Abstract
Examination of store-operated Ca2+ entry (SOC) in single, mechanically skinned skeletal muscle cells by confocal microscopy shows that the inositol 1,4,5-trisphosphate (IP3) receptor acts as a sarcoplasmic reticulum [Ca2+] sensor and mediates SOC by physical coupling without playing a key role in Ca2+ release from internal stores, as is the case with various cell types in which SOC was investigated previously. The results have broad implications for understanding the mechanism of SOC that is essential for cell function in general and muscle function in particular. Moreover, the study ascribes an important role to the IP3 receptors in skeletal muscle, the role of which with respect to Ca2+ homeostasis was ill defined until now.
Store-operated Ca2+ entry (SOC) in response to depletion of internal Ca2+ stores is important for maintaining normal cell Ca2+ homeostasis, but the precise mechanism is not understood fully (1, 2). In nonexcitable cells, the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) (3) appears to have a dual role: releasing Ca2+ from internal stores and mediating SOC by physical coupling with the plasma membrane (4, 5). A SOC mechanism has been identified in adult skeletal cells (6), which contain ryanodine receptors (RyRs)/Ca2+-release channels involved in excitation–contraction (E-C) coupling (7) and IP3Rs that may be involved in regulation of gene expression (8). However, it is not known whether the mechanism of SOC in skeletal muscle involves IP3Rs, the role of which in Ca2+ homeostasis remains controversial (7, 9–11).
Here we image extracellular Ca2+ in the sealed tubular (t)-system of mechanically skinned fibers (12, 13) to examine SOC. The sarcoplasmic reticulum (SR) and t-system remain functionally coupled in this preparation while allowing full experimental access to the interior of the cell. We found a fully operational SOC mechanism in this preparation and probed its mechanism by direct and rapid manipulation of the cytoplasmic environment.
Materials and Methods
The use of animals in this study was approved by the Animal Ethics Committee at La Trobe University. Adult cane toads were killed by double pithing, and the iliofibularis muscles were removed rapidly and placed in a Petri dish under a layer of paraffin oil. Single intact iliofibularis fibers were isolated and loaded with a physiological solution containing 112 mM NaCl, 3.3 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, 1 mM Fluo-5N (impermeant form, Molecular Probes), and 20 mM Hepes, pH 7.4, with a microcap pipette (Drummond Scientific, Broomall, PA) and then skinned mechanically to trap the dye in the sealed t-system as described (12, 13). Note that the presence of Fluo-5N in solution reduced the ionized [Ca2+] to ≈1.5 mM and made it possible to measure [Ca2+] changes in the sealed t-system in the micro- to millimolar range because of its relatively low sensitivity to [Ca2+] (KD ≈ 90 μM, also verified under our conditions). Dye-loaded preparations were moved to a custom-built experimental well that used a thin coverslip as a base and contained a standard myoplasmic solution. The standard myoplasmic solution contained 117 mM K+, 36 mM Na+, 1 mM Mg2+, 7 mM MgATP, 10 mM creatine phosphate, 46 mM hexamethylenediamine-N,N,N′,N′-tetraacetate, 60 mM Hepes, pH 7.1, 1 mM azide, and 4 mM CaEGTA/EGTA (200 nM Ca2+) to maintain a high level of Ca2+ in the t-system and SR. Before the beginning of the experimental protocol (see Fig. 1), this solution was substituted for a similar one with 4 mM EGTA and no added Ca2+ (<1 nM Ca2+). Heparin (from porcine intestinal mucosa, Catalog Nr H-3393, Sigma), a potent antagonist of IP3Rs (14) and IP3 were added to this solution as required. Caffeine (30 mM, an agonist of RyRs) (15) was added to a similar solution except where [Mg2+] was reduced to 75 μM to facilitate the rapid depletion of Ca2+ in the SR (16, 17).
Figure 1.
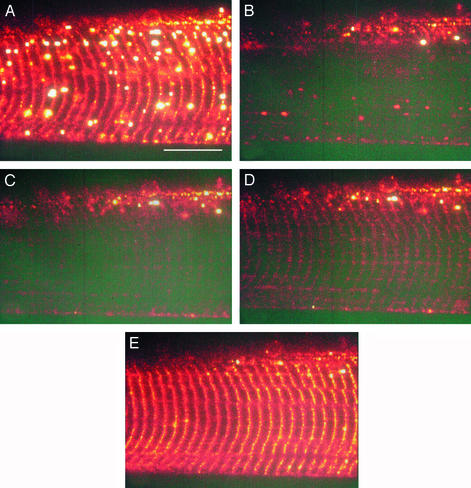
Ca2+ movements across the sealed t-system of a toad skeletal muscle fiber by SOC and Ca2+ reloading. Confocal images of the fluorescence of Fluo-5N trapped in the sealed t-system in the presence of standard myoplasmic solution containing <1 nM Ca2+ and 1 mM Mg2+ (A), 30 s after application of 30 mM caffeine in myoplasmic solution with 0.075 mM Mg2+ and <1 nM Ca2+ (B) and after 2 (C), 7 (D), and 12 (E) min in myoplasmic solution containing 200 nM Ca2+ are shown. Note that depleting the SR of Ca2+ with caffeine and low Mg2+ also resulted in the loss of Ca2+ from the sealed t-system indicating an operational SOC (B) and that Ca2+ can be reloaded into the Ca2+-depleted t-system (C–E). (Scale bar, 20 μm.)
Confocal imaging was in x-y mode averaging eight scans per line by using a Leica confocal laser (argon ion) scanning inverted microscope and a ×63, 1.4-numerical-aperture oil-immersion lens. Commercial confocal software (Leica) was used to analyze images. Fluorescence signals were converted to [Ca2+] by using the equation [Ca2+] = Kd⋅R/(Kd/[Ca2+]0 + 1 − R), where R = F/F0, F = fluorescence intensity in the t-system at 30 s after solution application, and F0 = fluorescence intensity in the t- system before solution application corresponding to a nominal initial [Ca2+]0 of 1.5 mM (see above). The fluorescence intensity in the t-system was measured as described in detail by Launikonis and Stephenson (18) by subtracting from the average fiber fluorescence intensity the background fluorescence between regularly spaced t-system elements where no tubules were present.
In some experiments, junctional t-system and SR were uncoupled by means of exposure to high intracellular [Ca2+] (16). Ca2+ uncoupling of the SR and t-system membranes was achieved by initially preequilibrating the preparation with a 0 ATP (rigor) solution containing 66 mM hexamethylenediamine-N,N,N′,N′-tetraacetate, 60 mM Hepes, 1.5 mM Mgtotal (1 mM [Mg2+]), 36 mM Na+, and 110 mM K+ for 1 min at 4°C and then to a similar solution with 1 mM CaCl2 added for a further 1 min at 4°C. For further details see Lamb et al. (16).
Otherwise, all experiments were performed at room temperature (22 ± 2°C). All average results are presented as the mean value ± SEM, and the statistical significance (P < 0.05) between results was assessed by using one-way ANOVA followed by Newman–Keuls posttest, two-way ANOVA, and Student's t tests as appropriate.
Results
To measure SOC in skeletal muscle we used mechanically skinned fibers where the t-system seals, forming a separate, closed extracellular compartment. Before mechanical skinning, the isolated intact fiber was exposed to a physiological solution containing 1 mM of the membrane impermeant form of Fluo-5N, a Ca2+-sensitive fluorescent dye (KD ≈ 90 μM; see Materials and Methods). Mechanical skinning traps Fluo-5N in the lumen of the sealed t-system, allowing the measurement of the now essentially finite extracellular Ca2+ pool while full experimental access to the myoplasm of the fiber is gained (12, 13). Fig. 1A shows a confocal image of a toad skinned fiber loaded with Fluo-5N in such a manner, bathed in a solution designed to minimize contractile movement during the ensuing caffeine application (the solution contained 4 mM EGTA and no added Ca; [Ca2+] < 1 nM) but otherwise mimicking the myoplasmic environment with high [K+], 1 mM Mg2+, 8 mM ATP, and 10 mM creatine phosphate, pH 7.1. The large vesicles evident in Fig. 1A are enlargements of the t-system unique to mechanically skinned fibers of toad, and we provide a detailed description elsewhere (13). A drop in t-system Ca2+ levels is indicated in Fig. 1B, which is an image scanned 30 s after the substitution for a myoplasmic solution containing 30 mM caffeine, 0.075 mM Mg2+, and 4 mM EGTA. The application of this solution will deplete the SR of Ca2+ (16, 17) rapidly under all conditions used in this study. The subsequent decrease in Fluo-5N fluorescence signal indicates an operational SOC mechanism in this preparation, as shown by the results presented in Figs. 2 and 3 where the SOC mechanism is interrupted. The Fluo-5N signals in Fig. 1 A and B indicate [Ca2+] of 1.5 mM and 36 μM, respectively. In nine preparations, t-system [Ca2+] dropped within 30 s from 1.5 mM to an average of 48 ± 14 μM (n = 9) after SR Ca2+ depletion (see Fig. 3). The t-system [Ca2+] dropped even further after longer times in the SR Ca2+-depleting solution, but accurate [Ca2+] measurements could not be made at relatively low levels of Fluo-5N fluorescence.
Figure 2.
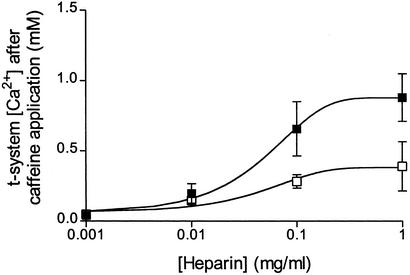
Summary of the effect of heparin (■) and the addition of IP3 (□; 10 μM for 10 μg/ml heparin and 100 μM for the 0.1 and 1 mg/ml heparin data points) to the heparin solution on SOC. Note that caffeine completely depletes the SR of Ca2+ under all conditions. The data represent means ± SEM from 13–16 cells under both sets of conditions and have been fitted to sigmoidal curves. The two sets of data with and without IP3 are statistically different (P < 0.05) from each other (two-way ANOVA). The data points for heparin ≥ 0.01 mg/ml are significantly different (P < 0.05) from controls and the initial [Ca2+] (one-way ANOVA).
Figure 3.
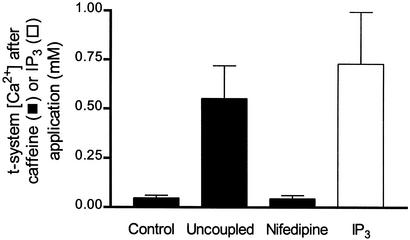
[Ca2+] in the t-system 30 s after the following interventions: control fibers after SR Ca2+ depletion with caffeine and low Mg2+; “high-Ca2+ uncoupled” fibers after SR Ca2+ depletion with caffeine and low Mg2+ (see Results); and fibers preincubated in 10 μM nifedipine after SR Ca2+ depletion with caffeine and low Mg2+ in the presence of nifedipine and fibers exposed to 500 μM IP3 in a myoplasmic solution containing 1 mM Mg2+ and 200 nM Ca2+ (no caffeine). The data represent means ± SEM for three to nine cells per condition. The data points for “uncoupled” fibers are significantly different (P < 0.01) from those for controls and nifedipine (one-way ANOVA), and there is no statistically significant difference (P > 0.5) between controls and nifedipine-treated fibers. The values for IP3-treated and uncoupled fibers are significantly smaller (P < 0.05) than the initial [Ca2+] (1.5 mM) in the t-system.
Fig. 1 C–E shows that Ca2+ can reaccumulate into the sealed t-system in a myoplasmic solution containing 1 mM Mg2+ and 200 nM Ca2+ after depletion. The estimated t-system Ca2+ concentrations in Fig. 1 C, D, and E are 39, 49, and 1,387 μM after 2, 7, and 12 min, respectively. This shows that the sealed t- system requires in the order of several minutes to reload with Ca2+ to physiological levels. In contrast, the Ca2+-depleted SR only requires <1 min to fully reload with Ca2+ to endogenous levels under the same conditions (data not shown). Previous studies with isolated t-system vesicles (19) have also shown that the rates of calcium transport by the t-system are two orders of magnitude lower than those of SR.
Because IP3Rs are strongly believed to mediate SOC in many nonexcitable cells (1, 2, 4, 5), it was important to know whether the IP3Rs play a role in SOC in skeletal muscle. To find out whether this was the case, the potent competitive IP3R antagonist heparin (14, 20, 21) was used in a series of experiments, and the results are summarized in Fig. 2. The protocol for these experiments was similar to that described for Fig. 1 A and B except that at each heparin concentration tested the preparation was allowed to equilibrate with heparin for 5 min before caffeine application (heparin was also present in caffeine solutions). Heparin inhibited SOC in a concentration-dependent manner (see filled symbols in Fig. 2). It is important to note that caffeine application caused complete depletion of the SR Ca2+ stores through the RyRs at each [heparin] (data not shown), thus enabling what would otherwise be maximal activation of SOC in the absence of heparin (which would be seen as a drop in t-system [Ca2+] to 48 ± 14 μM in these preparations). Furthermore, Fig. 2 (open squares) shows the effect of addition of exogenous IP3 to heparin-containing solutions of IP3 was included in the heparin solutions in the ratios (IP3/heparin): 10 μM:10 μg/ml; 100 μM:100 μg/ml; and 100 μM:1 mg/ml. At all [heparin] examined the addition of IP3 caused a reduction of the inhibitory effect of heparin on the loss of t-system Ca2+ when the stores were depleted. This is fully consistent with the action of heparin as a competitive IP3R blocker (22). Thus, the results in Fig. 2 point to graded SOC mediation by IP3Rs in skeletal muscle cells.
We have also found that high [IP3] (>0.2 mM) by itself could not deplete the SR of Ca2+ nor cause marked loss of t-system Ca2+ without an agent capable of causing SR-store depletion (Fig. 3). Nevertheless, there was a statistically significant decrease in t-system Ca2+ when IP3 was present compared with controls (Fig. 3).
It is well known that the t-system dihydropyridine receptor (DHPR) and RyR form a physical coupling in skeletal muscle, which is essential for skeletal-type coupling (7). In skinned skeletal muscle fibers, it is possible to sever this physical link by the application of high intracellular Ca2+, which induces subtle changes at the triad (16). Therefore, we used this uncoupling method to determine whether a physical coupling existed and was necessary [such as the proposed retrograde coupling involving calsequestrin (23)] for the normal function of SOC in skeletal muscle. The [Ca2+] gradient across the t-system was not different after uncoupling (data not shown), but importantly, depletion of SR Ca2+ with caffeine (16) greatly inhibited SOC (Fig. 3). Fig. 3 also shows a summary of the effect of the DHPR blocker nifedipine (10 μM) on SOC. Nifedipine had no effect on SOC, as also reported for adult mammalian skeletal muscle (6).
Discussion
SOC in Mechanically Skinned Skeletal Muscle Fibers.
This study has shown that there is a functional SOC mechanism in mechanically skinned skeletal muscle fibers of toad examined by confocal measurements of extracellular [Ca2+], using Fluo-5N trapped in the essentially finite pools of the sealed t-system. [Ca2+] in the sealed t-system dropped rapidly after the sudden depletion of SR Ca2+ in the presence of caffeine and 75 μM Mg2+ (Fig. 1). Importantly, when the SOC mechanism was inhibited by heparin (Fig. 2) or when the E-C coupling was interrupted by high Ca2+ treatment (Fig. 3), relatively little Ca2+ was lost from the sealed t-system over a 30-s period in the presence of 30 mM caffeine and 75 μM Mg2+, which are known to fully deplete the SR of Ca2+ under these conditions (16, 24). This shows that (i) neither 30 mM caffeine nor the reduction in [Mg2+] from 1 mM to 75 μM, by themselves, could cause thorough depletion of Ca2+ in the sealed t-system over this time period, and (ii) only preparations with functionally coupled membranes at the triad (see Coupling of the IP3R to the t-System Membrane) were able to elicit SOC.
The load of t-system Ca2+ could subsequently be reaccumulated via functional t-system Ca2+ pumps in a myoplasmic solution containing 200 nM Ca2+ and 1 mM Mg2+ (Fig. 1). Thus, the mechanically skinned fiber is a unique preparation containing both RyRs and IP3Rs [both previously implicated in SOC (4, 5, 23)] where SOC can be studied with full experimental access and control of the cytoplasm.
Role of the IP3R in Skeletal SOC.
We present evidence here that implicates the IP3R as the SR Ca2+ sensor necessary for SOC (Fig. 2). This evidence is twofold. First, heparin blocked SOC in a concentration-dependent manner. Second, the addition of exogenous IP3 to the heparin-containing solutions caused further loss of t-system Ca2+ (Fig. 2). This result shows that heparin alone is blocking the IP3R, and the addition of IP3 to the heparin-containing solution removes the blocking effect of heparin on the IP3R as one would expect considering that heparin is known to be a competitive IP3R blocker (14, 20–22). This implies that IP3 must bind to the IP3R for SOC function in skeletal muscle as suggested in other cells (4). Because SOC operates without exogenous IP3 in the freshly skinned muscle fibers, it indicates that sufficient endogenous IP3 is present in this preparation to activate a significant fraction of the IP3Rs. This is supported further by the relatively large concentration of heparin necessary for blocking SOC (Fig. 2). Indeed, it has been shown that there is a high level of IP3 production in the triad of amphibian skeletal muscle (25), and IP3Rs have been identified in muscle fibers (26) and localized at the triad in the SR (27, 28). The presence of IP3/IP3Rs at the triad in skeletal muscle led to the idea that they may be directly involved in E-C coupling (9, 10), but it now appears that IP3/IP3Rs play little role in normal E-C coupling (7, 11, 29, 30). Interestingly, the addition of 500 μM exogenous IP3 did cause a statistically significant loss of t-system Ca2+ without depletion of the SR Ca2+ stores (Fig. 3), although the t-system Ca2+ loss was much smaller than when SOC was fully activated. This result may suggest that at the endogenous level of IP3, not all IP3Rs are complexed with IP3, and that by increasing [IP3], some SOC channels become activated at the SR Ca2+-loading level in our preparations. Taken together, the results suggest a previously uncharacterized role for the IP3Rs at the triad in skeletal muscle as SR Ca2+-content sensors that is important for maintaining the long-term stability of E-C coupling (31).
Coupling of the IP3R to the t-System Membrane.
High Ca2+ exposure causes subtle morphological changes at the triad (16) that sever the “mechanical” coupling between the DHPRs and the RyRs (16). Because this mode of uncoupling junctional membranes is nonspecific for various protein–protein interactions, other types of physical coupling in existence across the triad (32) between the t-system and SR membranes are also likely to be disrupted by such high Ca2+ treatment. The marked loss of SOC after Ca2+ uncoupling indicates that the pathway between the IP3R and the t-system must have been severed. It is important to be aware that the SR maintains the ability to sequester and release Ca2+ with caffeine and that the t-system is also able to maintain a [Ca2+] ratio across its membrane of ≈106 (see Results and ref. 16). This indicates that the functional state of the membrane-imbedded proteins are affected little by high Ca2+ exposure. Therefore, presumably, the SOC mechanism would be unaffected by Ca2+ uncoupling if a diffusible messenger were responsible for mediating SOC. A physical coupling between the IP3R and plasma membrane has been shown in nonexcitable cells (4, 5), and this study suggests that a similar mechanism of SOC is occurring in skeletal cells.
Complete inhibition of SOC by the nonspecific IP3R blocker 2-aminoethoxydiphenylborane has also been observed in myotubes (31), but the authors suggested that there may be a retrograde action between the RyR and DHPR to account for SOC after they found that SOC was sensitive to but not inhibited completely by the genetic deletion of RyRs and the membrane scaffolding protein mg29 (31). The results, however, are also consistent with a physical coupling of the IP3R to the SOC channel in skeletal muscle without requiring a retrograde action between the RyR and DHPR, because it is likely that RyR and mg29 deletions affect the normal protein structuring at the triad, which may affect the coupling of the IP3R to the SOC channel.
Furthermore, the results of this study (Fig. 3) and that of Kurebayashi and Ogawa (6) found that SOC in adult skeletal muscle was insensitive to nifedipine, suggesting that the t-system SOC channel in skeletal muscle is not the DHPR. As in other cell types, the most likely candidates for SOC channels in skeletal muscle are channels formed by transient receptor potential (trp) proteins (4).
In conclusion, these results demonstrate an important functional role of the IP3R in Ca2+ homeostasis in skeletal muscle, which was not clear until now. Furthermore, this study provides direct support for the physical-coupling model of SOC operation (1, 2, 4, 5) and shows that IP3Rs are essential in mediating SOC, which plays a major role in Ca2+ homeostasis and the maintenance of longer-term E-C coupling (31).
Acknowledgments
This work was supported by grants from the Australian Research Council and the National Health and Medical Research Council of Australia.
Abbreviations
- SOC
store-operated Ca2+ entry
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- RyR
ryanodine receptor
- E-C
excitation–contraction
- t
tubular
- SR
sarcoplasmic reticulum
- DHPR
dihydropyridine receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Putney J W., Jr Cell. 1999;99:5–8. doi: 10.1016/s0092-8674(00)80056-2. [DOI] [PubMed] [Google Scholar]
- 2.Putney J W, Jr, Broad L M, Braun F, Lievremont J, Bird G S J. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M J. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 4.Kiselyov K, Xu X, Mozhayeva G, Kuo T, Pessah I, Mignery G, Zhu X, Birnbaumer L, Muallem S. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- 5.Ma H, Patterson R L, van Rossum D B, Birnbaumer L, Mikoshiba K, Gill D. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 6.Kurebayashi N, Ogawa Y. J Physiol (London) 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melzer W, Herrmann-Frank A, Lüttgau H C H. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- 8.Jaimovich E, Carrasco M A. Biol Res. 2002;35:195–202. doi: 10.4067/s0716-97602002000200010. [DOI] [PubMed] [Google Scholar]
- 9.Vergara J, Tsien R Y, Delay M. Proc Natl Acad Sci USA. 1985;82:6352–6356. doi: 10.1073/pnas.82.18.6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe P, Di Virgilio F, Pozzan T, Salviati G. Nature. 1985;316:347–349. doi: 10.1038/316347a0. [DOI] [PubMed] [Google Scholar]
- 11.Jaimovich E. J Muscle Res Cell Motil. 1991;12:316–320. doi: 10.1007/BF01738586. [DOI] [PubMed] [Google Scholar]
- 12.Launikonis B S, Stephenson D G. J Physiol (London) 2001;534:71–85. doi: 10.1111/j.1469-7793.2001.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Launikonis B S, Stephenson D G. Cell Biol Int. 2002;26:921–929. doi: 10.1006/cbir.2002.0942. [DOI] [PubMed] [Google Scholar]
- 14.Prakriya M, Lewis R S. J Physiol (London) 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann-Frank A, Lüttgau H C H, Stephenson D G. J Muscle Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- 16.Lamb G D, Junankar P R, Stephenson D G. J Physiol (London) 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launikonis B S, Stephenson D G. J Physiol (London) 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Launikonis B S, Stephenson D G. J Physiol (London) 2002;538:607–618. doi: 10.1113/jphysiol.2001.012920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo C, Gonzalez M E, Garcia A M. Biochim Biophys Acta. 1986;854:279–286. doi: 10.1016/0005-2736(86)90121-5. [DOI] [PubMed] [Google Scholar]
- 20.Supattapore S, Worley P F, Baraban J M, Sneyder S H. J Biol Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- 21.Parys J B, Sernett S W, Delisle S, Snyder P M, Welsh M J, Campbell K P. J Biol Chem. 1992;267:18776–18782. [PubMed] [Google Scholar]
- 22.Takei K, Shin R M, Inoue T, Kato K, Mikoshiba K. Science. 1998;282:1705–1708. doi: 10.1126/science.282.5394.1705. [DOI] [PubMed] [Google Scholar]
- 23.Shin D W, Pan Z, Kim E K, Lee J M, Bhat M B, Parness J, Kim D H, Ma J. J Biol Chem. 2003;278:3286–3292. doi: 10.1074/jbc.M209045200. [DOI] [PubMed] [Google Scholar]
- 24.Lamb G D, Posterino G S, Stephenson D G. J Physiol (London) 1994;474:319–329. doi: 10.1113/jphysiol.1994.sp020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo C, Jorquera J, Tapia V, Donoso P. J Biol Chem. 1993;268:15111–15117. [PubMed] [Google Scholar]
- 26.Moschella M C, Watras J, Jayaraman T, Marks A R. J Muscle Res Cell Motil. 1995;16:390–400. doi: 10.1007/BF00114504. [DOI] [PubMed] [Google Scholar]
- 27.Valdivia C, Vaughan D, Potter B V, Coronado R. Biophys J. 1992;61:1184–1193. doi: 10.1016/S0006-3495(92)81927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell J A, Carrasco M A, Adams D S, Drouet B, Rios J, Müller M, Estrada M, Jaimovich E. J Cell Sci. 2001;114:3673–3683. doi: 10.1242/jcs.114.20.3673. [DOI] [PubMed] [Google Scholar]
- 29.Pape P C, Konishi M, Baylor S M, Somlyo A P. FEBS Lett. 1993;235:57–62. doi: 10.1016/0014-5793(88)81233-x. [DOI] [PubMed] [Google Scholar]
- 30.Posterino G S, Lamb G D. J Muscle Res Cell Motil. 1998;19:67–74. doi: 10.1007/BF03257391. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z, Yang D, Nagaraj R Y, Nosek T A, Nishi M, Takeshima H, Cheng H, Ma J. Nat Cell Biol. 2002;4:379–383. doi: 10.1038/ncb788. [DOI] [PubMed] [Google Scholar]
- 32.Felder E, Protasi F, Hirsch R, Franzini-Armstrong C, Allen P D. Biophys J. 2002;82:3144–3149. doi: 10.1016/S0006-3495(02)75656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]