Abstract
Objective
To investigate whether gastrin has regenerative effect on the pancreas and in particular whether it prevents the atrophy of the distal pancreas after resection of pancreas in humans.
Summary Background Data
Although pancreatic regeneration after resection is well documented in animals, atrophy rather than regeneration of the distal remnant pancreas commonly occurs following pancreatoduodenectomy in humans. Of the many factors involving pancreatic regeneration, gastrin has been shown to have trophic effect on the pancreas in an animal model.
Methods
Between March 1999 and May 2000, a randomized prospective study was performed in 56 patients who underwent pylorus-preserving pancreatoduodenectomy for periampullary neoplasms. Patients were allocated to either a lansoprazole group or a control group. The lansoprazole members were given oral lansoprazole (30 mg/d) over 12 weeks postoperatively to induce hypergastrinemia. During the study period, 19 patients were excluded for different reasons; in the end a total of 37 patients (lansoprazole, n = 18; control, n = 19) were eligible for study. The volume of the distal pancreas as determined using thin-sectioned spiral CT data, nutritional status, and endocrine (insulin level, glucose tolerance test) and exocrine function (stool elastase) of the pancreas and serum gastrin levels were measured before surgery and 3 months after surgery. The two groups were clinically comparable.
Results
Serum gastrin level was elevated in the lansoprazole group. In this group, the mean volume of the distal pancreas was reduced by 10% after pylorus-preserving pancreatoduodenectomy, whereas severe pancreatic atrophy occurred in the control group. Postoperative insulin and stool elastase levels were higher in the lansoprazole group than in the control group.
Conclusions
This study is the first prospective randomized trial of induced hypergastrinemia on the regeneration of the pancreas in humans. It may be possible to use induced hypergastrinemia in the treatment or prevention of pancreatic insufficiency following resection or injury.
Pancreatoduodenectomy has become a standard operation for patients with malignant and benign diseases of the periampullary area. However, postoperative diarrhea, steatorrhea, and poor nutritional status are not infrequently seen after pancreatoduodenectomy. Pancreatic insufficiency and loss of distal stomach and duodenum are the main causes of this poor functional outcome.
Atrophy of the remnant distal pancreas after pancreatoduodenectomy is usual rather than exceptional. Little information is available in humans concerning the prevention of pancreatic atrophy, which has the sequela of pancreatic insufficiency. 1–3
Regeneration of the pancreas after resection has been relatively well documented in animal models. Some reports have demonstrated that pancreatic volume increases by about 30% to 70%, according to the extent of pancreatic resection, and that regeneration is complete within a 2- to 6-month period after resection. 4,5
Many potential regulatory factors for pancreatic regeneration have been studied. 6 Of these, several gut hormones have been shown to have a trophic effect on the pancreas. In particular, gastrin and cholecystokinin (CCK), which share a common amino acid sequence in their C-terminal 5 amino acids, have been widely studied through experimental models. 7–10 Xu et al. 9 reported that lansoprazole (proton pump inhibitor)-induced endogenous hypergastrinemia increases the pancreas weight and leads to amelioration of hyperglycemia and β-cell function after 90% partial pancreatectomy. We found a similar regenerative effect of endogenous hypergastrinemia on 66% resected pancreas in rat. 10 In the previous report, we proposed the possibility of clinical application of endogenous hypergastrinemia induced by a proton pump inhibitor, which is easily inducible in humans, for the stimulation of pancreatic regeneration.
For these reasons, we undertook this study to investigate whether gastrin has a similar regenerative effect on the human pancreas as it does in animal models, and whether this prevents the atrophy of the distal pancreas after pylorus-preserving pancreatoduodenectomy (PPPD).
METHODS
Study Design
Between March 1999 and May 2000, we conducted a randomized prospective study on 56 patients who underwent PPPD with presumed periampullary neoplasms in Seoul National University Hospital. We excluded three patients who had evidence of pancreatitis on physical examination, laboratory tests, and CT, because pancreatitis affected the pancreatic volume preoperatively due to swelling. Including 3 patients with pancreatitis, 11 of 56 patients were excluded: 3 did not consent to the study, 2 had severe pancreatic atrophy with a tortuous dilated pancreatic duct, 1 had combined colon resection, and 2 had prolonged delayed alimentation due to complications. Subjects were allocated into two groups preoperatively: a control group and a lansoprazole group (lansoprazole was given at a dosage of 30 mg/d for 12 weeks postoperatively to induce endogenous hypergastrinemia). Surgeons were unaware of the patient’s allocation. During the study period, 8 patients were excluded, leaving 37 patients eligible for study (18 patients in the lansoprazole group, 19 patients in the control group;Fig. 1).
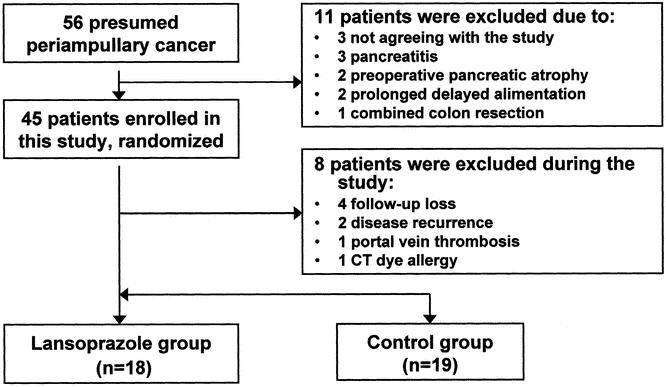
Figure 1. Patient allocations.
Gastrin Assay
Serum gastrin levels were measured immediately before surgery and after 1 week of oral diet postoperatively. After at least 8 hours of fasting, a blood sample was taken before and 15, 30, and 60 minutes after the ingestion of a modified Lundh test meal (300 mL, carbohydrate 75 g, protein 19.5 g, and fat 18.5 g). Serum gastrin was measured using a GammaDab (DiaSorin, MN).
Pancreas Volumetry
Pancrease volume was measured using spiral computed tomography (CT) with 5-mm consecutive section images and contrast opacification. After reconstructing the CT image data, a sectional area of the pancreas was selected using Image-Pro Plus (Media Cybernetics, MD), and three-dimensional volume of the distal pancreas was calculated using MATLAB (The MathWorks, MA). Image analysis and volumetry was done by a specialized radiologist who was unaware of the patient’s status. The volume of the remnant distal pancreas was measured 3 months after surgery using the same CT volumetry and was compared with the preoperative volume of the distal pancreas according to the surgical resection line.
Nutritional Status
Body weight and triceps skin-fold thickness were measured preoperatively and 1 and 3 months after surgery. To reduce the error caused by height differences, relative body weight (measured body weight/ideal body weight × 100 [%]), using a modified Broca’s formula ([height (cm) –100] × 0.9) was used for data analysis.
Evaluation of Pancreatic Function
The endocrine functions of the pancreas were evaluated by measuring the serum insulin level and performing an oral glucose tolerance test after a 75-g glucose challenge. The data were compared between the two groups, excluding the patients who had impaired glucose tolerance or diabetes mellitus preoperatively. Stool elastase I activity (μg unit per 1 g stool) was measured to evaluate pancreatic exocrine functions by using the monoclonal antibody and ELISA technique. Pancreatic endocrine and exocrine function tests were carried out preoperatively and 1 and 3 months after surgery, respectively. For the postoperative 3-month evaluation, the pancreatic function test was performed 1 week later after the completion of medication (lansoprazole).
Factors Influencing Pancreatic Volume Changes
To identify factors that might affect the postoperative change of pancreas volume, we analyzed age, sex, diagnosis, presence of preoperative jaundice, methods of pancreaticoenteric anastomosis, pancreatic leakage, use of somatostatin analog, and adjuvant chemoradiation.
Ethics
The study protocol was approved by the ethics committee of the Seoul National University Hospital and was conducted in accordance with the Declaration of Helsinki, as revised in 1983. Written and oral informed consent was obtained from all patients enrolled in this study.
Statistical Analysis
Comparisons were performed using the Mann-Whitney and chi-square tests. Results are expressed as mean ± standard error of the mean (SEM). Significance was accepted at the 5% level. All statistical analyses were performed with SPSS software.
RESULTS
Patients
The mean age of the patients was 58.4 years (range 33–81), and the male to female ratio was 21:16. Of the 37 patients, 16 had ampulla of Vater cancer, 13 had bile duct cancer, 6 had pancreas head cancer, and 2 had duodenal cancer. We found no significant difference between the two groups in terms of patient characteristics and perioperative parameters (Table 1).
Table 1. CLINICAL CHARACTERISTICS OF PATIENTS
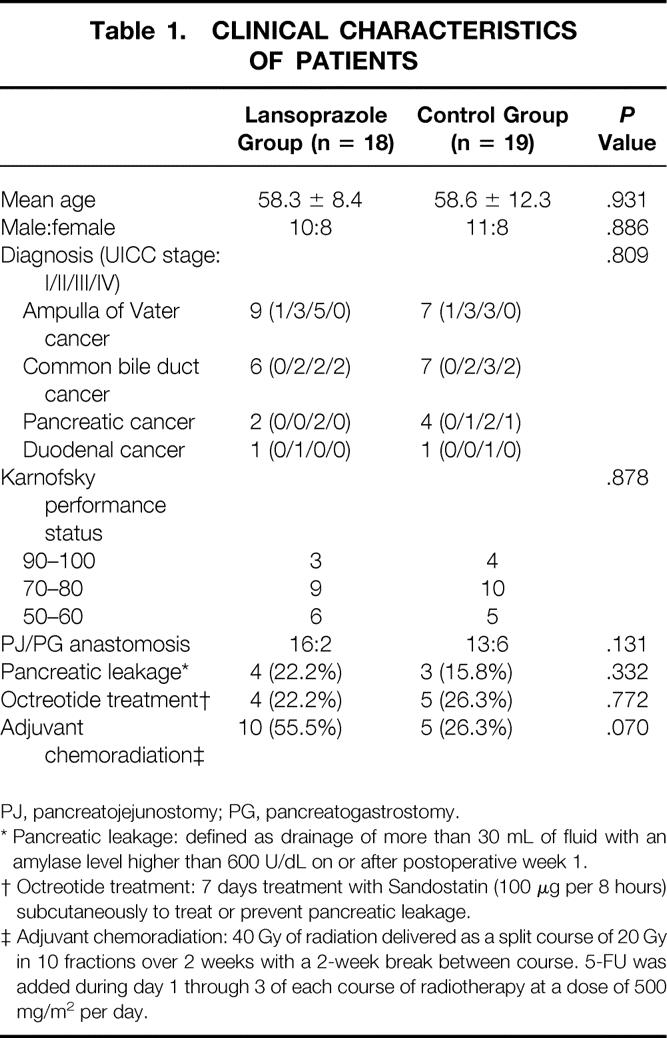
PJ, pancreatojejunostomy; PG, pancreatogastrostomy.
* Pancreatic leakage: defined as drainage of more than 30 mL of fluid with an amylase level higher than 600 U/dL on or after postoperative week 1.
† Octreotide treatment: 7 days treatment with Sandostatin (100 μg per 8 hours) subcutaneously to treat or prevent pancreatic leakage.
‡ Adjuvant chemoradiation: 40 Gy of radiation delivered as a split course of 20 Gy in 10 fractions over 2 weeks with a 2-week break between course. 5-FU was added during day 1 through 3 of each course of radiotherapy at a dose of 500 mg/m2 per day.
Gastrin Assay
Figure 2 shows preoperative and postoperative serum gastrin concentrations during modified Lundh meal test. No difference was found in the preoperative gastrin concentration of the two groups. The postoperative gastrin level of the lansoprazole group was approximately twice as high as the preoperative level (P < .001). In the control group, no differences were found between the preoperative and postoperative gastrin levels.
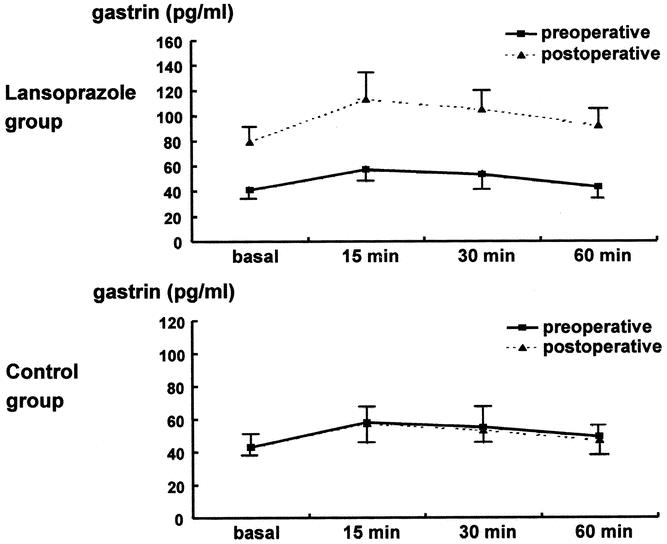
Figure 2. Effect of lansoprazole (proton pump inhibitor) administration on serum gastrin level.
Pancreas Volumetry
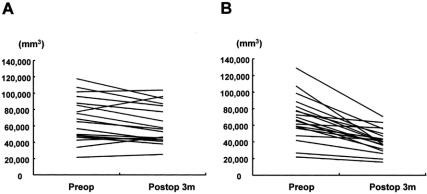
There was no significant difference in the mean preoperative volume of the distal pancreas between the control group and the lansoprazole group (Table 2, Fig. 3). Three months postoperatively, the remnant pancreatic volume decreased by 44% in the control group (P < .001). In the lansoprazole group there was no significant volume reduction of the distal remnant pancreas (10.7%, P = .108). Therefore, lansoprazole prevented the atrophy of the remnant distal pancreas after pancreatoduodenectomy.
Table 2. VOLUME CHANGE OF THE DISTAL (REMNANT) PANCREAS IN LANSOPRAZOLE AND CONTROL GROUPS
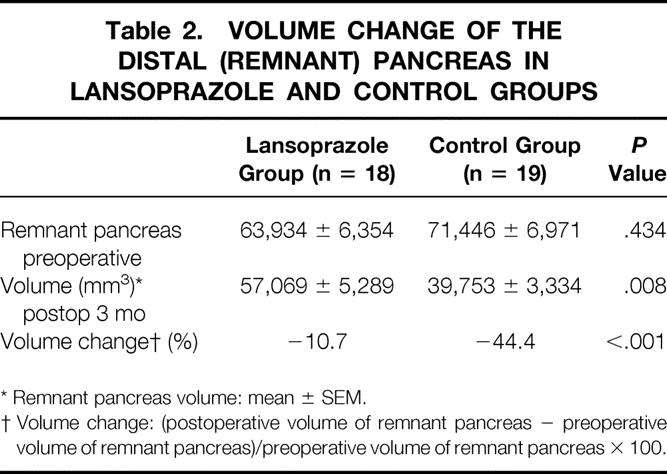
* Remnant pancreas volume: mean ± SEM.
† Volume change: (postoperative volume of remnant pancreas − preoperative volume of remnant pancreas)/preoperative volume of remnant pancreas × 100.
Figure 3. Individual volume changes of the distal pancreatic remnant in the lansoprazole group (A) and the control group (B).
Nutritional Status
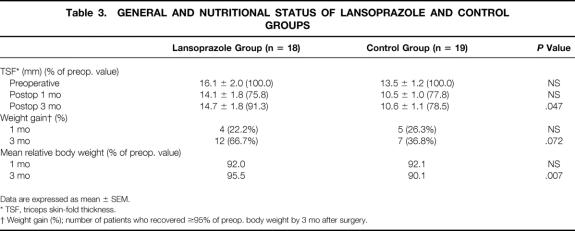
About 8% of relative body weight was lost 1 month after PPPD (compared to the preoperative weight) in both groups. After 3 months, 5% body weight loss was observed in the lansoprazole group, but 10% body weight loss was apparent in the control group (P = .007) (Table 3). The percentage of the patients who recovered more than 95% of their preoperative body weight 3 months postoperatively was 67% (12/18) in the lansoprazole group and 37% (7/19) in the control group (P = .072). Mean triceps skin-fold thickness 3 months after PPPD was lowered by 8.7% in the lansoprazole group and by 21.5% in the control group (see Table 3) (P = .047).
Table 3. GENERAL AND NUTRITIONAL STATUS OF LANSOPRAZOLE AND CONTROL GROUPS
Data are expressed as mean ± SEM.
* TSF, triceps skin-fold thickness.
† Weight gain (%); number of patients who recovered ≥95% of preop. body weight by 3 mo after surgery.
Pancreatic Endocrine and Exocrine Functions
In the oral glucose tolerance test, excluding the patients who had impaired glucose tolerance or diabetes mellitus before surgery, 1 of 16 patients (6.3%) had diabetes and 2 patient (12.5%) had impaired glucose tolerance in the lansoprazole group. In the control group, 1 of 15 patients (6.7%) had diabetes and 7 patients (46.7%) had impaired glucose tolerance. Therefore, 53.4% of the control group and 18.8% of the lansoprazole group had pancreatic endocrine insufficiency (Table 4). Although there was no statistically significant difference, we found a trend that endocrine functional deterioration was relatively prevented in the lansoprazole group compared to the control group. The serum insulin level also supported this preservation of pancreatic endocrine function in the lansoprazole group. The serum insulin level was higher in the lansoprazole group (21.1 ± 12.1 μU/mL, 101.0% of preoperative level) than in the control group (6.9 ± 1.6 μU/mL, 47.6% of preoperative level) 3 months postoperatively (Fig. 4).
Table 4. RESULTS OF ORAL GLUCOSE TOLERANCE TEST BETWEEN LANSOPRAZOLE AND CONTROL GROUPS
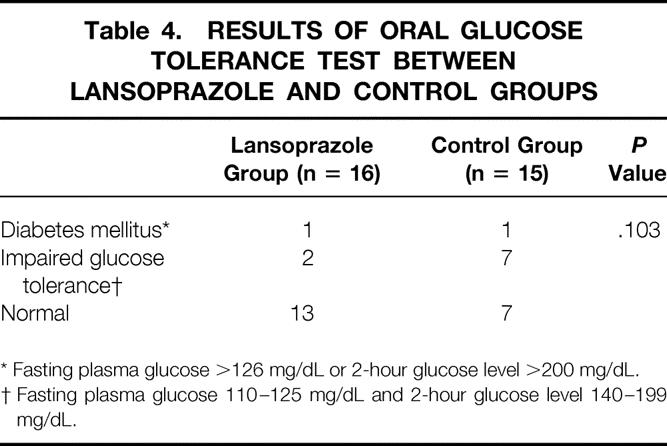
* Fasting plasma glucose >126 mg/dL or 2-hour glucose level >200 mg/dL.
† Fasting plasma glucose 110–125 mg/dL and 2-hour glucose level 140–199 mg/dL.
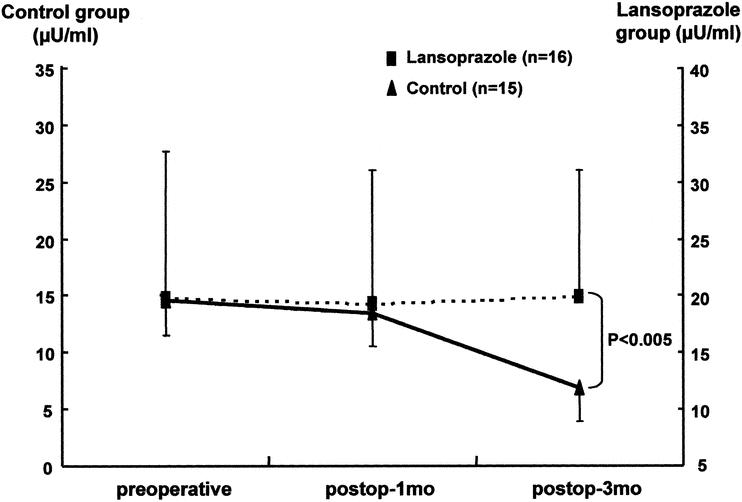
Figure 4. Serum insulin levels in the lansoprazole group and the control group.
The preoperative stool elastase level was 233 ± 46 μg/g in the control group and 203 ± 38 μg/g in the lansoprazole group (Fig. 5). After pancreatic resection, enzyme activity was lowered without a difference between the groups 1 month postoperatively. Three months postoperatively, the stool elastase level in the lansoprazole group was stationary at a level of 59 ± 12 μg/g, but in the control group it decreased to 24 ± 6 μg/g; the difference was significant (P = .009).
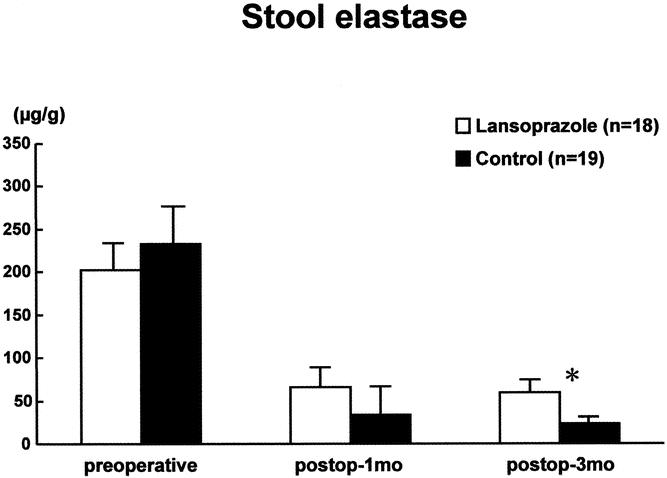
Figure 5. Stool elastase I levels in the lansoprazole group and the control group.
Factors Influencing Volume Change of Pancreas
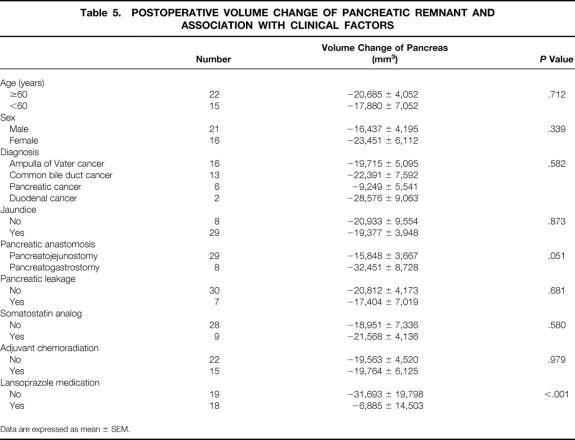
Of the nine clinical variables analyzed, only postoperative volume change of the pancreatic remnant was significantly related to lansoprazole treatment (P < .001) (Table 5). The method of pancreatic anastomosis approached statistical significance (P = .051). The other seven factors were not significantly associated with postoperative volume change of pancreas.
Table 5. POSTOPERATIVE VOLUME CHANGE OF PANCREATIC REMNANT AND ASSOCIATION WITH CLINICAL FACTORS
Data are expressed as mean ± SEM.
When we performed general linear model analysis to exclude the interaction between these variables, only lansoprazole treatment had statistical significance (P = .001), and the method of pancreatic anastomosis lost its marginal significance (P = .188).
DISCUSSION
Since Martinotti 11 reported the regeneration of pancreas in 1892, there have been many reports about pancreatic regeneration after injury or resection, especially in animal models. 7–10 Although the regenerative power of pancreas is not so intensive as the liver, 12 pancreatic regeneration seems to occur in some circumstances, and its associated factors are also known. 6
Of the many factors, gastrin is relatively easy to manipulate and can be converted to clinical use. Recently some studies have reported that hypergastrinemia, induced either medically (e.g., proton pump inhibitor) or surgically (e.g., fundectomy), has a trophic effect on the pancreas after pancreatic resection in animal models. 8–10,13,14
While there have been many reports about the growth and regeneration of pancreas in experimental animals, surprisingly, human studies on pancreatic regeneration are rare. Schlegel et al. 15 presented a case that showed complete restoration of the pancreatic volume 1 year after a distal pancreatectomy in a 57-year-old woman diagnosed with mucinous cystadenoma. Schonau et al. 16 reported that after 95% pancreatectomies in infants with nesidioblastosis, pancreatic endocrine and exocrine functions were satisfactory and the pancrease showed extensive regrowth. Others reported that pancreas regeneration with functional restoration may occur in tissue injury after pancreatitis as well as in pancreatic resection. 17,18
However, many reports show that remnant pancreatic atrophy, rather than regeneration, commonly occurs after surgical resection, especially after pancreatoduodenectomy in humans. 2,3,19 Remnant pancreatic atrophy is usual rather than exceptional after pancreatic head resection, which is the most common type of pancreatectomy. This pancreatic atrophy after pancreatic head resection results in exocrine and endocrine functional loss of the pancreas. 3,20,21
We previously suggested that postoperative atrophy of the distal pancreas after pancreatoduodenectomy occurs in humans because, at least in part, of the resection of the duodenum and the distal stomach, which are the sources of the trophic stimuli cholecystokinin and gastrin. 3 We confirmed that pylorus preservation decreased the extent of atrophy of the remnant pancreas after pancreatoduodenectomy by preserving the antrum secretion of gastrin compared to Whipple’s procedure. This suggests that gut hormones such as gastrin and CCK are related to the growth and regeneration of the pancreas.
In this study, we confirmed that atrophy of the pancreas after resection of the pancreas head occurs generally in humans; this is consistent with our previous findings in humans, 3,20 not showing extensive regrowth or regeneration as in animal models. 10 However, the amount of pancreatic atrophy in the lansoprazole group (the induced hypergastrinemia group) was lower than that of the control group, with better general status and pancreatic function in the lansoprazole group. These results suggest that induced hypergastrinemia after resection of the pancreas provokes pancreatic regrowth, prevents pancreatic atrophy, and therefore positively influences the restoration of pancreatic function and general nutritional status after pancreatoduodenectomy.
We found that there was not a great difference in pancreatic function and general status based on the degree of pancreatic volume change, despite considerable differences in the postoperative remnant pancreatic volume between the two groups. Even in the lansoprazole group, some patients showed severe pancreatic atrophy rather than regrowth, and there was no linear relationship between the volume change of pancreas and the change in pancreatic function or general nutritional status in a correlation test (data not shown). This means that there is individual variability in the growth response of pancreas to gastrin and in the relationship between pancreatic volume and pancreatic function. Because pancreas has very intricate relationships with other organs and sometimes itself, we do not think that manipulating only one hormonal milieu can affect every patient to the same degree. The individual variability in the growth response to the gastrin is at least partially due to the variability of the expression of gastrin receptor. Gastrin is expressed in the fetal pancreas and is likely to play a role in pancreas development and regeneration in humans. 22 Although some reports showed that the functional gastrin receptor exists in adult pancreas, 23 it is believed that gastrin receptor is present in adult pancreas minimally and its expression increases after damage, such as tumor, resection, or inflammation. 24,25
The status of gastrin receptor between rats and humans is slightly different; it is difficult to make a general statement about the intensity of gastrin’s trophic effect in humans and rats. However, we can suggest that metabolic demand to stimulate pancreas regrowth varies individually and has a great impact on the trophic driving force of gastrin.
According to Lankisch et al. 26 and Bozkurt et al., 27 after recovery from necrotizing pancreatitis, individual pancreatic enzyme secretion varied considerably during recovery in the follow-up period, and as little as 10% of the gland remaining would meet the functional demand of pancreas. Tsiotos et al. 2 insisted that pancreatic growth factors may not be upregulated in patients with larger remnants, as in pancreatoduodenectomy (about 50% resection of pancreas). Further studies, including the expression of the gastrin receptor in adult humans, are needed to predict the individual response to gastrin and the regrowth potential of pancreas after resection or injury.
Another consideration for the variability of pancreatic function is the technical problem of study. In human studies, restricting the study condition strictly is impossible, unlike animal models. Besides the possibility of influence of gastrin on the pancreatic test, 23 lifestyle factors, including diet, and other diseases or medications may affect the result of pancreatic function and general nutritional status. Although we tried to control many factors, including medications (except lansoprazole), and used more stable enzyme tests such as elastase I, 28 other factors that we could not control may have affected the pancreatic function test and general nutritional evaluation in this study.
Notwithstanding the many possible factors that could affect the results this study, we suggest that a mechanism of pancreatic regrowth or regeneration exists in humans, and gastrin plays a considerable role in that process. Other clinical factors associated with the functional loss of the pancreas after pancreatic resection have been believed to be accompanying pancreatitis, the patency of the pancreatic duct after pancreaticoenteric anastomosis, and the operative techniques. 20,29,30 In this study, we also investigated several clinical factors in an attempt to find any other factors that might have affected the postoperative changes in pancreatic volume. Although statistically significant factors could not be found, trends of pancreatic volume changes were observed according to the pancreaticoenteric anastomosis. Remnant pancreatic atrophy is more common in pancreatogastrostomy than in pancreatojejunostomy, and this result supports the results of previous reports showing that pancreatic insufficiency was more frequent in pancreatogastrostomy than in pancreatojejunostomy, 1,20 morphologically as well as functionally.
Some investigators argue that gastrin has trophic effects on pancreatic cancer, whereas others dismiss these effects. 31,32 These conflicting opinions on the tumor-stimulating effect of gastrin remain to be resolved. Therefore, before hypergastrinemia is used clinically, long-term follow-up is mandatory to determine its effect on tumor recurrence and metastasis in the hypergastrinemia group. Currently, no adverse trends have been observed in our study patients.
In conclusion, the results of this study suggest that induced hypergastrinemia prevents pancreatic atrophy after PPPD, probably via a mechanism of regenerative activity of the pancreas stimulated by gastrin. Although long-term follow-up is needed before clinical application, we propose that induced hypergastrinemia can be used in the treatment or the prevention of pancreatic insufficiency due to the pancreatic atrophy after pancreatic resection or pancreatitis in humans.
Footnotes
Supported by Seoul National University Research Fund (03-99-080).
Correspondence: Sun-Whe Kim, MD, FACS, Professor, Department of Surgery, Seoul National University College of Medicine, 28 Yongon-dong, Chongno-gu, Seoul, 110-744, Korea.
E-mail: sunkim@plaza.snu.ac.kr
Accepted for publication May 14, 2002.
References
- 1.Lemaire E, O’Toole D, Sauvanet A, et al. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000; 87: 434–438. [DOI] [PubMed] [Google Scholar]
- 2.Tsiotos GG, Barry MK, Johnson CD, et al. Pancreas regeneration after resection: does it occur in humans? Pancreas. 1999; 19: 310–313. [DOI] [PubMed] [Google Scholar]
- 3.Kim SW, Kim KW, Han JK, et al. Pylorus-preservation decreases the extent of atrophy of the remnant pancreas after pancreatoduodenectomy. HPB. 1999; 1: 65–70. [Google Scholar]
- 4.Pearson KW, Scott D, Torrance B. Effects of partial surgical pancreatectomy in rats. I. Pancreatic regeneration. Gastroenterology. 1977; 72: 469–473. [PubMed] [Google Scholar]
- 5.Oates PS, Morgan RG. Pancreatic growth after partial resection of normal and enlarged pancreas in rats fed soya flour. Am J Physiol. 1988; 255: G670–675. [DOI] [PubMed] [Google Scholar]
- 6.Sumi S, Tamura K. Frontiers of pancreas regeneration. J Hepatobiliary Pancreat Surg. 2000; 7: 286–294 [DOI] [PubMed] [Google Scholar]
- 7.Parekh D, Ishizuka J, Townsend CM Jr, et al. The effect of endogenous cholecystokinin released by bombesin and trypsin inhibitor on the regeneration of the pancreas. Ann Surg. 1993; 218: 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon TE, Morisset J, Wood JG, et al. Additive interaction of pentagastrin and secretin on pancreatic growth in rats. Gastroenterology. 1987; 92: 429–435. [DOI] [PubMed] [Google Scholar]
- 9.Xu G, Sumi S, Koike M, et al. Role of endogenous hypergastrinemia in regenerating endocrine pancreas after partial pancreatectomy. Dig Dis Sci. 1996; 41: 2433–2439. [DOI] [PubMed] [Google Scholar]
- 10.Kim SW, Kim KH, Park SJ, et al. Endogenous gastrin stimulates regeneration of the remnant pancreas after partial pancreatectomy. Dig Dis Sci. 2001; 46: 2134–2139 [DOI] [PubMed] [Google Scholar]
- 11.Condorelli A, Mancuso M, Ricceri R. Experimental study of the regenerative capacity of the pancreas after partial resection of the gland. Policlinico (Chir). 1963; 70: 163–180. [PubMed] [Google Scholar]
- 12.Bernuau D. Liver regeneration. Gastroenterol Clin Biol. 1993; 17: 361–363. [PubMed] [Google Scholar]
- 13.Chu M, Kullman E, Rehfeld JF, et al. Effect of chronic endogenous hypergastrinemia on the pancreatic growth and carcinogenesis in the hamster. Gut. 1997; 40: 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dembinski AB, Johnson LR. Growth of pancreas and gastrointestinal mucosa in antrectomized and gastrin-treated rats. Endocrinology. 1979; 105: 769–773. [DOI] [PubMed] [Google Scholar]
- 15.Schlegel RD, Tiscornia O, de Vedia y Mitre E, et al. Is there pancreatic regeneration? Morphological and functional certification after a corporocaudal splenopancreatectomy. Acta Gastroenterol Latinoam. 2000; 30: 107–113. [PubMed] [Google Scholar]
- 16.Schonau E, Deeg KH, Huemmer HP, et al. Pancreatic growth and function following surgical treatment of nesidioblastosis in infancy. Eur J Pediatr. 1991; 150: 550–553. [DOI] [PubMed] [Google Scholar]
- 17.Doubilet H, Mulholland JH. Eight-year study of pancreatitis and sphincterotomy. JAMA. 1956; 1620: 521–528. [DOI] [PubMed] [Google Scholar]
- 18.Pap A, Flautner L, Karacsonyi S, et al. Recovery of pancreatic function after distal resection for chronic pancreatitis: regeneration or merely functional amelioration? Mt Sinai J Med. 1987; 54: 409–412. [PubMed] [Google Scholar]
- 19.Sato N, Yamaguchi K, Yokohata K, et al. Long-term morphological changes of remnant pancreas and biliary tree after pancreatoduodenectomy on CT. Int Surg. 1998; 83: 136–140. [PubMed] [Google Scholar]
- 20.Jang JY, Kim SW, Park SJ, et al. Comparison of the functional outcome after pylorus preserving pancreatoduodenectomy between pancreatogastrostomy and pancreatojejunostomy. World J Surg. 2002; 26: 366–371. [DOI] [PubMed] [Google Scholar]
- 21.Tangoku A, Nishikawa M, Adachi A, et al. Plasma gastrin and cholecystokinin response after pylorus-preserving pancreatoduodenectomy with Billroth-I type of reconstruction. Ann Surg. 1991; 214: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomsky R, Langer F, Vortel V. Immunohistochemical demonstration of gastrin in mammalian islets of Langerhans. Nature. 1969; 223: 618–619. [DOI] [PubMed] [Google Scholar]
- 23.Saillan-Barreau C, Dufresne M, Clerc P, et al. Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes. 1999; 48: 2015–2021. [DOI] [PubMed] [Google Scholar]
- 24.Wank SA, Plsegna JR, de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci USA. 1992; 89: 8891–8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Povoski SP, Zhou W, Longnecker DS, et al. Stimulation of in vivo pancreatic growth in the rat is mediated specifically by the way of cholecystokinin-A receptors. Gastroenterology. 1994; 107: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 26.Lankisch PG, Lembcke B, Wemken G, et al. Functional reserve capacity of the exocrine pancreas. Digestion. 1986; 35: 175–181. [DOI] [PubMed] [Google Scholar]
- 27.Bozkurt T, Maroske D, Adler G. Exocrine pancreatic function after recovery from necrotizing pancreatitis. Hepato-Gastroenterology. 1995; 42: 55–58. [PubMed] [Google Scholar]
- 28.Sziegoleit A, Krause E, Klor HU, et al. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem. 1989; 22: 85–89. [DOI] [PubMed] [Google Scholar]
- 29.Sato N, Yamaguchi K, Chijiiwa K, et al. Duct-parenchymal ratio predicts exocrine pancreatic function after pancreatoduodenectomy and distal pancreatectomy. Am J Surg. 1998; 176: 270–273. [DOI] [PubMed] [Google Scholar]
- 30.Hyodo M, Nagai H. Pancreatogastrostomy (PG) after pancreatoduodenectomy with or without duct-to-mucosa anastomosis for the small pancreatic duct: short- and long-term results. Hepato-Gastroenterology. 2000; 47: 1138–1141. [PubMed] [Google Scholar]
- 31.Smith JP, Fantaskey AP, Liu G, et al. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995; 268: R135–141. [DOI] [PubMed] [Google Scholar]
- 32.Liehr RM, Melnykovych G, Solomon TE. Growth effects of regulatory peptides on human pancreatic cancer lines PANC-1 and MIA PaCa-2. Gastroenterology. 1990; 98: 1666–1674. [DOI] [PubMed] [Google Scholar]