Abstract
Objective
To determine the role of the microvascular endothelium in the regulation of regenerating liver mass after partial hepatectomy.
Summary Background Data
Angiogenesis is critical for both pathologic and physiologic processes. The ability of certain tissues, such as the liver, kidney, and spleen, to regenerate after injury is poorly understood. The liver will regenerate to its normal mass within 8 days of surgical excision. Because the authors have previously shown that the endothelial cell regulates tumor mass, we hypothesized that normal adult organ mass is also controlled by the endothelial cell.
Methods
Two-thirds partial hepatectomy was performed in 7- to 8-week-old C57 BL/6 mice, followed by systemic treatment with either the angiogenesis stimulator basic fibroblast growth factor (bFGF) (1 μg/g/d intraperitoneal) or the angiogenesis inhibitor TNP-470 (30 mg/kg/qod subcutaneous). Groups of three mice were then euthanized on postoperative days 2, 4, 6, and 8, and the livers were weighed and analyzed by immunohistochemistry.
Results
bFGF accelerated hepatic regeneration by 42%, 19%, 16%, and 16% on postoperative days 2, 4, 6, and 8, respectively. TNP-470 inhibited hepatic regeneration by 46%, 74%, 67%, and 64% on postoperative days 2, 4, 6, and 8, respectively. Immunohistochemistry revealed that bFGF and TNP-470 primarily affected the endothelial compartment. Specifically, bFGF increased endothelial proliferation and decreased endothelial apoptosis. TNP-470, in contrast, inhibited endothelial cell proliferation. The cessation of the regenerative process correlated with a decrease in endothelial proliferation and an increase in endothelial apoptosis.
Conclusions
The systemic administration of angiogenesis agents modulates the regeneration of hepatic mass primarily by affecting endothelial cell proliferation or apoptosis. Endothelial cell apoptosis is associated with the cessation of the regenerative process in control mice. These results suggest that the endothelial cell is one of the key mediators of regenerating adult tissue mass in this partial hepatectomy model.
The field of angiogenesis research, which began as an inquiry into neoplastic disease, now involves many other pathologic conditions. Several non-neoplastic diseases, including endometriosis, hemangioma, rheumatoid arthritis, and psoriasis, are mediated by angiogenesis. 1 While angiogenesis has been extensively studied in pathologic diseases, less is known about the role of angiogenesis in physiologic processes. Although angiogenesis has been shown to mediate the physiologic conditions of wound healing, exercising muscle, reproduction, and embryonic development, one physiologic phenomenon that is not well understood is the control of adult organ mass. 1 Certain tissues, such as the liver, spleen, and kidney, have the ability to regenerate after injury. 2–4 The regenerative capacity of these tissues is tightly controlled with a finite endpoint, similar to physiologic phenomena known to be angiogenesis-mediated.
The organ with the most profound ability to regenerate is the liver. Unlike the kidney or spleen, which may regenerate 44% to 100% after tissue loss over a period of up to 8 weeks, 5,6 the rodent liver will regenerate 100% of its lost mass within 8 days after surgical resection. 7 The liver is normally a quiescent organ, and the regulation of liver regeneration after injury is not clearly understood. We hypothesized that the control of physiologic organ mass was similar to the control of tumor mass. 8 Specifically, the proliferation of hepatocytes after partial hepatectomy, like the proliferation of neoplastic cells in tumors, requires the synthesis of new blood vessels to support the rapidly increasing mass.
Recent evidence supports our hypothesis that the control of organ mass, like tumor mass, may be angiogenesis-dependent. For example, the spleen has been noted to inhibit the autotransplantation of splenic tissue (splenosis) through a circulating factor. 9 After castration the mass of the prostate decreases and there is an associated decrease in the expression of vascular endothelial growth factor (VEGF) and the number of blood vessels before the loss of prostatic epithelial cells. 10 Finally, the growth of adipose tissue has been shown to be angiogenesis-dependent. 11
The control of hepatic mass also may be angiogenesis-mediated, since a complex paracrine interaction between hepatocytes and endothelial cells exists during liver regeneration. 12 For example, a peak in hepatocyte production of VEGF, an endothelial mitogen, corresponds to an increase of VEGF receptor expression on endothelial cells after partial hepatectomy. 12–14 The increased expression of VEGF and its receptors correlates with the rate of endothelial proliferation after partial hepatectomy. 15 Fibroblast growth factor and transforming growth factor-alpha, which stimulate endothelial cells, are secreted by hepatocytes 24 hours after partial hepatectomy. 16 Although hepatocytes do not typically express VEGF receptors, exogenously added VEGF has been shown to increase hepatocyte proliferation, suggesting a paracrine effect operating through the endothelial cell. 17,18
After partial hepatectomy, endothelial cells secrete hepatocyte growth factor, a potent hepatocyte mitogen that is also proangiogenic. 19,20 Transforming growth factor-beta is secreted by endothelial cells 72 hours after partial hepatectomy and has been shown to inhibit hepatocyte proliferation. 16 Thus, the endothelial cells and hepatocytes of the regenerating liver influence each other, and both populations are required for the regulation of the regenerative process. The relationship between the production of paracrine factors between hepatocytes and endothelial cells suggests a central role for angiogenesis in the control of regenerating liver mass.
To test our hypothesis that the control of organ mass, like tumor mass, is mediated by the endothelium, we developed a surgical liver regeneration model in mice. After partial hepatic resection, mice were treated with a known angiogenesis stimulator (bFGF) and inhibitor (TNP-470) to manipulate liver mass in vivo. We then performed immunohistochemistry to identify the cellular target and mechanism of the angiogenesis agents.
METHODS
Partial Hepatectomy
Using 7- to 8-week-old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME), we removed 67% of the liver under isoflurane anesthesia. Specifically, the left upper lobe, right upper lobe, and left lower lobe of the liver were excised distal to 5-0 silk ties. Three mice per group were then euthanized on postoperative days 2, 4, 6, and 8. Control animals consisted of three mice per group that were also euthanized on postoperative days 2, 4, 6, and 8. Control mice for the TNP-470 experiment received saline subcutaneously every other day. Control mice for the bFGF experiment received saline intraperitoneally every day. Regenerating livers were then weighed and fixed for immunohistochemistry. All experiments were repeated by a second group of independent investigators with similar results.
Angiogenesis Agents
bFGF, a selective stimulator of endothelial proliferation and migration, was administered (1 μg/g) intraperitoneally each day beginning on the day of 67% hepatectomy for up to 8 days. TNP-470 (AGM-1470, O-chloroacetyl-carbamoyl-fumagillol), a selective endothelial cell inhibitor, was administered (30 mg/kg) subcutaneously every other day beginning on the day of partial hepatectomy for up to 8 days. 21
PCNA Immunohistochemistry
Groups of three mice were killed on postoperative days 2, 4, 6, and 8. Regenerating livers were fixed in 10% formalin overnight and then washed with phosphate-buffered saline (PBS). The specimens were then embedded in paraffin and 5-μm sections were cut. To determine if bFGF and TNP-470 influenced hepatic regeneration by altering the proliferation of endothelial cells or hepatocytes, sections were stained with an antibody to proliferating cells. Specifically, cell proliferation was assessed by proliferating cell nuclear antigen (PCNA) immunostaining. After deparaffinization and rehydration, the sections were incubated with Citra buffer (Dako, Denmark) for 10 minutes at 90°C. Nonspecific binding sites were then blocked for 30 minutes at room temperature with TNB blocking buffer (NEN Life Sciences, Boston, MA). Primary PCNA antiserum 1:150 (Signet Laboratories, Dedham, MA) was added to the sections overnight at 4°C. Secondary goat antimouse IgG 1:400 antibody was then added for 30 minutes at room temperature. Next, the slides were incubated with streptavidin alkaline phosphatase (AP) solution (1:100), followed by biotinyl tyramide amplification diluent (1:50). After repeat incubation with AP solution (1:100) for 30 minutes at room temperature, the chromagen New Fuchsin (Biogenex, San Ramon, CA) was added. The slides were counterstained with Gill’s hematoxylin. After all incubations, except for the TNB blocking step, the sections were washed three times with TNT wash buffer (NEN Life Sciences). The TNB buffer, TNT buffer, biotinylated-tyramide solution, amplification diluent, and streptavidin-AP were used according to the instructions of the NEN TSA indirect kit (catalogue #NEL 700A, NEN Life Sciences).
TUNEL Immunohistochemistry
To study whether bFGF or TNP-470 modulated hepatic regeneration by changing the apoptotic rate of endothelial cells or hepatocytes, apoptosis during the regenerative process was studied. Cell apoptosis was determined by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end labeling (TUNEL). Slides were incubated with proteinase K to break aldehyde crosslinks, followed by 3% H2O2 in PBS to block endogenous peroxidase. Next, terminal deoxynucleotide transferase (TdT) was added to the slides, followed by antidigoxigenin-peroxidase and then DAB. PBS was used for each washing step. Finally, the slides were counterstained with Harris modified hematoxylin with acetic acid.
To confirm endothelial cell identification, because apoptotic cells may lose their morphology, different sections were doubly stained with fluorescent antibodies to CD31, an endothelial marker, as well as to TdT. Endothelial cells stained red; apoptotic cells stained green. Consequently, the identification of apoptotic endothelial cells was confirmed by yellow-staining cells.
After the completion of the immunostaining, proliferation and apoptosis were quantified as previously described. 22 Specifically, the number of PCNA-positive cells or TUNEL-positive cells were counted by two blinded independent investigators. The number of positively stained cells were counted per high-power field at 400× magnification. Ten random high-power fields were counted for each of the three mice per group by both investigators. The proliferation and apoptotic indexes were determined by dividing the number of positively stained cells per high-power field by the total number of cells of that type in the field.
Statistical Analysis
Statistical analysis of all results was performed using the Student two-tailed, unpaired t test for comparisons between groups. Differences were considered significant when P < .05 (group size, n = 3).
RESULTS
Murine Hepatic Regeneration After Partial Hepatectomy
Before treatment with angiogenesis agents, a model of hepatic regeneration in the mouse was established (Fig. 1A). Sixty-seven percent of the liver was resected in groups of three mice. The mice were then euthanized each day until liver mass reached the original liver weight of 1.19 ± 0.07 g. The majority of the hepatic regeneration occurred by postoperative day 4. The entire mass of the original liver was reconstituted by postoperative day 8.
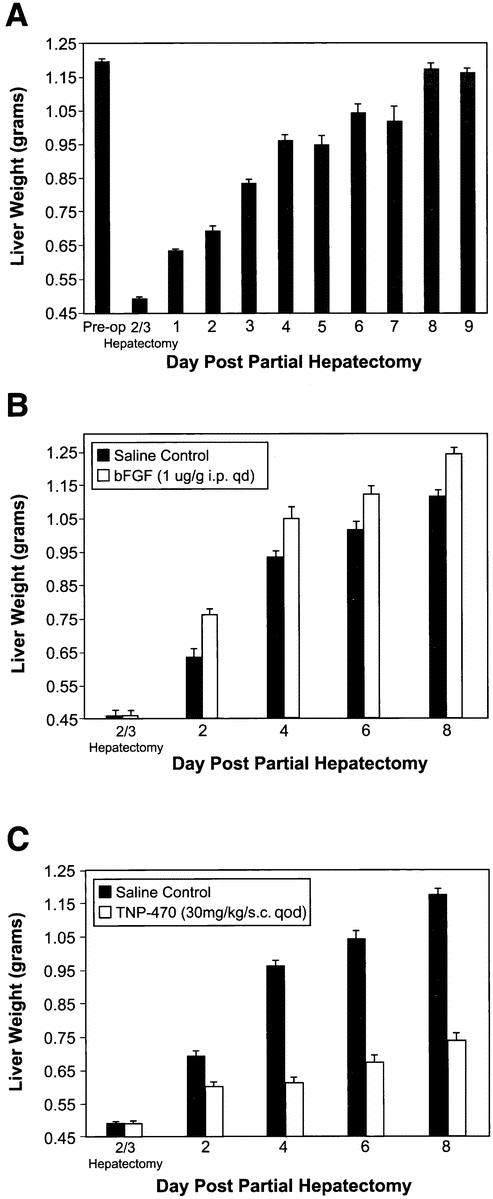
Figure 1. Effect of angiogenesis stimulators and inhibitors on hepatic regeneration. (A) Hepatic regeneration in mice. (B) Effect of bFGF (1 μg/g/qd intraperitoneal) on hepatic regeneration. (C) Effect of TNP-470 (30 mg/kg/qod subcutaneous) on hepatic regeneration.
Effects of bFGF on Hepatic Regeneration After Partial Hepatectomy
bFGF (1 μg/g) was injected intraperitoneally daily beginning on the day of surgery. Three mice per group were then sacrificed on postoperative days 2, 4, 6, and 8 (see Fig. 1B). Liver regeneration was accelerated by 42%, 19%, 16%, and 16% on postoperative days 2, 4, 6, and 8, respectively, compared to saline controls (P = .04).
To assess whether a more specific endothelial mitogen would have the same effect as bFGF on hepatic regeneration, VEGF165 was injected intraperitoneally (0.5 μg/g) into three mice each day beginning on the day of partial hepatectomy. After 8 days the weights of the livers treated with VEGF were 37% greater than the weights of control livers treated with saline (P = .03).
Effects of TNP-470 on Hepatic Regeneration After Partial Hepatectomy
After undergoing two-thirds partial hepatectomy, mice were injected with TNP-470 (30mg/kg) subcutaneously every other day beginning on the day of surgery (see Fig. 1C). Three mice per group were then euthanized on postoperative days 2, 4, 6, and 8. TNP-470 resulted in a 46%, 74%, 67%, and 64% inhibition of hepatic regeneration on postoperative days 2, 4, 6, and 8, respectively, compared to controls (P = .04).
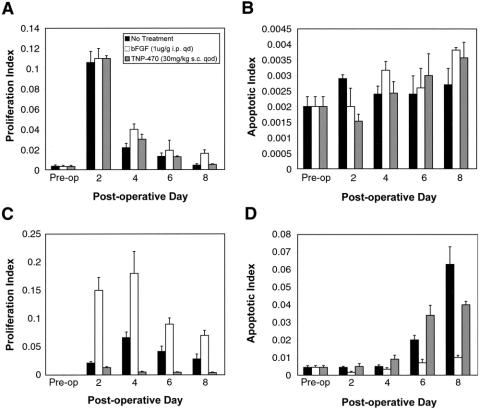
Effect of bFGF and TNP-470 on Endothelial Cell and Hepatocyte Proliferation After Partial Hepatectomy
Regenerating livers were stained for PCNA to identify cells undergoing proliferation (Figs. 2, 3). bFGF significantly increased endothelial cell proliferation by 87%, 67%, 66%, and 72% on postoperative days 2, 4, 6, and 8, respectively (P = .04). TNP-470, in contrast, significantly decreased endothelial cell proliferation by 37%, 92%, 88%, and 84% on postoperative days 2, 4, 6, and 8 after partial hepatectomy, respectively (P = .04). Although treatment with bFGF caused a small increase in hepatocyte proliferation at each time point, the effect did not reach statistical significance (P = .08). TNP-470 did not significantly affect hepatocyte proliferation (P = .9).
Figure 2. Effect of bFGF and TNP-470 on hepatocyte and endothelial proliferation and apoptosis. (A) Hepatocyte proliferation during hepatic regeneration. (B) Hepatocyte apoptosis during hepatic regeneration. (C) Endothelial proliferation during hepatic regeneration. (D) Endothelial apoptosis during hepatic regeneration.
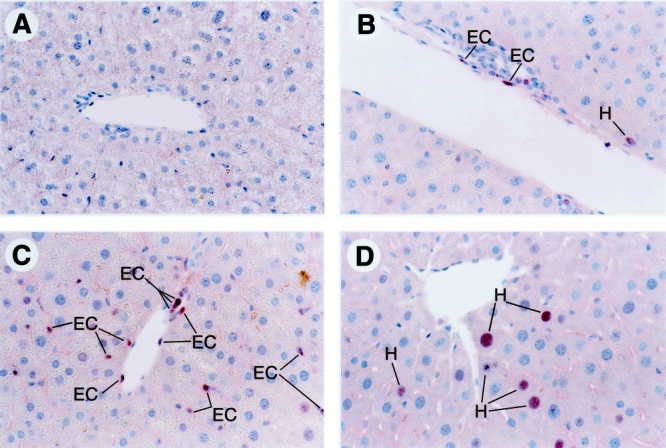
Figure 3. PCNA staining of regenerating liver on postoperative day 4 following partial hepatectomy, the time of maximal endothelial cell proliferation (400×). (A) Preoperative liver. (B) Normal regenerating liver on postoperative day 4. (C) Regenerating liver treated with bFGF. (D) Regenerating liver treated with TNP-470. EC, endothelial cell; H, hepatocyte.
Effect of bFGF and TNP-470 on Endothelial Cell and Hepatocyte Apoptosis After Partial Hepatectomy
Regenerating livers also were studied using the TUNEL assay to identify cells undergoing apoptosis (see Fig. 2). In control mice, hepatocyte apoptosis did not change during liver regeneration. However, endothelial cell apoptosis increased on postoperative days 6 and 8 as the regenerating liver completed the regenerative process. bFGF decreased endothelial apoptosis by 88%, 33%, 65%, and 87% on postoperative days 2, 4, 6, and 8, respectively (P = .04). bFGF treatment did not significantly influence hepatocyte apoptosis (P = .2). TNP-470 treatment did not significantly effect endothelial (P = .09) or hepatocyte (P = .1) apoptosis. Similar results were obtained with both single and double staining.
DISCUSSION
The endothelium is a candidate to mediate organ mass since all cells reside within the oxygen diffusion distance of 200 μm of a capillary. 1 The liver, which has the most profound regenerative capacity of any adult organ, is particularly suited for the endothelial control of its mass since each hepatocyte lies adjacent to at least two sinusoidal endothelial cells. Vascular endothelial cells acquire the same proliferative rate as bone marrow cells during periods of physiologic angiogenesis, such as reproduction, embryonic development, wound repair, and exercised muscle. 1 Unlike pathologic phenomena, however, endothelial cells during nonpathologic angiogenesis return to their normal resting state within days or weeks. Endothelial cells during hepatic regeneration behave similarly to endothelial cells in other physiologic processes known to be mediated by angiogenesis. Specifically, after partial hepatectomy endothelial cell proliferation rises, peaks at 4 days, and returns to normal after regeneration is complete.
Systemic treatment with proangiogenic factors accelerates liver regeneration and resulted in livers that were larger than the normal organ size. Although bFGF selectively stimulates endothelial cells, bFGF can stimulate fetal and adult hepatocytes in vitro. 23,24 Because of the complex paracrine interaction between endothelial cells and hepatocytes, bFGF may act primarily on endothelial cells, hepatocytes, or both. 25 However, our immunohistochemical analysis suggests that bFGF primarily targeted the endothelial compartment, because bFGF caused a large acceleration in endothelial proliferation compared to the small increase in hepatocyte proliferation. In addition, bFGF protected against endothelial, and not hepatocyte, apoptosis. Because the ratio of endothelial cells to hepatocytes is approximately 1:30 and hepatocytes are much larger than endothelial cells, a small increase in the hepatocyte proliferation index, as seen with the bFGF-treated group, may be responsible for the increase in liver mass when translated to the organ level. 7
TNP-470, an endothelial inhibitor, markedly suppressed liver regeneration. It is possible that TNP-470, in addition to directly inhibiting sinusoidal endothelial cells, may also be inhibiting endothelial cells indirectly through either positive or negative effects on hepatocytes or other cell types. While TNP-470 has been shown to inhibit the proliferation of some mesenchymal cells at a dose 100 to 10,000 times greater than the dose required for equivalent endothelial inhibition, TNP-470 has not been shown to be hepatotoxic in vitro or in vivo. 26–29 In these studies the action of TNP-470 appeared to be directed primarily at the endothelial compartment. Specifically, TNP-470 inhibited endothelial cell, and not hepatocyte, proliferation throughout the regeneration process.
Interestingly, liver regeneration does not typically cause pathology. While the regenerating liver protects the organism from the loss of hepatic function, the liver does not grow out of control but instead stops growing once it has reached its original size. The endothelial cell may be the physiologic break that stops the liver’s regenerative process because endothelial proliferation decreased while endothelial apoptosis increased on postoperative days 6 and 8 as the regenerating liver reached its preoperative mass. Endothelial apoptosis is known to stop another physiologic angiogenic process, ovarian luteolysis. 30
In summary, our studies of hepatic regeneration demonstrate that the endothelial cell is involved in the regulation of regenerating adult organ mass and suggests that the regulation of angiogenesis controls the regenerative process. First, endothelial apoptosis corresponds to the cessation of the regenerative process. Second, exogenously added angiogenesis stimulators and inhibitors will accelerate or inhibit hepatic regeneration, respectively. Finally, immunohistochemistry shows that the action of these agents is directed primarily toward the endothelial cell. This hepatic regeneration model suggests that, like tumors, adult organ mass may be controlled by the endothelium. Clinically, the ability to manipulate hepatic mass via the endothelium may prove efficacious after large hepatic resections as well as living related and split liver transplants. In addition, angiogenic agents may prove efficacious in hepatic diseases such as cirrhosis or focal nodular hyperplasia. The role of the endothelium in the control of hepatic mass also may provide clues to the mechanism of organ growth in general and to compensatory renal hypertrophy, prostate hypertrophy, and splenosis, specifically.
Acknowledgments
The authors thank Dr. David Zurakowski for his statistical expertise and Kristin Gullage for figure editing.
Footnotes
Supported by a Howard Hughes Medical Institute Postdoctoral Research Fellowship (A.K.G.). bFGF was the kind gift of Dr. Judy Abraham (Scios, Sunnyvale, CA). TNP-470 was the kind gift of Takeda Chemical Industries, Ltd. (Deerfield, IL).
Correspondence: Judah Folkman, MD, Department of Surgical Research, Children’s Hospital, Harvard Medical School, 300 Longwood Ave., Hunnewell 103, Boston, MA 02115.
E-mail: judah.folkman@tch.harvard.edu
Accepted for publication September 27, 2002.
References
- 1.Folkman J. Angiogenesis. In: Braumwald E, ed. Harrison’s textbook of internal medicine. New York: McGraw-Hill, 2001: 517–530.
- 2.Fausto N. Liver regeneration. Hepatology. 2000; 32: 19–31. [DOI] [PubMed] [Google Scholar]
- 3.Katz AI, Toback GF, Lindheimer MD. Independence of onset of compensatory kidney growth from changes in renal function. Am J Physiol. 1976; 230: 1067–1071. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez FE, Greco RS. Regeneration of the spleen after ectopic implantation and partial splenectomy. Arch Surg. 1980; 115: 772–775. [DOI] [PubMed] [Google Scholar]
- 5.Mulroney SE, Woda C, Johnson M, et al. Gender differences in renal growth and function after uninephrectomy in adult rats. Kidney Int. 1999; 56: 944–53. [DOI] [PubMed] [Google Scholar]
- 6.Pouche A, Chiodera P, Bosio P, et al. Experimental splenic regeneration. Surg Gynecol Obstet. 1986; 162: 25–29. [PubMed] [Google Scholar]
- 7.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997; 276: 60–65. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastasis by a Lewis lung carcinoma. Cell. 1994; 79: 315–328. [DOI] [PubMed] [Google Scholar]
- 9.Soutter AD, Ellenbogen J, Folkman J. Splenosis is regulated by a circulating factor. J Ped Surg. 1994; 29: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Is tissue mass regulated by vascular endothelial cells? Prostate as the first evidence [editorial]. Endocrinology. 1998; 139: 441–442. [DOI] [PubMed] [Google Scholar]
- 11.Rupnick MA, Panigrahy D, Langer R, et al. Adipose tissue mass is controlled by angiogenesis in ob/ob mice. Circulation. 1998; 98 (Suppl): I-455. [Google Scholar]
- 12.Ross MA, Sander CM, Kleeb TB, et al. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001; 34: 1135–1146. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001; 34: 683–689. [DOI] [PubMed] [Google Scholar]
- 14.Kraizer Y, Mawasi N, Seagal J. Vascular endothelial growth factor and angiopoietin in liver regeneration. Biochem Biophys Res Commun. 2001; 14: 209–215. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, El-Assal ON, Ono T. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001; 34: 690–698. [DOI] [PubMed] [Google Scholar]
- 16.Bucher NL. Liver regeneration: An overview. J Gastroenterol Hepatol. 1991; 6: 615–624. [DOI] [PubMed] [Google Scholar]
- 17.Assy N, Spira G, Paizi M, et al. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999; 30: 911–915. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi E, Sakisaka S, Matsuo K, et al. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001; 49: 121–129. [DOI] [PubMed] [Google Scholar]
- 19.Bussolino F, DiRenzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992; 119: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshito T, Hirao S, Matsumoto K, et al. Possible endocrine control by hepatocyte growth factor of liver regeneration after partial hepatectomy. Biochem Biophy Res Comm. 1991; 177: 330–335. [DOI] [PubMed] [Google Scholar]
- 21.Moulton KS, Heller E, Konerding MA, et al. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999; 99: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 22.Barnhill RL, Piepkorn MW, Cochran AJ, et al. Tumor vascularity, proliferation, and apoptosis in human melanoma micrometastases and macrometastases. Arch Dermatol. 1998; 134: 991–994. [DOI] [PubMed] [Google Scholar]
- 23.Ma TY, Kikuchi M, Sarfeh IJ, et al. Basic fibroblast growth factor stimulates repair of wounded hepatocyte monolayer: modulatory role of protein kinase A and extracellular matrix. J Lab Clin Med. 1999; 134: 363–371. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann B, Paul D. Basic fibroblast growth factor and transforming growth factor-alpha are hepatotrophic mitogens in vitro. J Cell Physiol. 1990; 142: 149–154. [DOI] [PubMed] [Google Scholar]
- 25.Baruch Y, Shoshany G, Neufeld, G, et al. Basic fibroblast growth factor is hepatotropic for rat liver in regeneration. J Hepatol. 1995; 23: 328–331. [PubMed] [Google Scholar]
- 26.Placidi L, Scott E, De Sousa G, et al. Interspecies variability of TNP-470 metabolism, using primary monkey, rat, and dog cultured hepatocytes. Drug Metab Dispos. 1997; 25: 94–99. [PubMed] [Google Scholar]
- 27.Placidi L, Scott E, Eckoff D, et al. Metabolic drug interactions between angiogenic inhibitor TNP-470 and anticancer agents in primary cultured hepatocytes and microsomes. Can Res. 1995; 55: 3036–3041. [PubMed] [Google Scholar]
- 28.Gervaz P, Scholl G, Padrum V, et al. Growth inhibition of liver metastases by the anti-angiogenic drug TNP-470. Liver. 2000; 20: 108–113. [DOI] [PubMed] [Google Scholar]
- 29.Kusaka M, Sudo K, Matsutani E, et al. Cytostatic inhibition of endothelial cell growth by the angiogenesis inhibitor TNP-470 (AGM-1470). Br J Cancer. 1994; 69: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Modlich U, Kaup F, Augustin HG. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest. 1996; 74: 771–780. [PubMed] [Google Scholar]