Abstract
Objective
To confirm evidence that breast-conserving treatment (BCT) does not impair the prognosis in breast cancer patients as compared to mastectomy and to argue that it be regarded as the treatment of choice in stage I and II disease.
Summary Background Data
Scientifically, survival rates in breast cancer have been shown to be stage-dependent, but independent of the extent of surgical breast tissue removal, as long as the resection margins are free of tumor infiltration.
Methods
Between 1984 and 1997, six different trials conducted by the Austrian Breast & Colorectal Cancer Study Group accrued a total of 4,259 women with hormone-responsive disease. The authors selected and compared three patient groups (n = 3,316) according to pathologic stage, age, and the surgical procedure applied.
Results
Over this interval, the BCT rate in the premenopausal node-positive subgroup experienced a highly significant increase from 27.2% to 73.2% overall. In the group of postmenopausal node-negative patients, the BCT rate grew significantly by 37.3% to 77.3% in total. With an overall BCT rate growing from 22.5% to 56.8% in postmenopausal node-positive women, those presenting with T1 tumors saw a significant increase from 35.1% to 65.9%. Mortality and local recurrence rates proved stable or even decreased considerably over time and in all subgroups.
Conclusions
The presented outcome of BCT rates, significantly improved over this 16-year period and in no way counterbalanced by higher local recurrence or death rates, reflects an excellent example of surgical quality control. BCT can safely be regarded as the standard of therapy for T1 and increasingly for T2 disease. Especially in multi-institutional adjuvant breast cancer trials, the highest priority should be given to breast-conserving procedures.
Scientifically, it has been well established that breast-conserving treatment (BCT) in patients with breast cancer does not impair overall prognosis as compared to mastectomy. Several randomized clinical trials in the past two decades have shown that survival rates in breast cancer are stage-dependent, but independent of the extent of surgical breast tissue removal, as long as the resection margins are free of tumor infiltration.
The particular importance the breast shows for female psychology and social interaction and the potential for feelings of mutilation and impaired physical integrity are another argument for carefully considering the implications connected with ablative surgical procedures. As a rational and informed basis for the shift in paradigms of invasiveness, the scientific community has felt the need to evidence the concept that local procedures have no detrimental impact on survival in such patients. Proof has been elaborated repeatedly by many investigators from different areas of the world. 1–3
The extent of tissue removed in breast cancer surgery, however, can be a predictor for local failure rate. Local relapse following mastectomy depends on tumor stage, nodal stage, and the biologic aggressiveness of the tumor and usually is an indicator of poor prognosis. By contrast, local recurrence after BCT does not necessarily have an impact on survival. In any case, the psychological and subjective burden of local failure following such interventions must never be underestimated. Therefore, BCT in general calls for adjuvant radiotherapy, although it has been shown that in a highly selected group of patients at very low risk for locoregional relapse, radiotherapy may indeed not be required. 4 This issue is under investigation in a number of prospective clinical trials.
By accepted academic standards, BCT combined with adjuvant radiotherapy should be regarded as the surgical treatment of choice in patients with stage I and II breast cancer, as stated by the Consensus Development Conference as early as 1990. 5 Yet in contrast to these well-established standards, for various reasons BCT has not been fully accepted in general practice. This unfortunate discrepancy is linked to specific factors with regard to both patients and physicians.
BCT rates are at considerable variance between different countries, but also within highly industrialized nations. For example, differences are reported to be marked between states in the United States. 6,7 In addition to geographic factors, the treatment level of a given hospital department is a key issue for the degree to which BCT is accepted. Women treated at university or teaching hospitals had doubled chances to have their breast preserved, while women over 70 and patients from rural areas were less likely candidates for BCT. 8
The most important factor influencing a patient’s decision on the surgical procedure to be used has proven to be the treating physician’s recommendation, which is rarely questioned in routine practice. 9 Thus, surgeons play the key role in patients’ decision-making processes.
Surgeons participating in clinical trials should be particularly well educated and trained at the highest level of medical knowledge. After all, one chief argument for performing clinical trials is that quality control is given considerable attention under such circumstances. This refers to primary trial endpoints as much as general medical care for patients, including quality control measurements applied to surgical techniques and the standards of histopathology.
Since 1984, the Austrian Breast & Colorectal Cancer Study Group (ABCSG) has been conducting nationwide, multicenter clinical trials in patients with stage I and II breast cancer. This framework has provided the opportunity, over one and a half decades, to investigate BCT rates in women participating in these investigations throughout Austria. To minimize potential selection bias, we selected subjects with identical basic prognostic characteristics (tumor and nodal stage, receptor status, and age) for the present analysis.
METHODS
Between 1984 and 1997, a total of 5,203 patients were recruited into nine different ABCSG trials. Our clinical study designs were approved by the relevant medical ethical committees in Austria. From the beginning, these designs were based on known hormone receptor status of the primary tumors. Six studies accrued a total of 4,259 participants presenting with receptor-positive breast cancer. The present analyses cover both premenopausal women showing nodal involvement and postmenopausal patients with and without axillary node metastases. The six trials under investigation here serve as the exclusive basis for the analyses. Individual trial designs are summarized in Table 1.
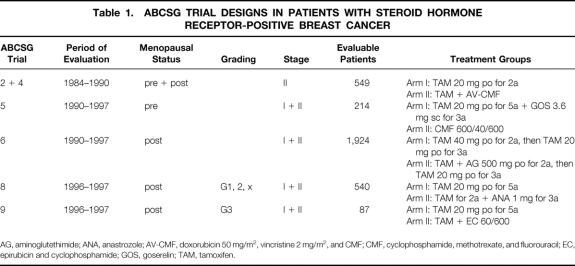
Table 1. ABCSG TRIAL DESIGNS IN PATIENTS WITH STEROID HORMONE RECEPTOR-POSITIVE BREAST CANCER
AG, aminoglutethimide; ANA, anastrozole; AV-CMF, doxorubicin 50 mg/m2, vincristine 2 mg/m2, and CMF; CMF, cyclophosphamide, methotrexate, and fluorouracil; EC, epirubicin and cyclophosphamide; GOS, goserelin; TAM, tamoxifen.
All trials called for patients to be randomized after submitting informed consent. Conservative and ablative surgical procedures were performed at the discretion of the responsible surgeon. The trials were not designed to increase the rate of breast conservation, yet the selection criteria for technical characteristics of BCT were thoroughly discussed in numerous Study Group meetings in an attempt to homogenize standards. In all trials, and in any single patient included, resection had to be performed with clear margins (R0), though the exact amount of required healthy tissue resected around the tumor was not further specified.
Complete axillary clearance was carried out in all patients with a minimum of level I or II dissection. An interdisciplinary team consisting of surgeons and radiotherapists analyzed the indication for postoperative irradiation in these trial participants with exclusively hormone-responsive tumors. However, radiotherapy was mandatory for premenopausal women undergoing BCT and high-risk, postmenopausal, mastectomized patients. Only postmenopausal women with favorable tumor characteristics were allowed to forego irradiation on account of our promising results in this patient cohort. 4 These patients are currently randomized between irradiation versus no irradiation in the framework of ABCSG Trial 8. 10
Tumor diameter was measured by the pathologist, and at least eight lymph nodes were examined histologically for patients to be eligible for trial participation. In all trials, eligibility criteria additionally included radically resected breast cancer and estrogen and/or progesterone receptor positivity, measured by either biochemical or immunocytochemical methods. Grading was determined according to Bloom and Richardson. 11 Patients were followed every 3 months for the first 3 years and every 6 months thereafter. Chest x-ray, liver sonography, mammography, and bone scan were performed on a regular follow-up basis and whenever clinically indicated.
As to statistical analysis, summary measurements are given as medium or mean ± standard deviation. Homogeneity in the figures was analyzed using the chi-square test in which two-sided P values lower than 0.05 were identified as significant. All analyses were carried out with the SAS 6.12 statistical package (SAS Institute, Inc., 1990, Cary, NC).
RESULTS
We compared three different groups of women presenting with hormone receptor-positive breast cancer according to their pathologic stage, age, and the surgical procedure applied:
447 premenopausal patients presenting with axillary lymph node metastases (participating in ABCSG Trials 2 and 5; 1984–1997)
1,667 postmenopausal patients without axillary node involvement (Trials 6, 8, and 9; 1990–1997)
1,202 postmenopausal patients with axillary node metastases (Trials 4, 6, 8, and 9; 1984–1997)
Premenopausal, Node-Positive Patients
A total of 447 participants were randomized in two trials involving premenopausal patients with positive nodes. ABCSG Trial 2 compared one treatment arm given tamoxifen with another given the antiestrogen in addition to a polychemotherapy regimen; Trial 5 investigated the efficacy of combination endocrine treatment versus standard CMF chemotherapy (see Table 1). Overall, 190 premenopausal women with nodal involvement (42.5%) underwent BCT and 257 (57.5%) modified radical mastectomy (MRM).
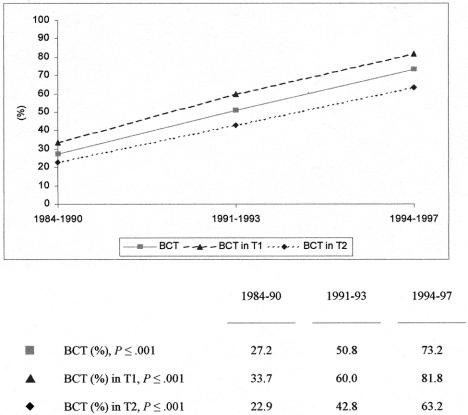
The development of these two surgical procedures within three different time intervals is depicted in Figure 1, with randomized patient numbers amounting to 235, 130, and 82, respectively. As shown between 1984 and 1990, 27.2% of the patients underwent BCT, rising to 50.8% in the 1991 to 1993 period and to 73.2% thereafter. The quantitative difference was highly significant (P ≤ .001). Within the three time intervals covering 16 years, the overall percentage of patients with T1 tumors increased from 40.4% to 46.2% and 53.7%. The mean numbers of investigated lymph nodes within the intervals were 12.1 (± 5.1), 14.8 (± 6.1) and 14.6 (± 5.0). Percentages of BCT rose from 33.7% to 60.0% and finally 81.8% (P ≤ .001) in T1 lesions, and from 22.9% to 42.8% and 63.2% (P ≤ .001) in T2 disease for the three time intervals, respectively. Of the 257 patients assigned to MRM, 162 patients had presented with a T2 tumor and 28 had shown more than 10 positive lymph nodes.
Figure 1. Surgical procedures in 447 premenopausal patients with axillary lymph node involvement within three time periods.
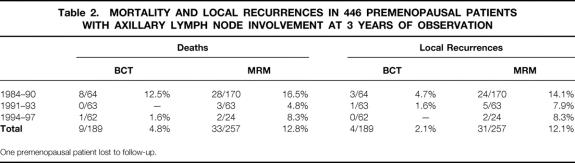
Table 2 gives actuarial data on mortality and local recurrence rates in this premenopausal cohort at 3 years of observation. A mean mortality total of 4.8% was identified in patients treated conservatively, reflecting a drop from 12.5% to 1.6% over time, versus a total of 12.8% deaths in mastectomized women. The local recurrence rate in patients treated with BCT decreased from 4.7% to 1.6% in the first two intervals and to nil in the final period. Overall, local recurrences were experienced by 2.1% versus 12.1% in the BCT and MRM sets, respectively. However, these percentages cannot directly be compared on account of different tumor and nodal status.
Table 2. MORTALITY AND LOCAL RECURRENCES IN 446 PREMENOPAUSAL PATIENTS WITH AXILLARY LYMPH NODE INVOLVEMENT AT 3 YEARS OF OBSERVATION
One premenopausal patient lost to follow-up.
Postmenopausal, Node-Negative Patients
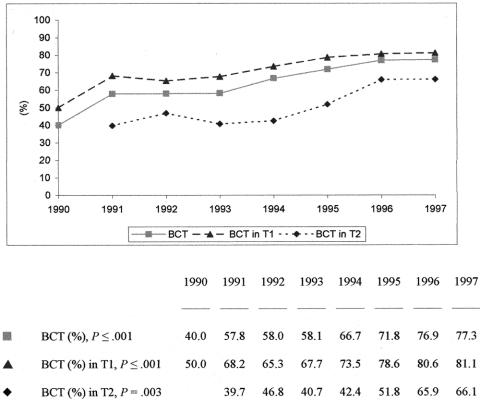
Between 1990 and 1997, 1,667 postmenopausal patients presenting with negative nodes were randomized in three different ABCSG trials, all of which were designed to investigate the efficacy of exclusive tamoxifen administration versus that of combination treatments with aminoglutethimide (Trial 6), anastrozole (Trial 8), or epirubicin and cyclophosphamide (Trial 9). Overall, the mean BCT rate in this cohort amounted to 66.1%. This 8-year period saw the percentage of conservative procedures rise significantly from 40.0% to 77.3%, as shown in Figure 2 (P ≤ .001). The percentage of patients with T1 increased from 64.1% to 74.7% within the same time period. Considering women with T1 lesions only, BCT was performed in 50.0% in 1990, rising to 81.1% by 1997 (P ≤ .001). In patients presenting with T2, percentages were 39.7% in 1991 and 66.1% in 1997, showing a significant difference over time (P = .003). Investigating the type of operation within three different age groups (50–60, 61–70, 71–80 years of age), we found rates of 73.0%, 63.7%, and 60.4% (P ≤ .001). Of the 568 radically treated patients, 262 had shown a T2 lesion.
Figure 2. Surgical procedures in 1,667 postmenopausal patients with negative lymph nodes.
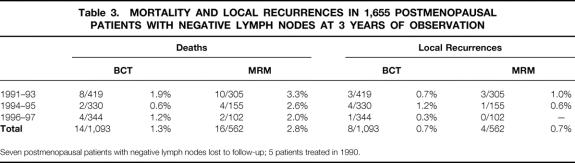
Roughly the same percentages of postmenopausal, node-negative participants in the BCT and MRM sets experienced local relapse of disease (<1% at 3 years), reflecting a highly beneficial tumor biology due to positive estrogen and/or progesterone receptor values. The mortality rate in conservatively treated women (1.3%) was also very low, representing a patient group with an excellent prognosis (Table 3).
Table 3. MORTALITY AND LOCAL RECURRENCES IN 1,655 POSTMENOPAUSAL PATIENTS WITH NEGATIVE LYMPH NODES AT 3 YEARS OF OBSERVATION
Seven postmenopausal patients with negative lymph nodes lost to follow-up; 5 patients treated in 1990.
Postmenopausal, Node-Positive Patients
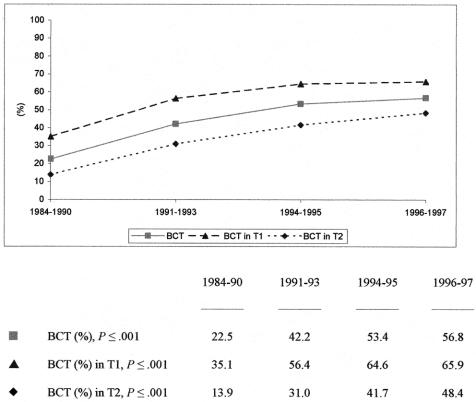
In our final analysis, 1,202 postmenopausal patients with lymph node metastases were included from four different trials conducted between 1984 and 1997 (Trials 4, 6, 8, and 9). The overall BCT rate in these patients was 41.1%; 541 women presented with a T1 lesion and 661 with a T2 lesion. We found a moderate increase in T1 tumors from 40.3% to 48.3% over time. The rate of conservative surgical procedures rose from 22.5% in 1984 to 1990 to 56.8% in 1996 to 1997 (Fig. 3). Patients presenting with T1 lesions experienced a significant increase from 35.1% to 65.9%, the percentage rising from 13.9% to 48.4% in those with T2 tumors. The mean numbers of investigated nodes in the time periods were 11.9 (± 4.8), 13.6 (± 4.9), 14.4 (± 5.5), and 15.5 (± 5.4), respectively. According to age groups (50–60, 61–70, 71–80), we identified BCT rates of 43.5%, 40.0%, and 37.5%, respectively. Of 711 patients treated with MRM, 466 had presented with a T2 lesion and 63 had more than 10 involved nodes.
Figure 3. Surgical procedures in 1,202 postmenopausal patients with lymph node metastases.
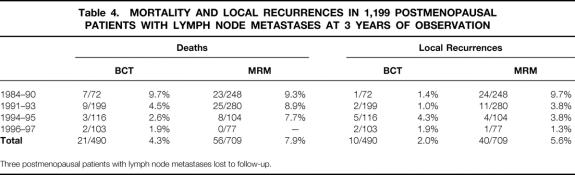
At 3 years, the local recurrence rate in the BCT set was 1.4% in the first observation period, 1% in the second, and 4.3% and 1.9% in the last two periods (Table 4). In node-positive patients, this development reflects an exceptional level of local quality control, due not only to the quality of surgery but also to postoperative endocrine treatment using tamoxifen.
Table 4. MORTALITY AND LOCAL RECURRENCES IN 1,199 POSTMENOPAUSAL PATIENTS WITH LYMPH NODE METASTASES AT 3 YEARS OF OBSERVATION
Three postmenopausal patients with lymph node metastases lost to follow-up.
DISCUSSION
These results indicate that during 16 years of clinical trials carried out by the ABCSG, surgical procedures in women with hormone-responsive breast cancer have undergone considerable change. In the mid-1980s, a mere 20% to 25% of all patients were treated conservatively; that rate increased to 73.2%, 77.3%, and 56.8% in the last observation period until 1997, depending on age, tumor diameter, and nodal status. This significant increase in the rate of BCT was not counterbalanced by an increasing frequency of local recurrences. At 3 years of observation, the local recurrence rates were 0% to 4.7% in premenopausal stage II breast cancer patients, less than 1.5% in postmenopausal stage I patients, and 1% to 4.3% in postmenopausal stage II patients. Clearly, the results point to a superior level of surgical quality control. They also reflect a beneficial tumor biology based on hormone-responsive tumors and postoperative treatment with tamoxifen, known to reduce local recurrences in patients with receptor-positive tumors. No significant difference was identified between patients treated conservatively or with mastectomy. The 3-year time interval was chosen on account of intertrial differences as to follow-up periods. We failed to identify any impact of increased BCT on overall survival. Overall, death and local recurrence rates were higher in patients treated with MRM on account of selection bias for those presenting with more advanced disease or poorer prognostic findings.
Of course, tumor size remains a key parameter in determining the surgical procedure to be used. In any of the time periods under investigation, the BCT rate was higher in T1 than in T2 lesions and lower in the presence than in the absence of nodal metastases. Other investigators have also demonstrated how T and N stages serve as selection criteria for BCT, both in early-stage and locally advanced disease, remaining unchanged by the recent use of neoadjuvant chemotherapy for improved BCT rates. 3,13,14
Comparing age groups in the last observation period, we found an increased amount of conservative interventions to be performed in premenopausal than in postmenopausal node-positive patients (81.8% in T1 and 63.2% in T2 vs. 65.9% and 48.4%, respectively). This difference between BCT rates depending on age and favoring premenopausal patients is interesting, since young age is an independent prognostic factor as well as a marker for adverse tumor biology. In the postmenopausal cohorts, we found a similar trend favoring younger women for BCT.
A marked overall increase has become noticeable in conservative procedures over the time periods investigated. There are several potential reasons for such a development within a nationwide trialists’ group operating in a small country. Explanations include better surgical training, improved awareness among surgeons and patients regarding the psychological, ablation-related trauma, and indirect effects related to increased media coverage. BCT rates may have also been ameliorated by the favorable results arising from published randomized trials, pressure from the public and individual patients (both pre- and postmenopausal), and probably also the presentation and discussion of such outcomes within the ABCSG.
The Study Group was founded in 1984 with the objective of conceptualizing and conducting nationwide multicenter trials dealing with adjuvant therapy in breast and bowel cancer patients. A network of 21 study centers was involved in the first generation of trials (1984–90), randomizing 1,118 subjects. 12,15 From 1991 to 1996, three further trials were implemented with a total of 71 participating centers and 3,356 recruited patients. Currently, 10 nationwide trials are in progress, with an approximate average of 950 annual accruals.
Participation in clinical trials in oncology is not only a potent way to address important medical questions with respect to study endpoints. In addition, it acts to maintain or even improve treatment quality in other aspects of primary patient care, including pathology, surgery, follow-up, and quality of life, depending on the way a protocol is designed. We believe it is imperative to avoid downregulation or limitations to such quality control aspects for the sake of increasing patient participation in clinical trials.
Only limited information exists in the literature dealing with the continuous development of BCT in multicenter clinical trials. For example, in large studies based on comparable criteria of patient selection (stage I and II disease, age, and/or receptor and nodal status), reported BCT rates range from 29.6% to 61.7% in premenopausal, node-positive women. 16–19 In postmenopausal patients, rates varying from 23.7% to 51% have been indicated in the presence of node positivity, 16,17,20,21 and a 18.7% rate has been produced in the absence of nodal metastases. 22 Other trials have reported BCT rates to be as low as 4.3% to 6.6% in receptor- and node-positive disease. 23,24 None of these investigations, however, has provided sufficient information to estimate how many patients are primarily suited for conservative surgery.
Rather, our general knowledge regarding BCT rates in large series is based on information from personal series and cancer registries. The Surveillance, Epidemiology, and End Results (SEER) Program assessed data from 19,661 white women with localized breast cancer who had been diagnosed between 1983 and 1986. The BCT rate ranged from 14.8% to 36.7%, being predicted by age, county characteristics, physician-to-population ratio, education and income levels, presence of a cancer center, and residence in a city of at least 100,000 inhabitants. In a multivariate analysis, regional variation was also shown to be a significant independent predictor for the type of surgery. 25 Another evaluation from a cancer database dealing with 157 hospitals and cases from 1988 to 1993 found the rate of BCT doubling from 7.3% to 14.3% over 6 years. Independent prognostic factors for BCT included age, private insurance coverage, hospital size, and treating surgeon’s graduation from medical school since 1981. 26
In a survey conducted in over 80,000 Medicare patients aged 65 to 79, and treated in 1986 and 1990, BCT percentages changed only insignificantly from 14.1% to 15%. Ten percent of hospitals performed 55% of all conservative surgical procedures. 27 The survey shows how important the surgeon’s opinion proves to be in the decision-making process. Improving BCT rates in the general population thus implies increased awareness among physicians treating breast disease, as well as improved access to BCT-specific training and education.
A study investigating the effect of state legislation with the aim of promoting BCT for women with early-stage breast cancer found a slight and transient effect on the degree to which this surgical procedure was put to use. 28 Neither specific legislation nor fully informed patient consent, which is to be applied uniformly, represents action that is powerful enough to increase the BCT rate. Again, the obvious reason is that the treating surgeon’s recommendation represents the most crucial factor. 9,29,30 The Colorado Center Cancer Registry, for example, indicated that 72% of patients presenting with T1 breast cancer in that state had been treated with MRM between 1986 and 1990. 9 A questionnaire sent to 175 general surgeons suggested that those who do not believe these two surgical modalities to be equivalent with respect to final outcome produce a higher rate of mastectomy in T1 cases. This also applies to surgeons who consider the two methods to be equivalent, but mastectomy still to be the gold standard. In precise terms, their rate is 15% to 20% higher than that of physicians who regard BCT as the treatment of choice. These data again act to stress the key role treating surgeons play in their patients’ decision. By contrast, medical contraindication is rarely an important factor for not performing organ-preserving treatment. 31
Local recurrences after BCT and following mastectomy have different biologic implications. Those developing after conservative surgery are commonly due to tumor cells left behind within the operative field or the presence of multifocality or multicentricity (often relating, in the patient’s mind, to the quality of surgery performed). Conversely, local recurrences after mastectomy are usually a sign of generalized disease. In particular, surgeons lacking sufficient training in the field may not want to risk taking the blame for surgical inaccuracy and therefore would give preference to mastectomy, the surgical procedure that in many ways is technically better defined. Certainly, however, such a perspective is ethically unacceptable.
Information concerning recent developments in medicine can be obtained, both by the physician in academic institutions and in nonacademic hospitals, from a variety of sources, including scientific publications, consensus meetings, professional societies, the Internet, and the press. Another approach, as we have argued, is to participate in cooperative trials that are not only dedicated to exploring new systemic therapies but also exert high quality control to applied surgical procedures, especially when small hospitals or private surgical practitioners are involved. The final goal is to ensure that every patient receives the best oncologic treatment—implying, for example, a BCT rate ranging from 80% to 90% in T1 cases. Our group has shown that by involving a large number of breast cancer patients in nationwide trials, it is indeed possible to take a step in this direction. However, patience is equally important for a number of years for local oncologic results to exert a genuine impact on the quality of surgery.
Acknowledgments
The authors gratefully acknowledge the support given by the ABCSG Trial Center affiliates, who have made various valuable contributions throughout the past 16 years of clinical breast cancer research in Austria.
APPENDIX
The Austrian Breast & Colorectal Cancer Study Group included the following investigators:
R. Jakesz, M. Gnant, E. Kubista, S. Taucher, and M. Seifert (authors), I. Agstner, C. Dadak, P. Dubsky, A. Galid, B. Gebhard, E. Hanzal, H. Helbich, E. Joura, D. Kandioler-Eckersberger, G. Locker, K. Oberhuber, M. Ploner, G. Reiner, S. Roka, M. Rudas, C. Sam, M. Schemper, G. Steger, C. Wenzel, and G. Wolf (University of Vienna); H. Samonigg, H.-J. Mischinger, and P. Steindorfer (authors), E. Andritsch, H. Bacher, T. Bauernhofer, A. Berger, H. Cervenka, A. El-Shabrawi, J. Freisinger, H. Hauser, J. Hebenstreit, G. Hofmann, A.-K. Kasparek, P. Konstantiniuk, G. Kosina, P. Krippl, L. Kronberger, I. Kuss, G. Luschin-Ebengreuth, R. Moser, H. Papadi, H. Pfeifer, F. Ploner, S. Reinisch, M. Riegler, G. Rosanelli, W. Schippinger, M. Schmid, W. Schwaiger, M. Smola, H. Stöger, M. Thalhammer, I. Thiel, P. Wagner, M. Wehrschütz, R. Winter, and G. Zehetleitner (University of Graz, Graz Hospital, and BHB Hospital Graz); B. Mlineritsch, R.-C. Menzel, and H. Hausmaninger (authors), E. Hell, H. Kogelnik, E. Moritz, C. Papp, R. Schandalik, M. Umlauft, and H. Waclawiczek (Salzburg, Braunau, Hallein and Oberndorf Hospitals, and BHB Hospital Salzburg); D. Depisch and W. Kwasny (authors), K. Haider and T. Payrits (Wiener Neustadt Hospital); R. Kolb (author, Waehring Lutheran Hospital Vienna); C. Tausch (author), M. Aufschnaiter, D. Heck, R. Klug, F. Kugler, and R. Schildberger (BHS Hospital Linz); M. Stierer (author), H. Matzinger and H. Spoula (Hanusch Medical Center Vienna); K. Renner and R. Schiessel (SMZ Ost Hospital Vienna); U. Schmidbauer and M. Wunderlich (BHS Hospital Vienna); M. Fridrik and G. Wahl (Linz General Hospital); D. Bauer, F. Hofbauer, and M. Lang (Oberpullendorf Hospital); G. Jatzko and V. Wette (BHB Hospital St. Veit/Glan); J. Omann, M. Starlinger, and A. Urbania (Klagenfurt Hospital); K. Keller, W. Schennach, and H. Zoller (Zams Hospital); E. Klug, K. Mach, and K. Steflitsch (Oberwart Hospital); J. Berger, R. Lenzhofer, and G. Winter (Schwarzach Hospital); and A. Haid, R. Koeberle, and G. Zimmermann (Feldkirch Hospital).
References
- 1.Veronesi U, Banfi A, Del Vecchio M, et al. Comparison of Halsted mastectomy with quadrantectomy, axillary dissection, and radiotherapy in early breast cancer: long-term results. Eur J Cancer Clin Oncol. 1986; 22: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Redmond C, Poisson R, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989; 320: 822–828. [DOI] [PubMed] [Google Scholar]
- 3.Lichter AS, Lippman ME, Danforth DN, et al. Mastectomy versus breast-conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at the National Cancer Institute. J Clin Oncol. 1992; 10: 976–983. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberger T, Gorlitzer M, Soliman T, et al. It is possible to omit postoperative irradiation in a highly selected group of elderly breast cancer patients. Breast Cancer Res Treat. 1998; 50: 37–46. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. NIH Consensus Development Conference Statement: treatment of early-stage breast cancer. Bethesda, MD: National Institutes of Health, 1990.
- 6.Guadagnoli E, Weeks JC, Shapiro CL, et al. Use of breast-conserving surgery for treatment of stage I and stage II breast cancer. J Clin Oncol. 1998; 16: 101–106. [DOI] [PubMed] [Google Scholar]
- 7.Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992; 326: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 8.Tate PS, McGee EM, Hopkins SF, et al. Breast conservation versus mastectomy: patient preferences in a community practice in Kentucky. J Surg Oncol. 1993; 52: 213–216. [DOI] [PubMed] [Google Scholar]
- 9.Tarbox BB, Rockwood JK, Abernathy CM. Are modified radical mastectomies done for T1 breast cancers because of surgeon’s advice or patient’s choice? Am J Surg. 1992; 164: 417–422. [DOI] [PubMed] [Google Scholar]
- 10.Jakesz R, Gnant M, Schmid M, et al. Abgeschlossene und derzeit laufende adjuvante Therapieprotokolle bei Patientinnen mit operablem Mammakarzinom (II). Acta Chir Austr. 1997; 29: 62–67. [Google Scholar]
- 11.Bloom HJG, Richardson WW. Histologic grading and prognosis in breast cancer. Br J Cancer. 1957; 11: 359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakesz R, Hausmaninger H, Haider K, et al. Randomized trial of low-dose chemotherapy added to tamoxifen in patients with receptor-positive and lymph node-positive breast cancer. J Clin Oncol. 1999; 17: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 13.Jakesz R, Hausmaninger H, Samonigg H, et al. Die Therapiestudien der Austrian Breast Cancer Study Group (ABC). Zbl Chir. 1998; 123 (suppl 5): 28–32. [PubMed] [Google Scholar]
- 14.Merajver S, Weber BL, Cody R, et al. Breast conservation and prolonged chemotherapy for locally advanced breast cancer: the University of Michigan experience. J Clin Oncol. 1997; 15: 2873–2881. [DOI] [PubMed] [Google Scholar]
- 15.Jakesz R, Samonigg H, Gnant M, et al. Very low-dose adjuvant chemotherapy in steroid receptor negative stage I breast cancer patients. Eur J Cancer. 1998; 34: 66–70. [DOI] [PubMed] [Google Scholar]
- 16.Hürny C, Bernhard J, Coates AS, et al. Impact of adjuvant therapy on quality of life in women with node-positive operable breast cancer. Lancet. 1996; 347: 1279–1284. [DOI] [PubMed] [Google Scholar]
- 17.Wallgren A, Bernier J, Gelber RD, et al. Timing of radiotherapy and chemotherapy following breast-conserving surgery for patients with node-positive breast cancer: International Breast Cancer Study Group. Int J Radiat Oncol Biol Phys. 1996; 35: 649–659. [DOI] [PubMed] [Google Scholar]
- 18.Scottish Cancer Trials Breast Group, ICRF Breast Unit, Guy’s Hospital, London. Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: The Scottish Trial. Lancet. 1993; 341: 1293–1298. [PubMed] [Google Scholar]
- 19.Fumoleau P, Devaux Y, Vo Van ML, et al. Premenopausal patients with node-positive resectable breast cancer: preliminary results of a randomised trial comparing 3 adjuvant regimens: FEC 50 × 6 cycles vs FEC 50 × 3 cycles vs FEC 75 × 3 cycles. Drugs. 1993; 45: 38–45. [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KI, Paterson AHG, Fine S, et al. Randomized trial of cyclophosphamide, methotrexate, and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: a report of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1997; 15: 2302–2311. [DOI] [PubMed] [Google Scholar]
- 21.Gerard JP, Hery M, Gedouin D, et al. Postmenopausal patients with node-positive resectable breast cancer: tamoxifen vs FEC 50 (6 cycles) vs FEC 50 (6 cycles) plus tamoxifen vs control: preliminary results of a 4-arm randomised trial. Drugs. 1993; 45: 60–67. [DOI] [PubMed] [Google Scholar]
- 22.Rutqvist LE, Cedermark B, Glas U, et al. Randomized trial of adjuvant tamoxifen in node negative postmenopausal breast cancer. Acta Oncol. 1992; 31: 265–270. [DOI] [PubMed] [Google Scholar]
- 23.Gundersen S, Hannisdal E, Søreide JA, et al. Adjuvant tamoxifen for pre- and postmenopausal women with estrogen receptor positive, node positive breast cancer: a randomized study. Breast Cancer Res Treat. 1995; 36: 49–53. [DOI] [PubMed] [Google Scholar]
- 24.Rivkin SE, Green S, O’Sullivan J, et al. Adjuvant CMFVP versus adjuvant CMFVP plus ovariectomy for premenopausal, node-positive, and estrogen receptor-positive breast cancer patients: a Southwest Oncology Group study. J Clin Oncol. 1996; 14: 46–51. [DOI] [PubMed] [Google Scholar]
- 25.Samet JM, Hunt WC, Farrow DC. Determinants of receiving breast-conserving surgery: The Surveillance, Epidemiology, and End Results Program, 1983–1986. Cancer. 1994; 73: 2344–2351. [DOI] [PubMed] [Google Scholar]
- 26.Kotwall CA, Covington DL, Rutledge R, et al. Patient, hospital, and surgeon factors associated with breast conservation surgery: a statewide analysis in North Carolina. Ann Surg. 1996; 224: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nattinger AB, Gottlieb MS, Hoffman RG, et al. Minimal increase in use of breast-conserving surgery from 1986 to 1990. Med Care. 1996; 34: 479–489. [DOI] [PubMed] [Google Scholar]
- 28.Nattinger AB, Hoffman RG, Shapiro R, et al. The effect of legislative requirements on the use of breast-conserving surgery. N Engl J Med. 1996; 335: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 29.Ward S, Heidrich S, Wolberg W. Factors women take into account when deciding upon type of surgery for breast cancer. Cancer Nurs. 1989; 12: 344–351. [PubMed] [Google Scholar]
- 30.Pierce PF. Deciding on breast cancer treatment: a description of decision behavior. Nurs Res. 1993; 42: 22–48. [PubMed] [Google Scholar]
- 31.Morrow M, Schmidt R, Hassett C. Patient selection for breast conservation therapy with magnification mammography. Surgery. 1995; 118: 621–626. [DOI] [PubMed] [Google Scholar]
Footnotes
Correspondence: Raimund Jakesz, MD, Department of Surgery, University of Vienna, Waehringer Guertel 18-20, A-1090 Vienna, Austria.
E-mail: raimund.jakesz@akh-wien.ac.at
Accepted for publication September 11, 2002.