoxin-resistant channels in primary nociceptive sensory neurons.
Stimulation of postsynaptic N-methyl-d-aspartate (NMDA) receptors plays an important role in spinal transmission of nociceptive signals and contributes to inflammatory nociceptive sensitization (1–7). Recently, a new spinal mechanism of nociceptor sensitization, retrograde sensitization of the primary sensory neuron, was proposed (8). Induction and maintenance of inflammatory nociceptor sensitization (hypernociception) were shown to depend on spinal cord presynaptic NMDA receptors. In the present study, we have investigated the importance of sodium tetrodotoxin-resistant (TTX-R Na+) channels, which are characteristic of peripheral nociceptive small C fibers, for the development of hypernociception induced by intrathecal (i.t.) administration of NMDA.
Intrathecal administration of prostaglandin E2 (PGE2), glutamate, and NMDA causes a bilateral long-lasting mechanical paw hypernociception (up to 6 h; refs. 9 and 10). It was previously shown that i.t. induced bilateral hypernociception was ipsilaterally inhibited by local s.c. injection of morphine or dipyrone. The doses of morphine and dipyrone used had no antinociceptive effect on the contralateral paw hypernociception (8, 9). In contrast with inhibitors of prostaglandin synthesis, which prevent the development of nociceptor sensitization, local administration of morphine or dipyrone directly antagonized ongoing hypernociception (10, 11). Peripheral ipsilateral blockade of mechanical hypernociception induced by i.t.-administered mediators constitutes a simple and straightforward behavioral test to show retrograde sensitization of primary sensory neurons.
In the present investigation, mechanical hypernociception was measured with an electronic version of the von Frey hair test, in which the force that evokes a behavioral withdrawal is automatically recorded by an electronic pressure meter. Bilateral paw hypernociception was induced by i.t. administration of NMDA or PGE2. Pretreatment of the paws with dipyrone or morphine, at doses shown previously to cause antinociception only in the injected paws, was used to show the sensitization of the primary sensory neurons.
Several studies have reported that inflammatory stimuli or mediators can significantly increase TTX-R Na+ channel NaV1.8 (SNS/PN3) mRNA expression and enlarge the amplitude of TTX-R Na+ channel currents in dorsal root ganglia (DRG; refs. 12–14). TTX-R Na+ channels are characteristically associated with fine primary sensory fibers (15, 16). By using antisense oligodeoxynucleotides (ODNs) to selectively “knock down” the expression of NaV1.8 (a specific and molecularly distinct TTX-R Na channel), it was shown that NaV1.8 present in primary afferent nociceptors contributes to peripheral sensitization induced by local PGE2 (15, 17). In the present study we reduced the expression of NaV1.8 mRNA by successive i.t. injections of specific antisense ODNs and compared the intensity of paw hypernociception induced by i.t. administration of NMDA with control animals (treated with mismatch ODNs followed by i.t. administration of NMDA).
Materials and Methods
Animals.
The experiments were performed on 180- to 200-g male Wistar rats housed in an animal care facility of the University of São Paulo and taken to the testing area at least 1 h before testing. Food and water were available ad libitum. All behavioral testing was performed between 9 a.m. and 4 p.m. Rats were used only once. Animal care and handling procedures were in accordance with International Association for Study of Pain (IASP) guidelines on the use of animals in pain research. Each experiment used four to six rats per group. All efforts were made to minimize the number of animals used and any discomfort.
Mechanical Nociceptive Test: Electronic Pressure Meter.
In a quiet room, rats were placed in acrylic cages (12 × 20 × 17 cm) with wire grid floors, 15–30 min before the start of testing. During this adaptation period, the paws were tested (probed) two to three times. The test consisted of evoking a hindpaw flexion reflex with a hand-held force transducer adapted with a 0.5-mm2 polypropylene tip (electronic von Frey hair; IITC Life Science, Woodland Hills, CA). A tilted mirror placed under the grid provided a clear view of the rat hindpaw. The investigator was trained to apply the tip in between the five distal footpads with a gradual increase in pressure. The stimulus was automatically discontinued and its intensity recorded when the paw was withdrawn. The maximum force applied was 80 g. The stimulus was repeated (up to six times, usually three) until the animal presented similar measurements (differences <10%). The end point was characterized by the removal of the paw in a clear flinch response after the paw withdrawal. The animals were tested before and after treatments. The results are expressed by the Δ withdraw threshold (in g) that was calculated by subtracting the average of the last three measurements after the treatments from the average of three measurements before treatments.
Drug Administration.
The agents used in this study were PGE2 (Sigma), indomethacin (Prodome, Campinas, São Paolo, Brazil), NMDA (Tocris, Washington, DC), dipyrone (Sigma), and morphine sulfate (Sigma). All drugs were dissolved in 0.15 M NaCl (saline). The stock solution of PGE2 (1 μg/μl) was prepared in 10% ethanol, and additional dilutions were made in saline; the final concentration of ethanol was 1%. The drugs were tested either by i.t. or intraplantar (i.pl.) injections. The i.t. administration was based on the technique in ref. 18. Each animal was lightly anesthetized by inhalation of 2.5% isoflurane (IsoFlo, USP, Abbott) and the dorsal fur was shaved. With the rat spinal column arched, a 30-gauge needle was directly inserted into the subarachnoid space between lumbars (L)4 and L5. All agents were slowly injected in a volume of 10 μl, using a 100-μl Hamilton microsyringe. The i.t. location of the needle tip was affirmed by a characteristic flick of the tail. The injection procedure from the beginning of the anesthetic inhalation until withdrawal of the needle took ≈3 min. The animals regained consciousness ≈1 min after discontinuing the anesthesia. The i.pl. administration was performed with 50 μl per paw with a 27-gauge hypodermic needle connected to a 100-ml Hamilton microsyringe. The needle was introduced s.c. near the third digit with its tip reaching the middle of the plantar hindpaw.
Antisense ODN.
The 18-mer ODNs used in this experiment were purchased from GIBCO/BRL. The antisense-ODN sequence, 5′-GGG GAG CTC CAT CTT CTC-3′, was directed against a unique sequence of the NaV1.8 as described (15). The mismatch-ODN sequence, 5′-GGG GTC TTC CAA GCT CTC-3′, corresponded to the antisense sequence except that six bases were changed (denoted by bold face). ODNs were lyophilized and reconstituted in nuclease-free water 0.9% NaCl to a concentration of 2 μg/μl. A search of the European Molecular Biology Laboratory and GenBank databases identified no sequences homologous to those used in this experiment. ODNs were administered i.t. (20 μg/10 μl) once daily for 3 days.
RT-PCR.
At the end of experiments, rats were killed with an overdose of Na pentobarbital, and the L4–L6 DRG or spinal cord was removed. The tissues were homogenized in 1.0 ml of trizol reagent, and 0.2 ml of chloroform was added to the samples before shaking vigorously for 30 s. The suspension was centrifuged at 13,000 × g at 4°C for 15 min. The aqueous phase was transferred to a fresh tube to which an equal volume of 2-propanol was added. After mixing, the samples were incubated for 15 min at −20°C. Samples were centrifuged at 13,000 × g at 4°C for 15 min. The RNA precipitate was washed with 0.5 ml of ethanol, and the preparation was suspended in 50 μl of diethyl pyrocarbonate-treated water containing 1 mM EDTA. Reverse transcription involved incubation of 10 μl of total RNA with dNTP, 200 units of superscript reverse transcriptase, first-strand buffer, and diethyl pyrocarbonate-treated water for 1 h at 37°C. The reaction was terminated by heating at 90°C for 5 min and cooling at 4°C for 5 min.
PCR amplification used primers to NMDA (forward: gag, ggt, ttc, gct, gat, gtc, ttc, ta; reverse: ccc, gct, cct, gtg, tgc, caa, act), NaV1.8 (forward: cag, ctt, cgc, tca, gaa, gta, tct; reverse: ttc, tcg, ccg, ttc, cac, acg, gag, act), and m-actin (forward: atg, cca, tcc, tgc, gtc, tgg, acc, tgg, c; reverse: agc, att, tgc, ggt, gca, cga, tgg) and was performed in the presence of TaqDNA polymerase, PCR buffer, and 1.5 mM MgCl2, dNTP mix, and DNase- and RNase-free water. The PCR was conducted in a Perkin–Elmer Cetus GeneAmp system 9600, starting with a 3-min incubation at 95°C, followed by 25 cycles of denaturation at 94°C for 60 s, annealing at 65°C for 60 s, and extension at 72°C for 2 min. The final extension was 72°C for 7 min. The number of cycles was optimized in pilot experiments to produce a light but well-defined band of NaV1.8 in control samples. The PCR products were separated on a 6% polyacrylamide gel. The 100-bp DNA ladder was used as a molecular size marker. Gels were run at 120 V for 45 min, stained with silver nitrate, and then scanned with each labeled band analyzed by integrated optical densitometry.
Data Analysis.
Data are presented as means ± SEM and analyzed statistically by using one-way ANOVA. When ANOVA showed significant differences between groups, Tukey's post hoc test was used to determine the specific pairs of groups between which statistically significant differences occurred. P < 0.05 was the accepted level for statistical significance. The number of observations (n) in each experiment is shown in the figure legends.
Results
Local Administration of Dipyrone Inhibits Mechanical Hypernociception Induced by s.c. Administration of PGE2.
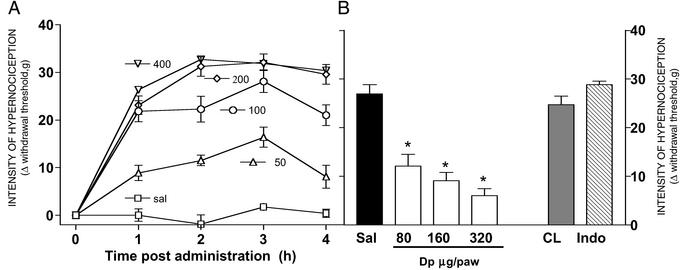
Fig. 1A shows the dose-dependent decrease in the paw withdrawal threshold induced by s.c. administration of PGE2 (0, 50, 100, 200, and 400 ng per paw in 50 μl) into the site of the application of the probe. A plateau of response was observed between 2 and 4 h after PGE2 administration (the R2, sum of squares, and df of the exponential regression of the dose–response curve at 3 h after PGE2 administration were 0.99, 102.8, and 2, respectively; Fig. 1A).
Figure 1.
Nociception plantar pressure test: peripheral effect of PGE2 and its peripheral blockade by dipyrone (Dp). (A) Dose–response curve (0–400 ng per paw) of hindpaw mechanical hypernociception induced by s.c. PGE2 measured at indicated time intervals. (B) Dose-dependent (80–320 μg per paw) antinociceptive effect of local pretreatment of dipyrone on the PGE2 (100 ng per paw; Sal, saline-pretreated control) nociceptor hypernociception induced by injection of PGE2 measured 3 h after administration. The CL bar shows the effect of high dose of dipyrone (320 μg per paw) administered into the contralateral paw, and the Indo bar shows the effect of indomethacin (300 μg per paw) administered into the ipsilateral paw. The * indicates significant difference (P < 0.001, Tukey) as compared with 50 ml of saline (filled bar). Values are means (±SEM) of five to seven rats per group.
Fig. 1B shows the lack of effect of i.pl. pretreatment with indomethacin (300 μg per paw, 30 min before PGE2) upon the PGE2-induced hypernociception. In contrast, dipyrone (80–320 μg per paw) applied 2 h and 30 min after PGE2 administration (100 ng per paw) inhibited (P < 0.001, ANOVA/Tukey) the PGE2-induced hypernociception in a dose-dependent manner. The lack of effect of the highest dose of dipyrone administration into the contralateral paw ruled out a possible systemic action of dipyrone (Fig. 1B).
Intrathecal Administration of PGE2 or NMDA Induces Bilateral Hindpaw Mechanical Hypernociception.
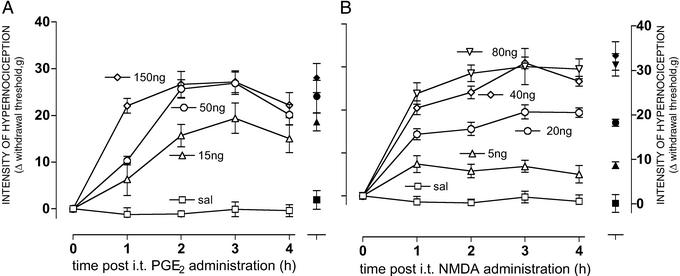
Intrathecal administration of PGE2 (15, 50, and 150 ng) or NMDA (5, 20, 40, and 80 ng) significantly decreased (P < 0.001; ANOVA/Tukey) the nociceptive withdrawal threshold of both paws compared with administration of vehicle (saline, 10 μl). The effect after i.t. administration was dose-dependent and with a plateau between 2 and 4 h after administration (the R2, sum of squares, and df of the exponential regression curve at 3 h after i.t. PGE2 or NMDA administration were 0.98, 11.7, and 1 or 0.99, 46.9, and 3, respectively; Fig. 2 A and B, respectively). There was no significant difference between the paw withdrawal threshold of right and left paws (ANOVA).
Figure 2.
Time course of mechanical nociceptor hypernociception induced in both rat paws by i.t. administration of PGE2 or NMDA. A and B are the time course of the mechanical nociceptor hypernociception induced by i.t. administration of PGE2 (0–150 ng per i.t.) or NMDA (0–80 ng per i.t.), respectively. In both images we show the intensity of hypernociception of the right paws (open symbols) and left paws (filled symbols, measured only in the fourth hour after challenge). Values are means (±SEM) of five to seven rats per group.
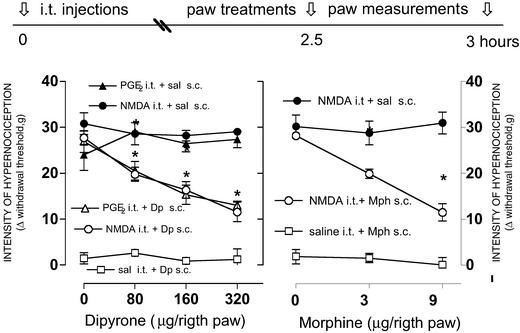
Subcutaneous Administration of Morphine or Dipyrone Suppresses Ipsilateral Hypernociception Induced by i.t. Administration of NMDA or PGE2.
Intrathecal administration of NMDA or PGE2 significantly decreased the mechanical withdrawal threshold in both paws. Local (i.pl. s.c.) administration of dipyrone (80, 160, and 320 ng per paw; Fig. 3 Left) or morphine (3 and 9 μg per paw; Fig. 3 Right) significantly decreased (P < 0.01, ANOVA/Tukey) the spinally induced hypernociception in the ipsilateral paw, compared with saline administration into the contralateral paw (20 ml per paw). Dipyrone or morphine was administered 30 min before measurements were taken, i.e., 3 h after i.t. administration of NMDA (40 ng) or PGE2 (50 ng). Neither the i.t. administration of saline (10 ml) nor the s.c. administration of dipyrone or morphine affected the mechanical withdrawal baseline (Fig. 3 Left and Right, respectively).
Figure 3.
Blockade by local (s.c.) administration of dipyrone or morphine of nociceptor hypernociception induced by i.t. administration of NMDA. Left and Right are the peripheral effect of dipyrone (Dp) or morphine (Mph), respectively, on the mechanical hypernociception induced by i.t. administration of PGE2 (filled triangles, right paws) or NMDA (filled circles, right paws). Saline (sal) controls (open squares) were injected in the left contralateral paws to Dp or Mph. Mph (0–9 μg per paw), Dp (0–320 μg per paw), and saline (20 μl) were injected in the right paw 2.5 h after the i.t. injections. Hypernociception was measured 3 h after i.t. injections. The * means significant differences from right paws (P < 0.001; ANOVA, Tukey). Values are mean ± SEM.
mRNA Expression of NaV1.8 Na Channel and NMDA Receptor on DRG and Spinal Cord.
The mRNA expression of rat NaV1.8 Na channel and NMDA receptor was assessed by RT-PCR. The expression of β-actin mRNA was used as a control for tissue integrity in all samples. L5 and L6 DRG tissue from rats treated with antisense ODN to NaV1.8 showed a decrease in expression of NaV1.8 Na channel mRNA when compared with the L5/L6 DRGs from rats treated with the same amount of mismatch ODN (see Fig. 5A, lanes 1–3). No labels of NaV1.8 Na channel expression were identified from the spinal cord samples of rats treated with mismatch ODN (Fig. 4A, lane 4). The densitometric analysis of the label images showed that the i.t. injections of antisense (20 mg once daily for 3 days), but not mismatch, significantly reduced the NaV1.8 mRNA expression in DRG when compared with control rats (Fig. 4C). In contrast, the NMDA receptor was expressed in both spinal cord (Fig. 4B, lane 4) and DRG (Fig. 4B, lanes 1–3), although the expression of NMDA receptor in the spinal cord was more intense than in the DRG.
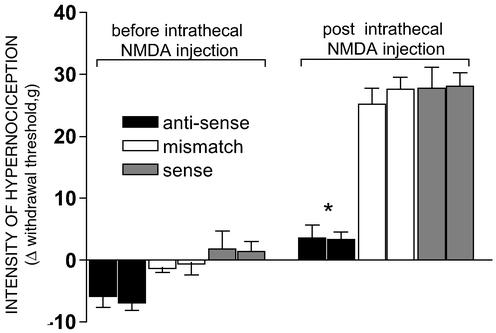
Figure 5.
Intrathecal pretreatment with a Na channel NaV1.8 antisense ODN blocks the nociceptive hypernociception induced by i.t. injection of NMDA. Rats received an i.t. injection of 18-mer ODNs, 20 mg in 10 μl, once daily for 3 days. Twelve hours after the last ODN injection, mechanical nociceptive hypernociception was measured before and 3 h after NMDA (40 ng) i.t. injection. The first and second bars of the treatment groups are the data of the left and right paws, respectively. The * means significant difference as compared with mismatch group (P > 0.001; Tukey). Values are mean ± SEM of six animals per group.
Figure 4.
PCR amplifications for TTX-R Na+ channel NaV1.8 and NMDA receptor expressed in DGR and spinal cord (Sp.C.). (A) No expression and reduction of the Na channel NaV1.8 in spinal cord of normal rats (4) and in DRG from rats pretreated with ODNs antisense to NaV1.8 Na channel (nos. 1 and 3), respectively. This channel was expressed on DRG from rats pretreated with mismatch (no. 2). (B) NMDA receptor was expressed as in spinal cord (no. 4) as in DRG (nos. 1–3). (C) The relative optical density of NaV1.8 labeling by using normal control DRG NaV1.8 labeling as 100%. The relative intensity of NaV1.8 from antisense-treated DRGs is significantly different (P < 0.05, unpaired t test) when compared with mismatch-treated DRGs. The expression of β-actin was used as control marker for both DRG and spinal cord tissues.
Knockdown of NaV1.8 Na Channel Prevents the Hypernociception Induced by i.t. Administration of NMDA.
Intrathecal administration of the antisense ODN to rat NaV1.8 Na+ channel slightly increased the mechanical withdrawal threshold (P < 0.05, ANOVA/Tukey) and completely prevented the hypernociception induced by a subsequent i.t. administration of 40 μg of NMDA (P < 0.001, ANOVA/Tukey). In contrast, the hypernociception induced in both paws by the i.t. administration of NMDA remained intact after the i.t. administration of a mismatch ODN (the antisense sequence with 3 bp changed) or the unique sequence (sense) of rat NaV1.8Na+ channel (Fig. 5).
Discussion
Like other methods of measuring mechanical hypernociception (15, 19, 20), the electronic pressure meter used in the present investigation was able to detect and quantify PGE2 nociceptor sensitization, as well as its blockade by i.pl. administration of dipyrone (21). In contrast to the von Frey test, the electronic pressure meter uses a single stimulation of increasing pressure instead of sequential applications of filaments of various stiffness. In the present study the term hypernociception instead of hyperalgesia or allodynia was used, because variants of a mechanical technique (von Frey or the electronic pressure meter) were not able to distinguish them. Although the differentiation between allodynia and hyperalgesia in human subjects may have clinical value, the molecular basis of any differences between allodynia and hyperalgesia remains obscure.
Sensitization of the nociceptors plays a major role in the development of acute and chronic overt inflammatory pain although plastic changes of spinal dorsal horn neurons are thought to modulate its intensity or persistence (6, 22, 23). The eicosanoids were the first family of molecules recognized as a distinct class of inflammatory mediators that contributes to overt inflammatory pain, by sensitizing the nociceptors in human subjects (24) and experimental animals (25). This finding contrasted with the former notion that nociceptor sensitization resulted from a subthreshold stimulation of nociceptors by a mixture of different mediators present in inflammatory exudates (26, 27). Our proposal that the mechanism of the analgesic action of aspirin-like drugs resulted from the prevention of this peripheral sensitization by inhibition of prostaglandin synthesis is broadly accepted today (28). During inflammatory reactions, central release of eicosanoids may also contribute to the amplification of nociceptive intensity or behavior (7, 20). It seems, however, that even the PGE2 release in the spinal cord involves the primary sensory neuron because its i.t. injection was blocked by i.pl. injection of morphine or dipyrone (10). In the present study, we confirmed that i.t. injection of NMDA, as well as PGE2, produces a characteristic bilateral rat hindpaw hypernociception (9, 10). It was also shown that peripheral morphine or dipyrone administration blocked ipsilateral without affecting the contralateral ongoing paw hypernociception. This result is a clear demonstration that NMDA was acting retrogradely at the presynaptic, primary sensory nerve-central terminals. The time course of hypernociception induced by i.t. PGE2-induced hypernociception paralleled the development of i.pl. injected PGE2, suggesting activation of a common metabolic pathway in the primary sensory neurons. Presynaptic NMDA receptors have already been described (29–31), and the mRNA of NMDA receptors is expressed in the DRG and spinal cord (12, 13) after inflammatory stimulation, as shown previously. In the present study, the i.t. antisense ODN (but not mismatch or sense ODN) treatment decreased Na channel NaV1.8 expression in the DRG and prevented the hypernociception induced by the i.t. administration of NMDA. Although the capacity of antisense ODN to reduce mRNA expression has been described (32), the mechanism by which antisense ODN specifically reduces the mRNA levels in the cell remains unclear. NMDA receptors are present in many excitable tissues but NaV1.8 Na channels have been detected only in DRG and spinal cord. These Na channels are essential for the development of peripheral PGE2-induced inflammatory hyperalgesia (12, 15) and are preferentially expressed in small diameter fibers of the DRG. Because this type of channel is characteristic of primary sensory neuron C fibers, this observation strongly supports a retrograde effect of i.t.-injected NMDA. Glutamate is thought to be the major spinal neurotransmitter that conveys nociceptive information from the periphery to the CNS. The presynaptic action of NMDA described here adds more weight to the participation of spinal glutamate as a modulatory agent in inflammatory mechanical hypernociception. It is interesting to note that, in contrast to NMDA administration, the mechanical hypernociception induced by i.t. administration of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) propionate was not inhibited by peripheral administration of morphine (9), stressing the participation of this receptor type in the sensitization of spinal secondary neurons.
Despite the distance and functional differentiation between peripheral and spinal terminals, the primary sensory neuron behaves as a highly integrated unit. Activation of NMDA presynaptic receptors lowered peripheral nociceptor threshold, an effect that was inhibited by administration of an analgesic into the paw. Thus, this integrated modulatory mechanism seems to be triggered by a single event occurring in a discrete area of the primary sensory neuron. Calcium oscillation is an integrating cellular mechanism described for several cell types, particularly for neurons (33–35), and may be involved in the induction and maintenance of hypernociception. This speculation is supported by a number of observations:
(i) Activation of the cAMP/Ca2+ pathway was proposed as the molecular basis of mechanical hypernociception (11).
(ii) Prostaglandin activation of G protein-coupled receptors alters the activity of Ca2+ channels via protein kinase A (PKA) or PKC, thus facilitating Ca2+ inflow (36, 37).
(iii) Pools in neuronal cytosolic Ca2+ concentration may originate from all parts of the endoplasmic reticulum or mitochondria by stimulation of membrane G protein receptor/phospholipid C/inositol 1,4,5-triphosphate (IP3) pathways, or by stimulation of IP3 or ryanodine receptors by Ca2+ itself (33–35).
(iv) Caffeine, which has been reported to promote Ca2+ release from endoplasmic reticulum (38), potentiates the mechanical hypernociception induced by i.pl. PGE2, calcium ionophore, or 8Br-cAMP (11).
Cytosolic Ca2+ oscillation seems to be an efficient manner of intracellular “long-distance” signaling, which results from the rhythmic activation of a “calcium-induced calcium release” process (33). When this process becomes regenerative, an explosive intracellular increase in calcium concentration generates a calcium wave that spreads throughout the cell (33). At the sensory nerve terminal, neuronal Ca2+ oscillations may be responsible for presynaptic glutamate release. Also, glutamate, by acting retrogradely on NMDA receptors (9), promotes calcium influx and may initiate Ca2+ oscillations (39). The Ca2+ influx by presynaptic NMDA receptor and the subsequent cytosolic Ca2+ oscillation may be essential to initiating or maintaining hypernociception induced by inflammatory mediators. The cAMP transduction cascade may also mediate the PGE2-induced inhibition of K+ currents in rat sensory neurons (40). However, despite the mechanism underlying the alterations of cytosolic K+ concentration, it has been shown that the antinociceptive effects of dipyrone, morphine, nitroglycerin, and Db-cGMP are antagonized by tolbutamide and glibenclamide, ATP-dependent K+ channel inhibitors (41, 42). It is a plausible hypothesis that the lowering of K+ in a discrete neuronal area may interrupt Ca2+ oscillation (43). This event would explain, as shown here, why the administration of dipyrone or morphine given in the periphery inhibits the hypernociception induced by i.t.-injected NMDA or PGE2.
Concomitant with the ionic mechanisms, which control the nociceptor threshold (neuronal depolarization), the induction of a change in nociceptive behavior depends on the expression of functional TTX-R Na+ channels. Although specific receptors trigger G protein/PKA or PKC metabolic pathways, it is not yet established whether it is biochemical activation or a lowering of the nociceptor threshold that is required for the TTX-R Na+ channels to become functional. A neuronal depolarization would be sufficient if these channels are already expressed in the nociceptor. Phosphorylation of TTX-R Na+ channels has been suggested as one of the mechanisms underlying inflammatory hyperalgesia (44).
We are aware that the retrograde sensitization of the primary sensory neuron may help explain secondary hyperalgesia and referred pain symptoms that are generally assumed to result from central sensitizing mechanisms (45, 46). However, the diffusion of glutamate in the dorsal horn would cause retrograde sensitization of contiguous presynaptic terminations of primary peripheral sensory neurons as well as of convergent visceral and cutaneous neurons.
In summary, we report that the hypernociception induced by i.t. administration of NMDA or PGE2 depends on the expression of NaV1.8 Na channels in the primary sensory neuron and that this hypernociception is inhibited by peripheral treatment with morphine or dipyrone. Taken together, these findings indicate that glutamate and PGE2, injected intrathecally, activate presynaptic receptors to sensitize the primary sensory neuron. These results agree with the suggestion that glutamate, released spinally by primary sensory neurons, acts retrogradely on presynaptic NMDA receptors, facilitating the induction or maintenance of peripheral hypernociception.
Acknowledgments
We thank I. R. Santos and S. R. Rosa for excellent technical assistance. This study was supported by grants from the Fundaçao de Amparo à Pesquisa do Estado de São Paulo, Brazil.
Abbreviations
- NMDA
N-methyl-d-aspartate
- i.pl.
intraplantar
- PGE2, prostaglandin E2
i.t., intrathecal
- ODN
oligodeoxynucleotide
- TTX-R Na+
sodium tetrodotoxin-resistant
- DRG
dorsal root ganglia
- L
lumbar
References
- 1.Aanonsen L M, Lei S, Wilcox G L. Pain. 1990;41:309–321. doi: 10.1016/0304-3959(90)90008-2. [DOI] [PubMed] [Google Scholar]
- 2.Haley J E, Wilcox G L. In: Hyperalgesia and Allodynia. Willis W, editor. New York: Raven; 1992. pp. 281–293. [Google Scholar]
- 3.Woolf C J, Doubell T P. Curr Opin Neurobiol. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 4.Dubner R, Ruda M A. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- 5.Minami T, Nishihara I, Uda R, Ito S, Hyodo M, Hayaishi O. Pain. 1994;57:225–231. doi: 10.1016/0304-3959(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 6.Urban M O, Gebhart G F. Proc Natl Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malmberg A B, Yaksh T L. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 8.Minami T, Matsumura S, Okuda-Ashitaka E, Shimamoto K, Sakimura K, Mishina M, Mori H, Ito S. Brain Res. 2001;895:178–185. doi: 10.1016/s0006-8993(01)02069-8. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira S H, Lorenzetti B B. Neuropharmacology. 1994;33:1479–1485. doi: 10.1016/0028-3908(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira S H, Lorenzetti B B. Inflamm Res. 1996;45:499–502. doi: 10.1007/BF02311085. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira S H, Nakamura M. Prostaglandins. 1979;18:191–200. doi: 10.1016/0090-6980(79)90104-7. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Cummins T R, Ishikawa K, Dib-Hajj S D, Black J A, Waxman S G. NeuroReport. 1998;9:967–972. doi: 10.1097/00001756-199804200-00003. [DOI] [PubMed] [Google Scholar]
- 13.Waxman S G, Dib-Hajj S, Cummins T R, Black J A. Proc Natl Acad Sci USA. 1999;96:7635–7639. doi: 10.1073/pnas.96.14.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akopian A N, Sivilotti L, Wood J N. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 15.Khasar S G, Gold M S, Levine J D. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- 16.Gold M S. Proc Natl Acad Sci USA. 1999;96:7645–7649. doi: 10.1073/pnas.96.14.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold M S, Reichling D B, Shuster M J, Levine J D. Proc Natl Acad Sci USA. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papir-Kricheli D, Laufer R, Gilon C, Chorev M, Selinguer Z, Devor M. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- 19.Moller K A, Johansson B, Berge O G. J Neurosci Methods. 1998;84:41–47. doi: 10.1016/s0165-0270(98)00083-1. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira S H, Lorenzetti B B, Correa F M. Eur J Pharmacol. 1978;53:39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzetti B B, Ferreira S H. Eur J Pharmacol. 1985;114:375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox G L. IASP: Refresher Courses on Pain Management—An Updated Review (Refresher Course Syllabus) Seattle: Intl. Assoc. Study Pain; 1999. pp. 572–591. [Google Scholar]
- 23.Millan M J. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira S H. Nat New Biol. 1972;240:200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- 25.Moncada S, Ferreira S H, Vane J R. In: Inflammation. Ferreira S H, Vane J R, editors. Berlin: Springer; 1978. pp. 588–616. [Google Scholar]
- 26.Lynn B. In: Textbook of Pain. Wall P D, editor. London: Churchill Livingstone; 1984. pp. 19–33. [Google Scholar]
- 27.Handwerker H O, Reeh P W. In: Hyperalgesia and Allodynia. Willis W D Jr, editor. New York: Raven; 1992. pp. 107–115. [Google Scholar]
- 28.Roberts L J, Morrow J D. In: Goodman & Gilman's: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 2001. pp. 687–731. [Google Scholar]
- 29.Liu H, Mantyh P W, Basbaum A I. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Wang H, Sheng M, Jan L Y, Jan Y N, Basbaum A I. Proc Natl Acad Sci USA. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrero I, Miras-Portugal M T, Sánchez-Prieto J. Nature. 1992;360:163–166. doi: 10.1038/360163a0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Cook J, Nickel J, Yu R, Stecker K, Myers K, Dean N M. Nat Biotechnol. 2000;18:862–867. doi: 10.1038/78475. [DOI] [PubMed] [Google Scholar]
- 33.Usachev Y M, Thayer S A. BioEssays. 1999;21:743–750. doi: 10.1002/(SICI)1521-1878(199909)21:9<743::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 34.Johenning F W, Zochowski M, Conway S J, Holmes A B, Koulen P, Ehrlich B E. J Neurosci. 2002;22:5344–5353. doi: 10.1523/JNEUROSCI.22-13-05344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzuto R. Curr Opin Neurobiol. 2001;11:306–311. doi: 10.1016/s0959-4388(00)00212-9. [DOI] [PubMed] [Google Scholar]
- 36.Bevan S. In: Textbook of Pain. Wall P D, Melzack R, editors. London: Harcourt; 1999. pp. 85–103. [Google Scholar]
- 37.Godfraind T, Govoni S. Trends Pharmacol Sci. 1995;16:1–4. doi: 10.1016/s0165-6147(00)88961-0. [DOI] [PubMed] [Google Scholar]
- 38.Kuba K. J Physiol (London) 1980;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe D B, Brown T H. J Neurophysiol. 1994;72:471–474. doi: 10.1152/jn.1994.72.1.471. [DOI] [PubMed] [Google Scholar]
- 40.Evans A R, Vasko M R, Nicol G D. J Physiol (London) 1999;516:163–178. doi: 10.1111/j.1469-7793.1999.163aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soares A C, Leite R, Tatsuo M A, Duarte I D. Eur J Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues A R, Duarte I D. Br J Pharmacol. 2000;129:110–114. doi: 10.1038/sj.bjp.0703038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schermerhorn T, Sharp G W. Cell Calcium. 2000;27:163–173. doi: 10.1054/ceca.2000.0107. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald E M, Okuse K, Wood J N, Dolphin A C, Moss S J. J Physiol (London) 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonica J J. In: Hyperalgesia and Allodynia. Willis W, editor. New York: Raven; 1992. pp. 17–43. [Google Scholar]
- 46.Lamotte R H. In: Hyperalgesia and Allodynia. Willis W, editor. New York: Raven; 1992. pp. 175–185. [Google Scholar]