Abstract
Objective
To compare the radiogenic effects on microcirculation in healthy and malignant pancreatic tissue.
Summary Background Data
Vascular injury is an important effect of radiotherapy, which has been suggested for antiangiogenic tumor therapy.
Methods
An established model of ductlike pancreatic cancer (DSL6A) was used. Investigation was performed in 12 healthy and 24 tumor-bearing Lewis rats. The tumors were locally irradiated with 15 Gy in 12 animals 4 weeks after intraperitoneal inoculation. Additionally, local radiation of the normal pancreas was performed in six healthy animals. Intravital microscopy of tumor and normal pancreatic microcirculation was performed 5 days after radiation. Relevant parameters were erythrocyte velocity and functional vessel density. Tumor apoptosis and the fraction of vital tumor cells were estimated histologically 5 and 12 days after radiation.
Results
Local radiation with 15 Gy caused a pronounced impairment of blood flow and functional capillary density in the normal pancreas 5 days after radiation, while the tumor blood flow was not significantly changed. A significant reduction in the fraction of vital tumor cells and a significant increase in tumor apoptosis were observed 12 days after radiation.
Conclusions
Local radiation impairs blood flow in healthy pancreas but not in pancreatic cancer tissue. Tumor cell death is the leading consequence of radiation injury in malignant pancreatic tissue without affecting the vascular system of the tumor. The authors conclude that external beam radiation does not appear to be a useful adjunct for a vascular-targeted therapy in pancreatic carcinoma but causes distinct hypoperfusion in the healthy pancreas.
Numerous histologic and ultrastructural studies have shown that radiation can impair the vascular system, leading to late complications in irradiated normal tissue. 1,2 Radiogenic injury to the microcirculation has been shown to be pronounced in various tissue types, such as hematopoietic tissue, gonads, and the gastrointestinal tract, 3 whereas vascular injury of the normal pancreas by radiation has not been studied.
It has recently been recognized that therapeutic radiation has potential implications for damage to the tumor vascular system, since the endothelium demonstrates a loss of clonogenity and might not develop resistance to radiation. 4 The fact that the tumor microcirculation is a limiting factor for tumor growth has stimulated the search for vascular-targeted therapies focusing not only on inhibition of angiogenesis but also on obliteration of tumor vasculature. 4 The reaction of tumor microcirculation to radiation is poorly understood. It is unknown whether the radiation-induced damage to microcirculation in normal tissue is comparable to the microvascular changes in tumors originating from this tissue. In the present study, the different radiogenic in vivo effects were studied using a new model of pancreatic carcinoma developed specifically to study tumor microcirculation. 5 In contrast to previous studies, this rat model allows direct observation of microcirculation after local radiation of normal pancreas and pancreatic carcinoma. We demonstrate that vascular and morphologic changes after radiation are different in normal pancreas compared to pancreatic carcinoma.
METHODS
Tumor Cell Line
An established cell line of pancreatic carcinoma of the rat, DSL6A, was used. 6 Histologically this is a desmoplastic ductlike carcinoma that resembles human pancreatic cancer, in contrast to the acinar morphology of most other types of rat tumors of the exocrine pancreas. 6
Tumor Inoculation
In previous studies, we investigated the tumor microcirculation after orthotopic tumor inoculation. 5,7 At this implantation site, radiation of healthy pancreatic tissue does not exclude a subsequent change of tumor blood flow, since both malignant and normal tissue have common afferent and efferent blood vessels. Therefore, we decided to implant the tumor underneath the peritoneum of the abdominal wall, which allows the tumor to invade the muscles of the abdominal wall and to form a vascular supply from the surrounding muscle tissue. This implantation site helps to avoid the influence of adjacent tissue injury on tumor microcirculation, since surrounding muscle tissue has a high resistance to radiation, unlike healthy pancreas. 3
Twelve male Lewis rats (300–350 g) were used for the investigation of normal pancreatic microcirculation. Twenty-four other animals (120–150 g) were used for tumor inoculation. Each animal was anesthetized with intraperitoneal pentobarbital (10 mg/kg; Nembutal, Sanofi, FRG) and intramuscular ketamine (40 mg/kg; Ketanest, Parke-Davis, Berlin, FRG). Two sterile polymethylmethacrylate (PMMA) plates (11 mm; Glasflex, Stirling, NJ, USA) were applied using sandwich technique on the peritoneum and the outer surface of the abdominal wall musculature, but beneath the skin. The plates were connected by fine sutures (Prolene 5.0; Ethicon, Norderstedt, FRG). The tumor of approximately 1 mm3 was harvested from a subcutaneous parent tumor of a syngeneic rat and interposed intraperitoneally between the plates. One lead plate (10 mm, thickness 1 mm) was used to protect the abdominal organs from radiation (Fig. 1). Another PMMA plate was placed on the lead to prevent intraperitoneal adhesions. The abdomen was closed and the animal was allowed to waken. After tumor implantation, the animals were randomly allocated to radiation or nonradiation groups.
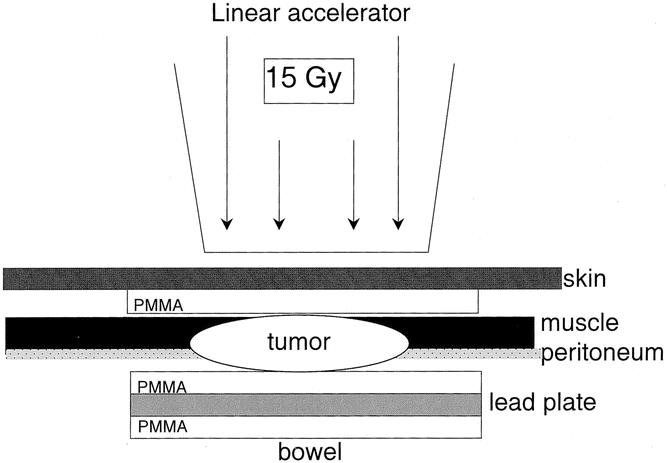
Figure 1. Tumor implantation. Two PMMA plates were applied using sandwich technique on the peritoneum and the outer surface of the abdominal wall musculature, but beneath the skin. The tumor (approximately 1 mm3) was harvested from a subcutaneous parent tumor of a syngeneic rat and interposed intraperitoneally between the plates. One lead plate was used to protect the abdominal organs from radiation. Another PMMA plate was placed on the lead to prevent intraperitoneal adhesions.
Local Irradiation
To be able to compare the antitumoral and vascular-targeted effect of radiation, we defined the optimal dose of radiation that caused a sufficient tumor cell destruction of approximately 50% tumor cells. The dose of 15 Gy has been found to satisfy this aim in vivo. 8 After 4 weeks the animals were reanesthetized as described above. The tumor area of 12 tumor-bearing rats were locally irradiated (electrons, 15 Gy) using a linear accelerator (Mevatron; Siemens, Erlangen, FRG). The abdominal organs of these animals were protected from the radiation damage by a lead plate. The radiogenic effects were investigated 5 and 12 days after radiation in six animals each.
Local radiation of the right upper abdomen was performed to radiate the pancreatic head in six healthy animals. Investigation of radiogenic injury was performed in this group 5 days after radiation only because of the high risk of injury in adjacent organs at the later phase after radiation. Additionally, 12 tumor-bearing and 6 healthy animals were not irradiated and were used as controls.
Intravital Microscopy 5 Days After Radiation
The investigation of microcirculation in pancreatic carcinoma and normal pancreas was performed as previously described. 6 Five days after radiation all animals were reanesthetized as described above. A catheter (ID 0.5 mm; B. Braun AG, Melsungen, FRG) was inserted into the left internal jugular vein for venous access. Another catheter was placed into the right carotid artery for blood sampling and monitoring of cardiovascular parameters. The abdomen was opened using a midline incision. The inner PMMA plate and the lead plate of tumor-bearing rats were carefully removed. The tumor was macroscopically identified. The tumor area in tumor-bearing animals or the pancreatic head in healthy rats was immobilized in a temperature-controlled (37°C) immersion chamber containing Ringer’s solution.
The entire setup was placed under a fluorescence microscope (Leica GmbH, Wetzlar, FRG). The fluorescence filter block I3 (excitation filter BP 450–490; emission filter LP 520) was used. After the preparation was completed three or four microscopic fields per animal were randomly chosen. After 30 minutes stabilization time, all animals received an intravenous injection of 1 mL/kg FITC-labeled autologous erythrocytes (hematocrit 50%) for the measurement of erythrocyte velocity. 9 Subsequently, 50 mg/kg FITC-labeled albumin (Sigma Chemicals Co., St. Louis, MO, USA) was infused to obtain maximal contrast of vessel morphology and to evaluate vessel density. The microcirculatory images were transmitted by a video camera (CF 8/1, Kappa GmbH, Gleichen, FRG) to a monitor (PVM-1440 mol/L, Sony, Tokyo, Japan) and recorded on a video recorder (AG-7350-E, Panasonic, Osaka, Japan) for subsequent off-line analysis.
After the end of the experiment animals were killed by an overdose of pentobarbital. The investigated tissue was harvested and fixed in 10% formalin for further histologic studies.
Assessment of Microcirculatory Parameters 5 Days After Radiation
The evaluation of microcirculatory parameters was performed off-line using special software (Capimage, Zeintl GmbH, Heidelberg, FRG). 10 Erythrocyte velocity was used as a highly sensitive parameter to assess changes in microvascular blood flow. It was assessed by the frame-to-frame method. In addition, the number of blood vessels that are passed by erythrocytes or fluorescent plasma reflects the functional vessel density (e.g., the density of perfused blood vessels). For the evaluation of vessel density, the number of perfused blood vessels were counted in four fields in each experiment and expressed as vessels per 1 mm2.
Histologic Changes and Apoptosis 5 and 12 Days After Radiation
Tissue specimens were embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin (Fluka, Steinheim, FRG) and eosin (RdH Chemicals, Seelze, FRG). Histologic changes were examined by an investigator unaware of the treatment mode by light microscopy (Zeiss GmbH, Jena, FRG). A histology score ranging from 0 (absent) to 4 (severe) was applied to the following parameters: edema, necrosis, fibrosis, and leukocyte infiltration.
Apoptosis was stained by the TUNEL method (In Situ Cell Death Detection Kit AP, Roche, Mannheim, FRG). The sections were counterstained by hematoxylin and examined by a light microscope (Zeiss GmbH). The total cross-sectioned tumor surface was transmitted by a color video camera (DBS GmbH, Hamburg, FRG) to a computer and measured using special software (Capimage). The digitalized histologic images of standard cross-sectioned tumor surface (0.735 mm2) were used to measure the number of vital tumor cells and apoptotic cells, which were counted using special software. The fraction of vital tumor cells was expressed as cells per 1 mm2. Apoptotic rate was expressed as the percentage of apoptotic cells relative to the total cell number.
Statistics
All data are given as mean ± SD. The Mann-Whitney test was used to compare the differences between the groups. P < .05 was considered significant.
RESULTS
Healthy Pancreas
Control nonradiated animals demonstrated regular microangioarchitecture and normal erythrocyte velocity, as well as higher capillary density compared to previous reports 11 (Figs. 2, 3).
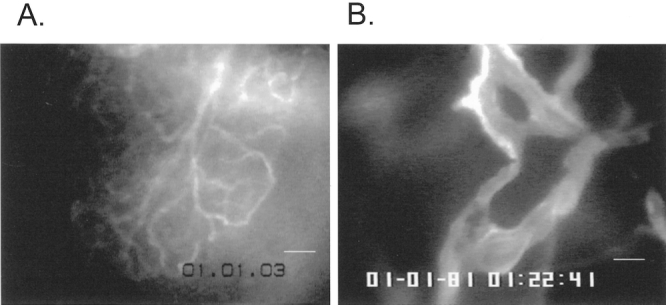
Figure 2. Microcirculation of normal exocrine pancreas and pancreatic carcinoma. (A) Normal pancreatic microcirculation represents a dense capillary network supplied by individual arterioles and drained by individual venules (white line, 25 μm). (B) Solid tumors are characterized by features such a low vessel density, lack of vascular differentiation, heterogeneous vessel distribution, and irregular vessel diameter (intravital plasma staining with FITC-labeled albumin; white line, 25 μm).
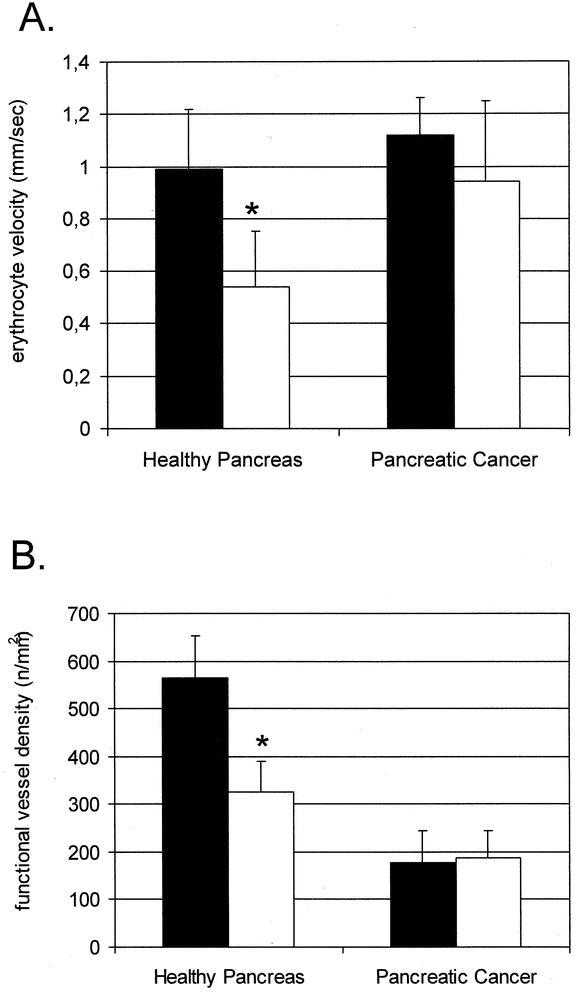
Figure 3. Erythrocyte velocity (A) and functional vessel density (B) 5 days after radiation. (A) Nonradiated normal animals demonstrated a regular erythrocyte velocity that was not significantly different from the erythrocyte velocity in nonradiated tumors. Radiation caused a significant decrease in erythrocyte velocity in normal pancreatic vessels. There were no changes of erythrocyte velocity in tumor vessels 5 days after radiation. (B) Nonradiated animals demonstrated a high capillary density that was significantly decreased 5 days after radiation. Radiation did not cause a significant change in vessel density in tumor vessels. *Significant differences compared to nonradiated normal tissue; ▪, no radiation; □, radiation 15 Gy.
After radiation with 15 Gy, inspection of irradiated normal pancreas revealed macroscopically cyanotic changes of pancreatic tissue and the duodenal loop as well as an atonic dilatation of duodenum 5 days after radiation. Subsequently, intravital microscopy showed that these changes were the sequelae of a pronounced impairment of pancreatic blood flow. As shown in Figure 3A, erythrocytes moved through capillaries with a velocity that was significantly reduced (0.54 ± 0.14 mm/s) compared to nonirradiated animals (0.99 ± 0.23 mm/s) (P = .006). Moreover, the functional capillary density (number of perfused capillaries) was significantly decreased (179 ± 65 n/mm2) compared to the control group (565 ± 89 n/mm2) (P = .001) (see Fig. 3B). Analysis of histologic sections demonstrated that there were no changes 5 days after radiation such as pancreatic edema, necrosis, or leukocyte infiltration. Apoptotic rates in normal pancreatic tissue remained under 1% and showed no significant differences compared to the control group (P > .05).
Pancreatic Carcinoma
Tumor Microcirculation Without Radiation (4 Weeks Plus 5 Days After Inoculation)
The tumors were identified at the site of implantation by gross inspection before intravital microscopy. They could be distinguished from the surrounding tissue due to their solid structure, with a typical white color and round form. After intravital microscopy, the vitality of tumor tissue was confirmed by histology. At intravital microscopy, the tumors reached a size of cross-sectioned diameter between 5 to 8 mm. Neither intratumoral necrosis nor metastasis was present in the surrounding tissue, which would appear at later stages of tumor growth in this model. The microangioarchitecture of solid tumors was characterized by typical features such as a lack of vascular differentiation, heterogeneous vessel distribution, and irregular vessel diameter (see Fig. 2). Within solid tumors, avascular regions exist next to well-vascularized areas, and frequently vascular sinusoids and lacunes can be identified (see Fig. 2). The vascular system of pancreatic cancer demonstrated a significantly lower overall vessel density than healthy pancreas (P = .007) (see Fig. 3).
Five Days After Radiation
As shown in Figure 3, the mean erythrocyte velocity was not significantly different between nonradiated (1.12 ± 0.21 mm/s) and radiated (0.94 ± 0.31 mm/s) groups (P > .05). Although there was a marked heterogeneity of distribution of erythrocyte velocity in tumor blood vessel (range 0.15–2.5 mm/s), the mean red blood cell velocity in tumor tissue did not differ significantly from healthy pancreas. In contrast to normal tissue, radiation did not cause a significant change in functional vessel density in pancreatic carcinoma.
Twelve Days After Radiation
The gross inspection of irradiated tumors did not reveal any changes in tumor tissue. Visualization of tumor blood vessels by intravital microscopy was not feasible 12 days after radiation due to inflammation and fibrosis.
Histology
Radiation caused morphologic changes such as leukocyte infiltration by mononuclear cells and fibrosis; they were already observed 5 days after radiation and progressed to pronounced alterations 12 days after radiation. The extent of these morphologic changes was heterogeneously distributed between the different tumors as well as between the different areas of the same tumor.
There were no significant changes of the tumor surface area both 5 and 12 days after radiation (Fig. 4A). The reduction of the fraction of vital tumor cells (see Fig. 4B) was not significant 5 days after radiation. However, there was a dramatic decrease in the fraction of vital tumor cells, from 1,305 ± 242 cells/mm2 in the control group to 607 ± 184 cells/mm2, 12 days after radiation (P = .010). The control group showed an apoptotic rate lower than 1%. Radiation caused a significant increase in tumor apoptosis, from 0.4 ± 0.1% in the control group to 3.7 ± 3.3% 5 days after radiation (P = .004) and to 3.2 ± 3.5% 12 days after radiation (P = .017).
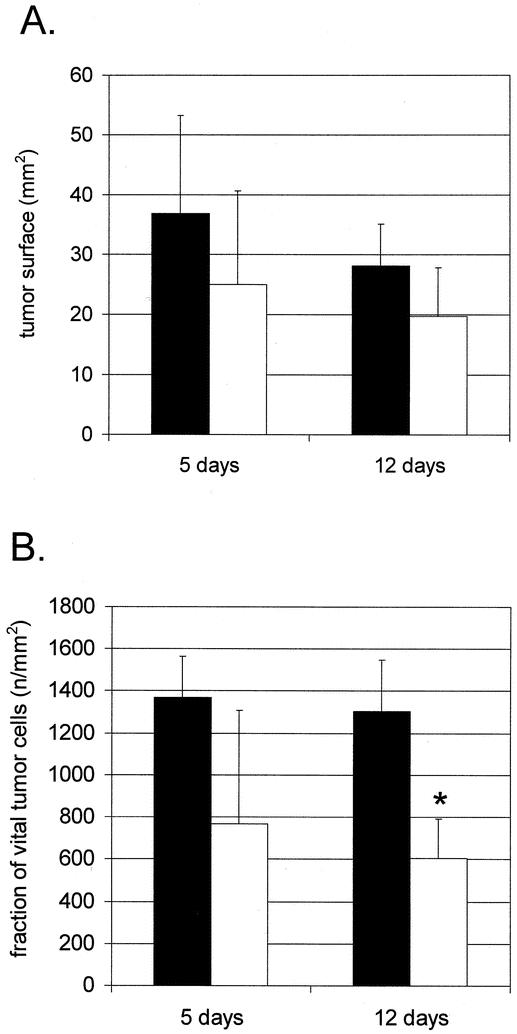
Figure 4. Cross-sectioned tumor surface (A) and fraction of vital tumor cells (B). (A) Cross-sectioned tumor surface was not significantly different both 5 and 12 days after radiation. (B) Although tumor cell density tended to decrease, there was no significant difference in the vital tumor cell fraction 5 days after radiation. However, radiation led to a significant decrease in the fraction of vital tumor cells 12 days after radiation. *Significant differences compared to nonradiated tumors; ▪, no radiation; □, radiation 15 Gy.
DISCUSSION
Pancreatic cancer is the second most common gastrointestinal malignancy and the fifth most common cancer in Western countries. 12,13 Approximately 80% to 85% of all patients with pancreatic cancer have clinically apparent metastatic disease or radiographic evidence of major vascular involvement at first presentation. 12,13 Because of its nonspecific initial symptoms, delay in diagnosis is common, and most patients present with disease that is beyond the scope of surgical cure. Despite surgery, only a few patients with ductal adenocarcinoma survive 5 years. 13 This disease represents an exceptional challenge in oncology.
In the present study, the radiogenic effects on normal pancreatic microcirculation and on pancreatic carcinoma were studied using a new model developed specifically for in vivo studies of tumor microcirculation. 5,14 In most previous models for the investigation of radiogenic effects in pancreatic cancer, tumor inoculation was achieved by standard subcutaneous implantation of tumor cells or tumor fragments in animals. 15,16 One advantage of the present model is the intraperitoneal tumor inoculation, which ensured a more habitual microenvironment for pancreatic cancer than the subcutaneous implantation site. The use of a lead setup protected the abdominal organs against radiation damage. Furthermore, the separation of pancreatic and tumor microcirculation by nonorthotopic implantation reduced the impact of radiogenic changes on the surrounding tissue, since muscle tissue is less sensitive to radiation than the pancreas. 3
Radiation is widely used for the treatment of malignant tumors. The direct cytotoxic effect on proliferating cells and the injury to tumor vasculature are two important effects of radiation. Both normal and malignant tissues take up the electromagnetic energy, which activates biochemical reactions, resulting in a profound reorganization of cellular homeostasis. The results of our study indicated that both normal pancreas and pancreatic carcinoma demonstrated a pronounced reaction to external beam radiation, but based on different mechanisms. High-dose radiation with 15 Gy induced dramatic cell death in tumor tissue, which was proven by increased tumor apoptosis and by a decreased fraction of vital tumor cells. At the same time, the change of cross-sectioned tumor surface area was not changed. As shown by Walter et al., 17 the reduction in tumor size is a valid parameter of tissue injury only in later phases after radiation.
However, normal pancreas did not show increased cell death after radiation, since normal exocrine and endocrine pancreatic cells are not sensitive to radiation-induced DNA injury. Kovacs 18 investigated the changes in pancreatic parenchyma in rats from day 1 to day 150 after radiation. Similar to our results, this study described a latency of changes in the normal pancreas until 15 days after radiation. The late stage of radiogenic damage was characterized by capillary obliteration and subsequent acinar atrophy. 18 The author concluded that the impairment of the vascular system might be a leading feature in the radiation-induced injury of normal pancreatic tissue. In the present study, intravital microscopy demonstrated that a single dose of radiation resulted in a substantial impairment of capillary blood flow and a decrease in functional capillary density by 5 days after radiation.
This observation may have clinical implications regarding intraoperative radiotherapy (IORT) with or without subsequent external beam radiation. Several clinical studies have demonstrated no change in complication rates after IORT plus external beam radiation compared to control subjects. 19–21 However, other investigators described an increase of complications. Kasperk et al. observed the leakage of pancreaticojejunal anastomosis in 2 of 35 patients treated with IORT. 22 Nishimura et al. reported that 14% of 126 patients developed gastrointestinal ulcers after IORT. 23 Ishikawa et al. noted gastrointestinal bleeding in 8% of patients after IORT plus external beam radiation with a dose exceeding 50 Gy. 24 Direct comparison between these studies is difficult due to different patient selection and radiation regimens.
We believe that intraoperative and postoperative radiation can potentially influence the surgical complication rate due to an impairment of microcirculation in the healthy pancreas. The functional results of the present investigation correspond well to numerous histologic and ultrastructural studies showing a general pattern of vascular changes in irradiated normal tissue such as swelling of capillary endothelial cells, platelet aggregation, capillary thrombosis, and capillary leakage and extravasation. 4 Notably, the microcirculation of pancreatic cancer did not show a similar effect. This observation is clear evidence that malignant cells can use other biochemical pathways to revise the intercepted electromagnetic energy. This observation is important for the therapy of cancer, since therapeutic irradiation has been suggested as a potential tool for the obliteration of tumor vasculature. 4
It is generally accepted that a tumor’s radiosensitivity depends on its oxygenation, and hypoxia protects tumor cells from radiation damage. 25,26 The major cause of tumor hypoxia is a lack of adequate vascularization, since tumors cannot initiate efficient neovascularization. 26 The pancreatic carcinoma of the cell line DSL6A used in the present study demonstrated a similarly insufficient vascularization. As shown previously, the mean vessel density of this carcinoma is significantly lower compared to the capillary density in the healthy pancreas, and the vessel distribution shows a heterogeneous pattern. 5 Interestingly, the radiogenic damage to tumor tissue has shown a similar heterogeneity in the tumor 12 days after radiation. Histologic changes of various degrees were recorded, while some tumor areas had no morphologic changes at the same time. It is possible that the tumor areas with a lower vessel density were more sensitive to radiation than the tumor areas with a higher vessel density. Despite the high vascular density in normal pancreas, no death of nonmalignant cells could be detected 12 days after radiation, underlining that normal pancreatic tissue is relatively insensitive to radiation due to its low proliferation rate.
In summary, local radiation with 15 Gy caused different effects on normal and malignant pancreatic tissue. Radiation impairs the blood flow in healthy tissue only, while radiation-induced tumor cell death is the leading feature in pancreatic cancer tissue. The present study demonstrates that radiation cannot be used for a vascular-targeted therapy of experimental pancreatic carcinoma; moreover, it can cause a distinct hypoperfusion in healthy pancreatic tissue.
Acknowledgments
The authors thank Dr. R. Nobiling for the support in the performance of intravital microscopy and computer-assisted evaluation of histologic images; Dr. W. Gross for the help in the computer-assisted analysis of video and digital images and in the statistical data analysis; Dr. K. Weber for critical reading and discussion during manuscript preparation; and Ms. C. Bernardi for excellent technical assistance.
Footnotes
Supported by a grant from Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 405.
Correspondence: Eduard Ryschich, MD, Department of Surgery, University of Heidelberg, Im Neuenheimer Feld 110, 69120 Heidelberg, Germany.
E-mail: eduard_ryschich@med.uni-heidelberg.de
Accepted for publication August 21, 2002.
References
- 1.Buell MG, Harding RK. Proinflammatory effects of local abdominal irradiation on rat gastrointestinal tract. Dig Dis Sci. 1989; 34: 390–399. [DOI] [PubMed] [Google Scholar]
- 2.Panes J, Anderson DC, Miyasaka M, et al. Role of leukocyte-endothelial cell adhesion in radiation-induced microvascular dysfunction in rats. Gastroenterology. 1995; 108: 1761–1769. [DOI] [PubMed] [Google Scholar]
- 3.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999; 82: 385–395. [DOI] [PubMed] [Google Scholar]
- 4.Hallahan DE, Chen AY, Teng M, et al. Drug-radiation interactions in tumor blood vessels. Oncology. 1999; 13: 71–77. [PubMed] [Google Scholar]
- 5.Schmidt J, Ryschich E, Daniel V, et al. Vascular structure and microcirculation of experimental pancreatic carcinoma in the rat. Eur J Surg. 2000; 166: 328–335. [DOI] [PubMed] [Google Scholar]
- 6.Pettengill OS, Faris RA, Bell RHJ, et al. Derivation of ductlike cell lines from a transplantable acinar cell carcinoma of the rat pancreas. Am J Pathol. 1993; 143: 292–303. [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt J, Ryschich E, Maksan SM, et al. Influence of gastrointestinal hormones on tumor microcirculation of experimental pancreatic cancer in the rat. Dig Surg. 2000; 17: 250–255. [DOI] [PubMed] [Google Scholar]
- 8.Ryschich E, Loeffler T, Schmidt J, et al. Concurrent Taxol- and radiation therapy of experimental pancreatic cancer [abstract]. Gastroenterology. 1999; 116: A494. [Google Scholar]
- 9.Mithofer K, Schmidt J, Gebhard MM, et al. Measurement of blood flow in pancreatic exchange capillaries with FITC-labeled erythrocytes. Microvasc Res. 1995; 49: 33–48. [DOI] [PubMed] [Google Scholar]
- 10.Klyscz T, Junger M, Jung F, et al. Cap image—a new kind of computer-assisted video image analysis system for dynamic capillary microscopy. Biomed Tech. 1997; 42: 168–175. [DOI] [PubMed] [Google Scholar]
- 11.Klar E, Endrich B, Messmer K. Microcirculation of the pancreas. A quantitative study of physiology and changes in pancreatitis. Int J Microcirc. 1990; 9: 85–101. [PubMed] [Google Scholar]
- 12.Harris J, Bruckner H. Adjuvant and neoadjuvant therapies of pancreatic cancer: a review. Int J Pancreatol. 2001; 29: 1–7. [DOI] [PubMed] [Google Scholar]
- 13.Cooperman AM, Kini S, Snady H, et al. Current surgical therapy for carcinoma of the pancreas. J Clin Gastroenterol. 2000; 31: 107–113. [DOI] [PubMed] [Google Scholar]
- 14.Ryschich E, Schmidt J, Löffler T, et al. A new model for in vivo analysis of the radiogenic effects on tumor microcirculation of experimental pancreatic cancer [abstract]. Langenbecks Arch Chir. 1998;Suppl.1750.
- 15.Marincola FM, Drucker BJ, Siao DY, et al. The nude mouse as a model for the study of human pancreatic cancer. J Surg Res. 1989; 47: 520–529. [DOI] [PubMed] [Google Scholar]
- 16.Kelland LR, Steel GG. Dose-rate effects in the radiation response of four human tumour xenografts. Radiother Oncol. 1986; 7: 259–268. [DOI] [PubMed] [Google Scholar]
- 17.Walter J, Maurer Schultze B. Regrowth, tumor cell proliferation and morphological alterations of the adenocarcinoma EO 771 following a single dose of 30 Gy 60Co gamma-rays. Strahlenther Onkol. 1987; 163: 687–694. [PubMed] [Google Scholar]
- 18.Kovacs L. Histological examination of pancreatic parenchymal changes induced by an experimental fractionated local exposition to X-rays. Strahlentherapie. 1976; 152: 455–468. [PubMed] [Google Scholar]
- 19.Farrell TJ, Barbot DJ, Rosato FE. Pancreatic resection combined with intraoperative radiation therapy for pancreatic cancer. Ann Surg. 1997; 226: 66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobelbower RR, Merrick HW, Khuder S, et al. Adjuvant radiation therapy for pancreatic cancer: a 15-year experience. Int J Radiat Oncol Biol Phys. 1997; 39: 31–37. [DOI] [PubMed] [Google Scholar]
- 21.Zerbi A, Fossati V, Parolini D, et al. Intraoperative radiation therapy adjuvant to resection in the treatment of pancreatic cancer. Cancer. 1994; 73: 2930–2935. [DOI] [PubMed] [Google Scholar]
- 22.Kasperk R, Klever P, Andreopoulos D, et al. Intraoperative radiotherapy for pancreatic carcinoma. Br J Surg. 1995; 82: 1259–1261. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura Y, Hosotani R, Shibamoto Y, et al. External and intraoperative radiotherapy for resectable and unresectable pancreatic cancer: analysis of survival rates and complications. Int J Radiat Oncol Biol Phys. 1997; 39: 39–49. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa H, Suzuki Y, Nakayama Y, et al. Intraoperative radiotherapy and bypass surgery for unresectable pancreatic cancer. Hepato-Gastroenterology. 2000; 47: 1151–1155. [PubMed] [Google Scholar]
- 25.Gray JH, Conder AD, Ebert M, et al. The concentration of oxygen dissolved in tissue at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953; 26: 638–648. [DOI] [PubMed] [Google Scholar]
- 26.Vaupel P. Hypoxia in neoplastic tissue. Microvasc Res. 1977; 13: 399–408. [DOI] [PubMed] [Google Scholar]


