Abstract
Neuroendocrine mechanisms that mediate male aggression toward infants are poorly understood. Although testosterone is known to enhance aggression in other social contexts, evidence that it modulates aggression toward infants is equivocal. We have found that male progesterone receptor knockout (PRKO) mice exhibit no infanticidal behavior and little aggression toward young. Male PRKO mice also display significantly enhanced parental behaviors. In wild-type mice, blockade of PR induces a behavioral phenotype similar to that of the PRKO males, whereas progesterone exacerbates aggressive tendencies toward infants. Aggressive behaviors directed toward adult males, by contrast, are unaffected by progesterone, PR antagonism, or PR gene deletion. Previously thought to be of diminished importance in male animals, PRs play a critical and specific role in modulating infant-directed behaviors in male mice.
Adult male animals display a repertoire of behaviors toward conspecific infants that can include some combination of parental care, indifference, and aggression. Parental care is the least commonly observed of these behaviors among male mammals, though it can predominate in male Djungarian hamsters, Mongolian gerbils, cotton-top tamarins, prairie voles, some strains of mice, and humans. In most strains of laboratory mice, however, indifference or overt aggression toward infants is most common; parental care is usually only observed after careful sensitization of the adult male to pups.
The neuroendocrine mechanisms mediating aggressive behavior toward infants remain obscure. Because testosterone (T) has long been known to enhance intermale aggression, the aggression of adult males toward offspring and absence of paternal care have also been considered T-dependent behaviors. High T levels, however, do not necessarily decrease paternal behavior in many species (1, 2) and even promote paternal behavior in the California mouse, Peromyscus californicus (3) via aromatization to estrogen (4). Furthermore, T levels do not correlate with paternal behavior in common laboratory mice (5). In humans, some fathers experience a decrease in T levels immediately after birth of a child (6, 7); however, its association with paternal behaviors is not known.
The inconsistency of reported associations between T and infant-directed male behaviors lead us to consider the possibility that this type of male aggression may be governed by neuroendocrine regulatory mechanisms that differ from those controlling intermale aggression. We specifically assessed the possibility that male aggression toward infants may be modulated by progesterone, via the intracellular progesterone receptor (PR). Traditionally viewed as a hormone that controls female reproductive behavior and physiology, recent work has suggested that progesterone may also influence male reproductive behaviors (8) as well as male-specific development of the hypothalamus (9). These findings, together with the observations that progesterone can inhibit parental behaviors in female rodents (10), prompted us to examine whether this steroid and its cognate receptors play a similar role in diminishing paternal behavior and/or enhancing infant-directed aggression in males. Here we report the novel finding that aggression toward infants and the suppression of paternal behavior are mediated by progesterone and PR.
Materials and Methods
The Animal Care and Use Committee of Northwestern University approved all animal protocols.
Parental Behavior Test.
Mated males aged 10–24 weeks were caged with their mates throughout gestation and parturition. Virgin males were isolated at weaning and caged singly until testing at age 8–22 weeks. All males were given nesting cotton and allowed to build a nest overnight. After a 1-h acclimation period, a pup, aged 3–7 days, was placed in the farthest corner from the nest. The latency to contact, latency to pick up the pup (if applicable), and the time to retrieve pup to nest was recorded. To determine the parental behavior index score points were awarded in the following manner: +1 for contacting pup, +1 for picking up pup, +4 for retrieving pup to nest, +1 for nurturant behaviors such as licking and crouching continuously for at least 2 min after retrieval. A perfect score of 7 indicates the highest level of paternal care; a score of 0 indicates no paternal behavior, e.g., attacking of the pup. The total test time was 10 min. When pups were attacked, the test was stopped immediately and the pup was removed. Pups that were attacked were not used in subsequent tests. Scores on the behavioral index were compared using a Mann–Whitney U test for independent samples (mated males) and Kruskal–Wallis one-way ANOVA for genotype differences (virgin males).
Isogenic (ISO) mice were generated by mating heterozygous littermates. No more than two litters from each pair were used to control for any possible changes in allelic composition from the PR knockout (PRKO) breeding colony.
PR and RU486 Administration.
Silastic implants filled with progesterone in sesame oil were implanted. Progesterone was suspended in sesame oil (Sigma) at a concentration of 25 mg/ml. This concentration has been shown to deliver a physiological dose of progesterone in mice for at least 28 days (39). Silastic medical-grade tubing was cut into 1-cm segments and filled with either vehicle or hormone. The ends of the implant were sealed with Silastic medical adhesive (Silicone Type A, Dow Corning). Control capsules contained sesame oil vehicle. Capsules were allowed to cure overnight before implantation. Progesterone capsules were implanted at 7 weeks of age and the mice were tested 14 days after implantation. RU486 pellets were purchased from Innovative Research of America (Sarasota, FL). The pellets released 0.5 mg/day and were implanted s.c. at 7 weeks of age. Control pellets contained inert components. Males were tested for behavior 14 days after implantation. Tests were performed by an observer blind to treatment group.
Hormone Measurements.
Animals were deeply anesthetized with CO2, and 2-cm horizontal incisions were made with sharp scissors just below the xiphoid process. The rib cage was bisected, exposing the heart. A 21-gauge needle was inserted into the right ventricle, and blood was withdrawn. Blood was centrifuged, and plasma was frozen at −20°C for later RIA.
Intermale Aggression Tests.
All tests were performed during the dark phase (4–8 hr after lights off) of the light–dark cycle. Males were isolated at weaning and caged singly until testing. Aggressive acts were defined as lunging, chasing, tail rattling, biting, wrestling, and mounting. An aggressive bout was defined as a series of behavioral interactions including at least one aggressive behavior; a new aggressive bout was scored if more than 3 sec elapsed between behavioral acts. Three behavioral paradigms, described below, were used to measure aggression: homogeneous set test in a neutral cage, resident–intruder, and standardized opponent test in resident–intruder paradigm. The duration for each test was 15 min. Latency to onset, number of bouts, and cumulative duration of fighting were recorded for each test. If aggression was not displayed a latency of 900 sec was recorded. Most males were used in just one paradigm. Males that were used in multiple aggression tests were used in the following order: neutral cage paradigm, resident–intruder paradigm, standardized intruder paradigm. All tests were videotaped and scored by an observer blind to genotype and/or treatment. Aggression data were analyzed by Kruskal–Wallis one-way ANOVA or Mann–Whitney U test for effects of genotype, test day, or treatment differences, followed, if applicable, by post hoc pair wise comparisons. In the neutral cage paradigm, pairs of males of the same genotype were placed in a clean neutral cage on either side of a cardboard divider. After a 5-min adaptation period, the divider was removed and aggression was recorded. Tests were done using the same pairs on two consecutive nights. No significant differences in latency to onset, number of bouts or cumulative duration were recorded between the first and second night of testing. In the resident–intruder paradigm, each experimental male was placed in the home cage of an individually housed male. Tests were also done using males of the same genotype as resident and intruder. No significant differences were recorded. In the standardized opponent resident–intruder paradigm, each male was tested in his home cage against a group-housed olfactory bulbectomized C57BL/6 male intruder. OBX males rarely display aggression because male aggression is mainly regulated by olfactory cues. Because OBX males still have intact gonads they can elicit aggression from the experimental male, but rarely display aggression themselves.
Intermale Aggression After Castration.
At the time of castration, males were implanted s.c. with a T capsule. T was suspended in sesame oil at a concentration of 4 mg/ml, and 20 μl was injected into a 1-cm length of silastic tubing and sealed. Control capsules contained sesame oil. Males were tested by using the resident–intruder with a standardized intruder paradigm described above 15–20 days after castration.
Results and Discussion
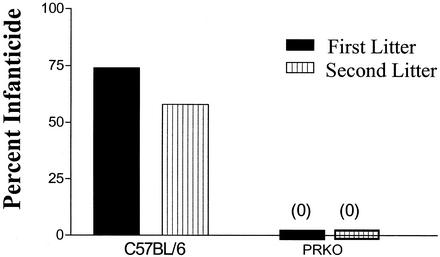
We examined the parental behavior of mice lacking PR to determine whether activation of this receptor influences paternal behavior and aggression toward young. We found a complete absence of infanticide in sexually experienced male PRKO mice (Fig. 1). Males of the C57BL/6 strain, by contrast, were found to commit infanticide with the birth of their first litter at a frequency of 74%. With the birth of a second litter the frequency dropped to 58% in C57BL/6 males, whereas infanticide remained absent in PRKO males.
Figure 1.
Frequency of infanticide in mated male mice. Mated C57BL/6 (n = 19) males committed infanticide of their first litters 74% of the time. Instances of infanticide dropped to 58% for second litters (n = 31). Mated PRKO males did not commit infanticide (n = 60).
On presentation with a pup, males exhibit one of three strikingly different behaviors: aggression, indifference (no physical contact initiated), or paternal care. We used a standard pup presentation paradigm (11) to obtain a measure of the frequency of aggressive behavior, and an index of paternal behavior in mated male mice. The PRKO males attained nearly perfect paternal care scores (6.7 ± 0.13 on a scale of 0–7), and only 8% exhibited aggressive behavior toward the pup placed in the cage. The majority of the C57BL/6 male fathers could not be tested in this paradigm, having killed their litters. Nevertheless, even those C57BL/6 male mice that did not commit infanticide with their second litters scored significantly lower in the paternal care test (4.6 ± 1.2/7.0; P < 0.008) and attacked pups with twice the frequency of PRKO males (data not shown).
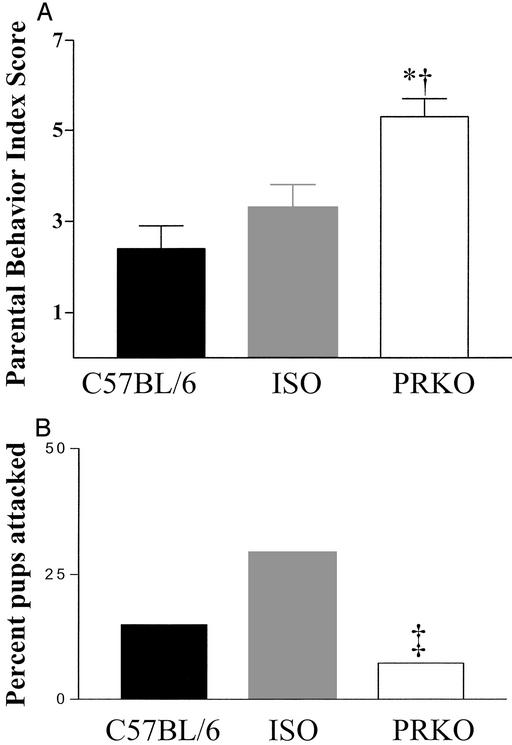
Because a variety of factors, including prior social experience and strain, can influence paternal behavior (12) we examined levels of aggression toward infants and parental behaviors in socially isolated, virgin males. In this test we included males that were the same combination of strains present in the PRKOs (C57BL/6 and 129SvEv) but express wild-type PR (denoted as ISO control). In the pup presentation paradigm, virgin PRKO males isolated at weaning exhibited a significantly higher degree of paternal behavior (Fig. 2) than C57BL/6 and ISO males. PRKO males retrieved pups 63% of the time compared with 23% in C57BL/6 and 44.1% in ISO. Virgin PRKO males exhibited significantly reduced aggression toward young compared with virgin C57BL/6 and ISO males (Fig. 2). Thus PRKO males exhibit extremely low levels of aggression toward infants and a high degree of paternal care.
Figure 2.
Virgin PRKO males displayed more paternal behavior and less infant-directed aggression than males expressing PR. (A) Scores on the paternal behavior index for PRKO (n = 50) were significantly higher than C57BL/6 (*, P < 0.001; n = 26) and ISO (†, P < 0.01; n = 34) strains. (B) PR-expressing strains, C57BL/6 and ISO, attacked pups during the behavior tests 15% and 29.4%, respectively. PRKO virgin males attacked pups in 7.3% of the tests (χ2 = 6.8; ‡, P < 0.03). Scores on the behavioral index were compared by using Kruskal–Wallis one-way ANOVA for genotype differences.
Males that do not display aggression toward young may simply be less aggressive overall and, conversely; males that are aggressive toward young may be generally more aggressive in a variety of behavioral settings. In some avian species, for example, it has been demonstrated that males experience a tradeoff, or negative correlation, between levels of overall aggression and paternal behavior (13). Males that spend more time participating in territorial aggression spend less time contributing to paternal care. This tradeoff between aggression and paternal care has not been extensively studied in mammals. To test the hypothesis that ablation of PR results in the specific reduction in aggression toward infants, and not a general reduction in aggressive behavior, we assessed intermale aggression in C56BL/6, PRKO, and ISO genotypes by using three different standard behavioral paradigms: resident–intruder, standardized intruder, and neutral cage (14). In all three paradigms, the latency to onset of aggression, number of attacks, and cumulative duration of fighting, did not differ significantly with genotype (Table 1). These data suggest that PR ablation does not result in a tradeoff of paternal behavior and territorial aggression. Specifically, PRKO mice display normal levels of aggression toward adult males, even though they also exhibit little aggression toward infants and robust paternal behavior in the pup-presentation tests.
Table 1.
Intermale aggression tests
| Paradigm | Genotype | Latency, sec | Duration, sec | Bouts, no. per 10 min | n | P value |
|---|---|---|---|---|---|---|
| Neutral cage | C57BL/6 | 260.6 ± 112.3 | 211.2 ± 54.8 | 9.8 ± 2.7 | 12 | >0.05 |
| ISO | ND | ND | ND | >0.05 | ||
| PRKO | 243.6 ± 51.3 | 103.5 ± 19.25 | 10.94 ± 1.6 | 9 | >0.05 | |
| Resident–intruder | C57BL/6 | 107 ± 52.1 | 292.8 ± 89.7 | 9.67 ± 2.4 | 6 | >0.05 |
| ISO | 67.9 ± 34.9 | 210.9 ± 29.6 | 31.4 ± 4.4 | 8 | >0.05 | |
| PRKO | 137.8 ± 44.3 | 123.4 ± 19.7 | 15.9 ± 1.9 | 9 | >0.05 | |
| OBX | C57BL/6 | 143.2 ± 63.1 | 268.3 ± 86.7 | ND | 6 | >0.05 |
| ISO | 400 ± 57.4 | 143.6 ± 28.7 | ND | 16 | >0.05 | |
| PRKO | 305 ± 94.6 | 107 ± 30.9 | ND | 10 | >0.05 |
No significant differences among genotypes were observed during the three intermale aggression paradigms. ND, not determined; OBX, olfactory bulbectomized.
T's regulation of aggressive behaviors has been extensively studied, and it is well known that castration reduces aggression levels in males of most species. To further assess the possibility that the PRKO behavioral phenotype represents a generalized reduction in T-dependent aggressive behaviors, we castrated both C57BL/6 and PRKO mice and treated some castrates of each genotype with T. In behavior tests, both C57BL/6 and PRKO mice exhibited a reduction in intermale aggressive behaviors after castration, and T treatment restored aggression to a similar degree in both genotypes (Table 2). These findings provide further evidence that the reduced aggression toward infants and enhanced parental behaviors observed in the PRKO male are behaviors modulated specifically by PR, and do not represent a generalized reduction in T-dependent aggressive behaviors.
Table 2.
Intermale aggressive behaviors after castration
| Treatment | Genotype | Latency, sec | Duration, sec | Bouts, no. per 10 min | n |
|---|---|---|---|---|---|
| Sham + oil | C57BL/6 | 157 ± 75 | 305 ± 95 | 9.8 ± 2.7 | 6 |
| PRKO | 393 ± 110 | 101 ± 45.2 | 13.7 ± 3.8 | 9 | |
| C + O | C57BL/6 | 299.8 ± 122.6 | 78.3 ± 51.5 | 8.1 ± 2.1 | 6 |
| PRKO | 269.7 ± 106 | 59.8 ± 44.8 | 7.9 ± 2.9 | 9 | |
| C + T | C57BL/6 | 132.4 ± 30 | 182.2 ± 28.2* | 18.4 ± 2† | 5 |
| PRKO | 249.7 ± 104 | 127.4 ± 45 | 21.9 ± 5.7‡ | 9 |
No difference in intermale aggression was observed between C57BL/6 and PRKO males in response to castration. Males were tested by using the resident–intruder with standardized intruder paradigm. C, castrated; O, sesame oil.
P < 0.04 vs. oil treatment.
P < 0.004 vs. oil treatment.
P < 0.02 vs. oil treatment.
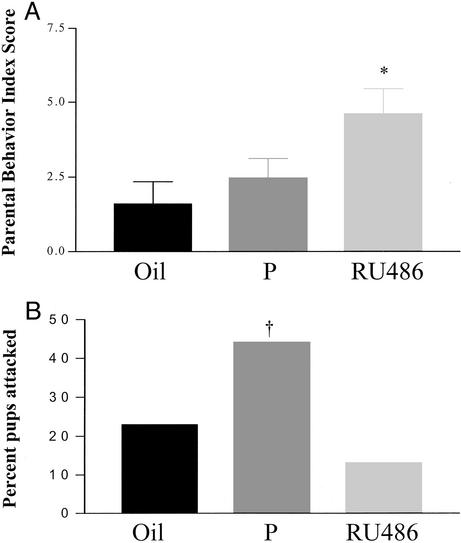
Although inhibition of aggression toward young is a necessary prerequisite for paternal care, males that are not aggressive may not exhibit paternal care. It is possible that different mechanisms mediate the inhibition of aggression and the onset of paternal care (15). In an effort to distinguish whether this might be the case, C57BL/6 males were treated with progesterone for an extended period, and aggression toward pups and paternal behavior were assessed. C57BL/6 males treated with progesterone displayed similarly low levels of paternal responsiveness (2.5 ± 0.6 of 7.0) compared with controls (1.6 ± 0.74 of 7.0), and exhibited a significantly greater frequency of aggression toward young (treated 44%, control 23%) (Fig. 3). Blockade of PR by using the antagonist RU486 significantly increased paternal behavior compared with both untreated and progesterone-treated males (treated 4.6 ± 0.8 of 7.0, untreated 1.6 ± 0.75 of 7.0; P < 0.05) and decreased aggression toward young (treated 13%, untreated 23%). Moreover, RU486 reversed the increase in aggressive behavior toward young seen in the progesterone-treated animals (Fig. 3B). Although different mechanisms may exist to regulate inhibition of paternal aggression and the onset of paternal care, this work suggests that PRs are involved in regulating both behaviors.
Figure 3.
Treatment with progesterone inhibits and RU486 enhances paternal behavior. (A) Virgin C57BL/6 mice treated with progesterone exhibited similar levels of paternal behavior compared with oil-treated controls (n = 22). RU486 treatment significantly increased paternal behavior in the same strain compared with oil- and progesterone-treated animals (*, P < 0.05; n = 15) blind to treatment group. (B) Virgin C57BL/6 males treated with progesterone attacked pups in 44% of the tests compared with 23% for control-treated animals (χ2 = 3.19; †, P < 0.037). RU486-treated males attacked pups in 13% of the tests.
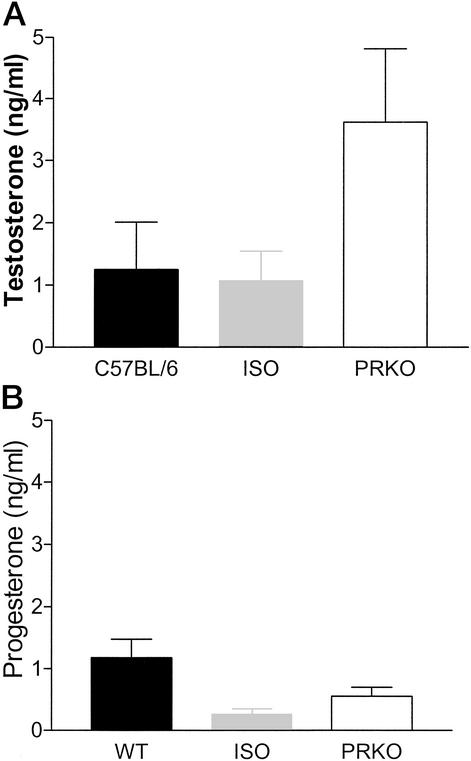
We considered the possibility that altered signaling through PRs might produce hormonal disruptions that secondarily influence aggressive behavior and paternal responsiveness. Changes in T and progesterone levels do not appear to be responsible for behavioral differences observed in PRKO males, as plasma T and progesterone concentrations were not significantly different in PRKO and C57BL/6 mice (Fig. 4). As mentioned above, aromatization of T to E has been demonstrated to enhance paternal behavior in some species. However, serum E levels did not vary between the genotypes (PRKO 11.9 ± 1.9 pg/ml; C57BL6 10.78 ± 1.9 pg/ml; P > 0.05). Likewise, a role for prolactin (PRL) in this regard also does not appear likely. PRL is important in the expression of maternal and paternal behavior in mammals. Induction of PRL receptors in the brain of male rats induces paternal behavior (16) and increased PRL levels have been correlated with the onset of paternal behavior in a variety of naturally paternal species (11, 17). PRL is higher in men during the final 3 weeks of pregnancy (7). Although PRL may enhance or help maintain paternal behavior in these strains of mice, the increase in paternal care and decreased aggression toward young observed in this study cannot be attributed to an increase of PRL secretion because we found that plasma PRL levels are normal in PRKO males (PRKO 12.9 ± 0.86 ng/ml; C57BL/6 19.4 ± 4.9 ng/ml; P > 0.05). However, it remains a possibility that deletion of PR may lead to altered signaling through the PRL receptor.
Figure 4.
Plasma concentrations for T and progesterone in males of three genotypes. (A) T levels did not differ significantly among genotypes (C57BL/6 1.25 ± 0.76, n = 10; ISO 1.06 ± 0.49, n = 10; PRKO 3.61 ± 1.2, n = 14). (B) Progesterone levels did not significantly differ among C57BL/6, ISO, or PRKO males (C57BL/6 1.183 ± 0.3, n = 10; ISO 0.256 ± 0.09, n = 5; PRKO 0.56 ± 0.14, n = 3).
We also considered the possibility that enhanced paternal behavior in PRKO mice may be a consequence of arrested or delayed sexual maturation. Juvenile rats and prairie voles can exhibit some parental-like behaviors, such as contacting and carrying pups (18), and thus the parental behaviors that we observed in the PRKO animals could reflect the continued expression of this type of juvenile attention to pups. However, we have found that testicular weights and hormonal markers of puberty are not different between age-matched PRKO and C57BL/6 mice (unpublished observation), providing evidence that the display of paternal behaviors in PRKO mice is not secondary to any disturbance in growth and maturation that is produced by PR deletion.
We have demonstrated that PR activation mediates infanticide, aggressive behavior toward infants, and reduction of parental behavior in male mice. Although these behavioral tendencies are characteristic of most male laboratory mice, they are rarely displayed by normal females. Because PRs in the brain (19) and circulating progesterone concentrations are, if anything, greater in adult females versus males, this suggests that sexually differentiated signal transduction and integrative mechanisms downstream from the PR mediate the male responses to progesterone. This idea is supported by the findings that female responses to progesterone are both qualitatively and quantitatively different from those observed in males; progesterone treatments in females can reduce maternal responsiveness and increase maternal aggression toward adult male intruders (20, 21), but do not similarly increase infant-directed aggression.
Behavioral responses to progesterone in males may in turn depend on androgen-mediated masculinization of brain structures during prenatal and neonatal neurodevelopment. Recent findings in sex-reversed transgenic mice suggest that sexual differentiation of infanticidal and parental behaviors is at least partially sex chromosome dependent; infanticide is highest in XY males, somewhat lower in XX males with a SRY locus on the X chromosome, and absent in XX females, with the prevalence of parental behaviors ranked in the reverse order (22). Recent observations in neonatal male rats (23) and mice (24) suggest that one consequence of the neonatal androgen surge on the developing male preoptic area is a transient induction of PR gene expression. Thus, the predisposition of the adult male to exhibit a progesterone-mediated sexually differentiated behavior, such as infanticide, may be established via the early organizational effects of androgens on PR-expressing neurons in this sexually dimorphic nucleus (9).
The neural mechanisms that mediate progesterone-dependent, infant-directed behaviors remain to be elucidated. PR activation may sustain or suppress activity in neurotransmitter circuitries that have been implicated in parental behaviors, such as those that produce vasopressin (2, 25, 26) and oxytocin (27). Activated PR may also modulate signaling through other steroid receptor-mediated signaling pathways, such as those mediated by estrogen receptors (ERs) (28, 29). Progesterone action is mediated by two distinct forms of PR, PR-B, and the N-terminally truncated isoform, PR-A. In human breast cancer cells, both isoforms can function as ligand-activated transcriptional regulators of progesterone-responsive target genes; moreover, the PR-A isoform can additionally operate as a repressor of steroid hormone receptor actions. For example, progesterone activated PR-A can modulate estrogen action by preventing ERα-mediated gene transcriptional activation as indicated by reporter gene expression (28, 29). In our studies we have observed a decrease in infanticide among male mice lacking PRs, whereas a recent report by Ogawa et al. (30) documented a significant increase in infanticide among male ERα knockout (ERKOα) mice. These opposite behavioral findings in the ERKOα and PRKO genotypes, taken together with the foregoing evidence for PR-A-ERα cross-talk, provide some circumstantial evidence that PR-dependent male aggression toward young may be exerted via the ability of activated PRs to antagonize ERα-mediated transcriptional regulation in vivo.
The potential adaptive significance of aggression toward young and infanticide exhibited in wild animal populations under specific environmental and social conditions has been debated (31, 32). Infant-directed aggression may be a pathological response to overcrowding or other environmental stresses, and may therefore be considered a maladaptive behavior. Under appropriate circumstances, however, infanticidal behavior can increase a male's reproductive success and therefore constitute an adaptive behavior (33). That infanticide in mice may be a product of natural selection is underscored by the fact that it is both a heritable and male-specific behavioral trait (34).
Adaptive significance notwithstanding, infant-directed aggressive behaviors are displayed in wild populations amidst natural settings that are vastly different from the controlled physical and social environment of the laboratory mouse. The extent to which PRs may function to enhance aggression toward infants in other species and in spontaneous social circumstances remains largely unknown. Although male Cynomolgus monkeys exhibit increased male-male aggression after medroxyprogesterone acetate (MPA) treatments (35), the specific effects of this progestin on infant-directed aggression in male primates have not been examined. Interestingly, a study of females in an island colony of stumptail macaques demonstrated that MPA specifically increases aggression toward subadults, juveniles, and infants; male monkeys were not similarly examined (36). In humans, MPA is used clinically to treat male paraphilic sex offenders, as it is generally found to reduce libido, paraphilic erotic fantasies, and associated behavioral urges (37). It does not, however, appear to reduce aggressive, impulsive, or antisocial behavior, and we are not aware of any studies that have specifically addressed the possibility that MPA may actually increase aggressive impulses or behaviors toward children.
Aggression is a general descriptor for many different types of agonistic behaviors, with subtypes often being distinguishable on the basis of the aggressor, target, social circumstance, motivational state, developmental stage, or adaptive context. Although all of these subtypes of aggression are recognized, studies of the neural substrates of aggression have disproportionately focused on male–male paradigmatic aggressive behaviors, most often based on territoriality, e.g., the resident–intruder paradigms. Increasing attention is now being paid to the likelihood that the neuroendocrine mechanisms governing subtypes of aggressive behaviors are dissociable. Recent pharmacological studies (38), for example, have been conducted in an attempt to parse specific types of aggressive behaviors on the basis of their sensitivity to various classes of neuroactive drugs. We have used a combination of gene deletion and pharmacological approaches to demonstrate that the neuronal mechanisms governing the expression of one type of aggression, namely infant-directed aggression, is at least partially separable from other types of male aggression: activation of PRs in adult male mice enhances aggression toward an infant without a commensurate increase in aggression toward other adult males. Our findings further suggest other gene products that specifically mediate infant-directed behaviors may lie downstream of the PR, and work is underway toward their identification.
Acknowledgments
We thank Dr. Alessandro Guidotti for help with serum progesterone measurements and Mrs. Brigitte Mann for assistance with all other hormone measurements. This work was supported by National Institute of Child Health and Human Development Program Project Grant PO1 HD21921.
Abbreviations
- PR
progesterone receptor
- KO
knockout
- T
testosterone
- ISO
isogenic
References
- 1.Clark M M, Galef B G. J Comp Psychol. 1999;113:388–395. doi: 10.1037/0735-7036.113.4.388. [DOI] [PubMed] [Google Scholar]
- 2.Lonstein J S, De Vries G J. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- 3.Trainor B, Marler C. Horm Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- 4.Trainor B, Marler C. Proc R Soc London Ser B. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svare B, Bartke A, Gandelman R. Horm Behav. 1977;8:372–376. doi: 10.1016/0018-506x(77)90011-3. [DOI] [PubMed] [Google Scholar]
- 6.Berg S J, Wynne-Edwards K E. Mayo Clin Proc. 2001;76:582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- 7.Storey A, Walsh C, Quinton R, Wynne-Edwards K. Evol Hum Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- 8.Witt D, Young L, Crews D. Physiol Behav. 1995;57:307–313. doi: 10.1016/0031-9384(94)00247-3. [DOI] [PubMed] [Google Scholar]
- 9.Quadros P, Lopez V, De Vries G, Chung W, Wagner C. J Neurobiol. 2002;51:24–32. doi: 10.1002/neu.10040. [DOI] [PubMed] [Google Scholar]
- 10.Siegel H I, Rosenblatt J S. Horm Behav. 1975;6:223–230. doi: 10.1016/0018-506x(75)90009-4. [DOI] [PubMed] [Google Scholar]
- 11.Reburn C J, Wynne-Edwards K E. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- 12.Elwood R. Behav Neural Biol. 1986;46:54–63. doi: 10.1016/s0163-1047(86)90894-0. [DOI] [PubMed] [Google Scholar]
- 13.Ketterson E, Nolan V, Jr, Wolf L, Ziegenfus C. Am Nat. 1992;140:980–999. [Google Scholar]
- 14.Ogawa S, Chester A E, Hewitt S C, Walker V R, Gustafsson J A, Smithies O, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.vom Saal F. Physiol Behav. 1985;34:7–15. doi: 10.1016/0031-9384(85)90069-1. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi K, Tanaka M, Ohkubo T, Doh-ura K, Fujikawa T, Sudo S, Nakashima K. Neuroendocrinology. 1996;63:559–568. doi: 10.1159/000127085. [DOI] [PubMed] [Google Scholar]
- 17.Brown R, Murdoch T, Murphy P, Moger W. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- 18.Lonstein J S, De Vries G J. Behav Brain Res. 2000;114:79–87. doi: 10.1016/s0166-4328(00)00192-3. [DOI] [PubMed] [Google Scholar]
- 19.Scott R, Wu-Peng X, Pfaff D. J Neuroendocrinol. 2002;14:175–183. doi: 10.1046/j.0007-1331.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 20.Svare B, Miele J, Kinsley C. Horm Behav. 1986;20:194–200. doi: 10.1016/0018-506x(86)90017-6. [DOI] [PubMed] [Google Scholar]
- 21.Albert D J, Jonik R H, Walsh M L. Neurosci Biobehav Rev. 1992;16:177–192. doi: 10.1016/s0149-7634(05)80179-4. [DOI] [PubMed] [Google Scholar]
- 22.Reisert I, Karolczak M, Beyer C, Just W, Maxson S, Ehret G. Behav Genet. 2002;32:103–111. doi: 10.1023/a:1015297622509. [DOI] [PubMed] [Google Scholar]
- 23.Wagner C, Nakayama A, De Vries G. Endocrinology. 1998;139:3658–3661. doi: 10.1210/endo.139.8.6223. [DOI] [PubMed] [Google Scholar]
- 24.Wagner C K, Pfau J L, De Vries G J, Merchenthaler I J. J Neurobiol. 2001;47:176–182. doi: 10.1002/neu.1025. [DOI] [PubMed] [Google Scholar]
- 25.Young L, Nilsen R, Waymire K, MacGregor G, Insel T. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Liu Y, Young L, Insel T. J Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 27.Francis D, Young L, Meaney M, Insel T. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 28.Wen D, Xu Y-F, Mais D, Goldman M, McDonnell D. Mol Cell Biol. 1994;14:8356–8364. doi: 10.1128/mcb.14.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulac-Jericevic B, Mullinax R A, DeMayo F J, Lydon J P, Conneely O M. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Washburn T, Taylor J, Lubahn D, Korach K, Pfaff D. Endocrinology. 1998;139:5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 31.Svare B, Broida J, Kinsley C, Mann M. In: Infanticide: Comparative and Evolutionary Perspectives. Hausfater G, Hrdy S, editors. New York: Aldine; 1984. pp. 387–400. [Google Scholar]
- 32.vom Saal F. In: Infanticide: Comparative and Evolutionary Perspectives. Hausfater G, Hrdy S, editors. New York: Aldine; 1984. pp. 401–424. [Google Scholar]
- 33.vom Saal F, Howard L. Science. 1982;215:1270–1272. doi: 10.1126/science.7058349. [DOI] [PubMed] [Google Scholar]
- 34.Perrigo G, Belvin L, Quindry P, Kadir T, Becker J, van Look C, Niewoehner J, vom Saal F. Behav Genet. 1993;23:525–531. doi: 10.1007/BF01068143. [DOI] [PubMed] [Google Scholar]
- 35.Zumpe D, Bonsall R, Kutner M, Michael R. Horm Behav. 1991;25:394–409. doi: 10.1016/0018-506x(91)90010-f. [DOI] [PubMed] [Google Scholar]
- 36.Linn G, Steklis H. Physiol Behav. 1990;47:403–408. doi: 10.1016/0031-9384(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 37.Lehne G. In: Handbook of Sexology. Sitsen J, editor. Vol. 6. New York: Elsevier; 1988. pp. 516–525. [Google Scholar]
- 38.Parmigiani S, Ferrari P, Palanza P. Neurosci Biobehav Rev. 1998;23:143–153. doi: 10.1016/s0149-7634(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 39.Milligan S, Cohen P. Reprod Fertil Dev. 1994;6:235–239. doi: 10.1071/rd9940235. [DOI] [PubMed] [Google Scholar]